Abstract
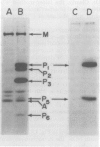
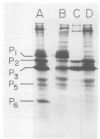
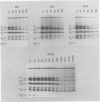
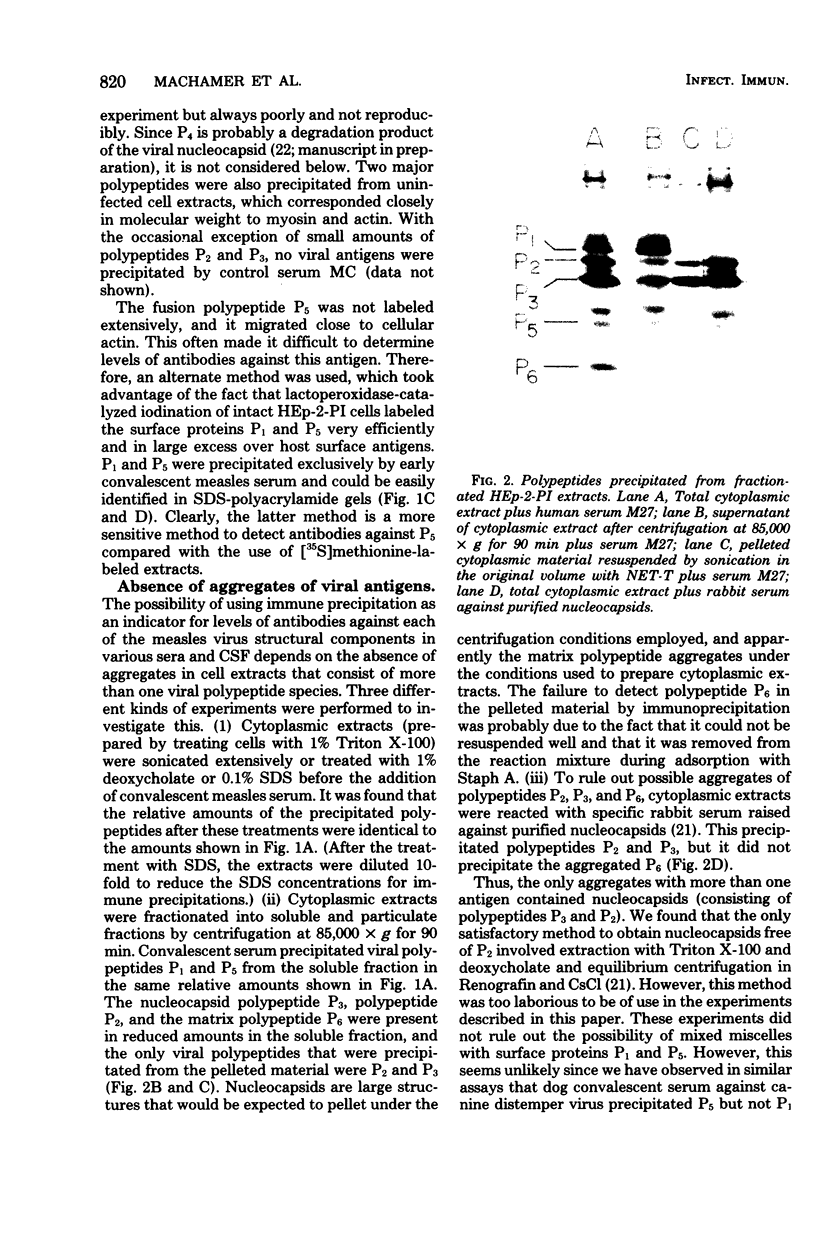
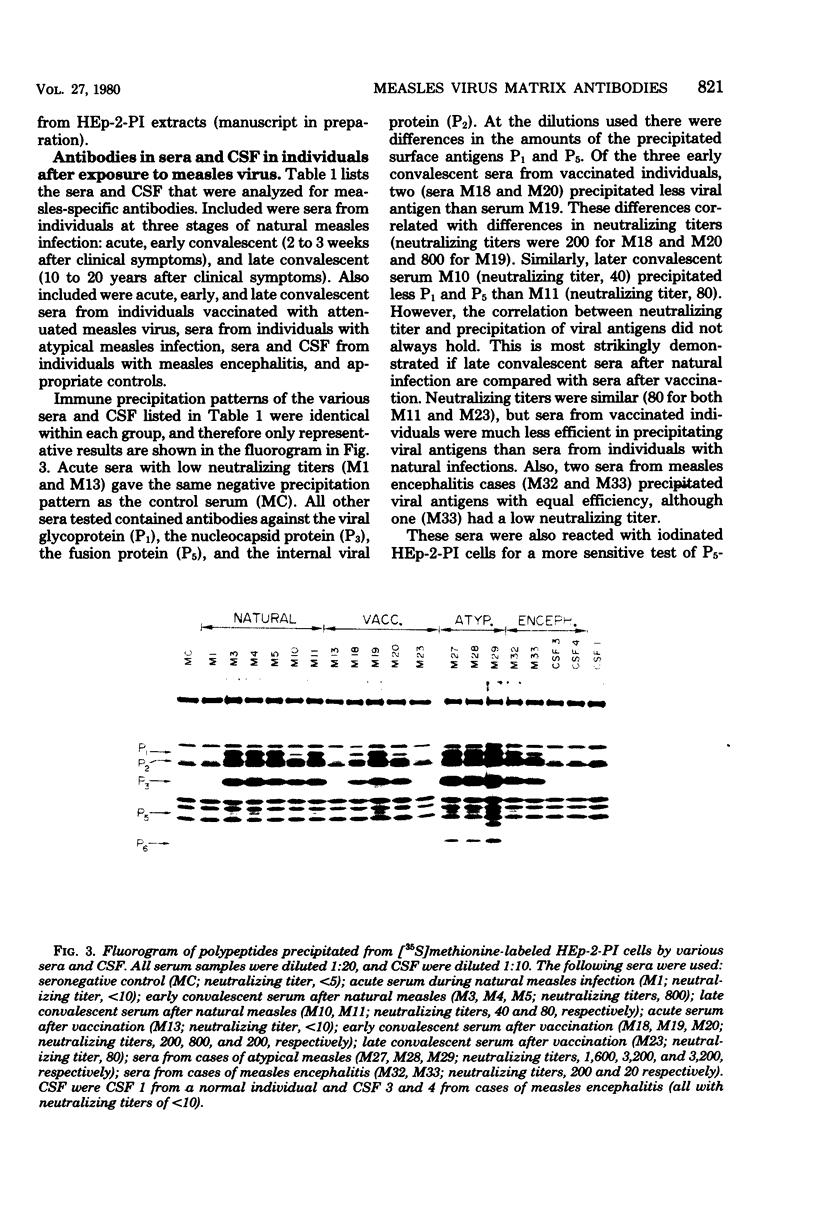
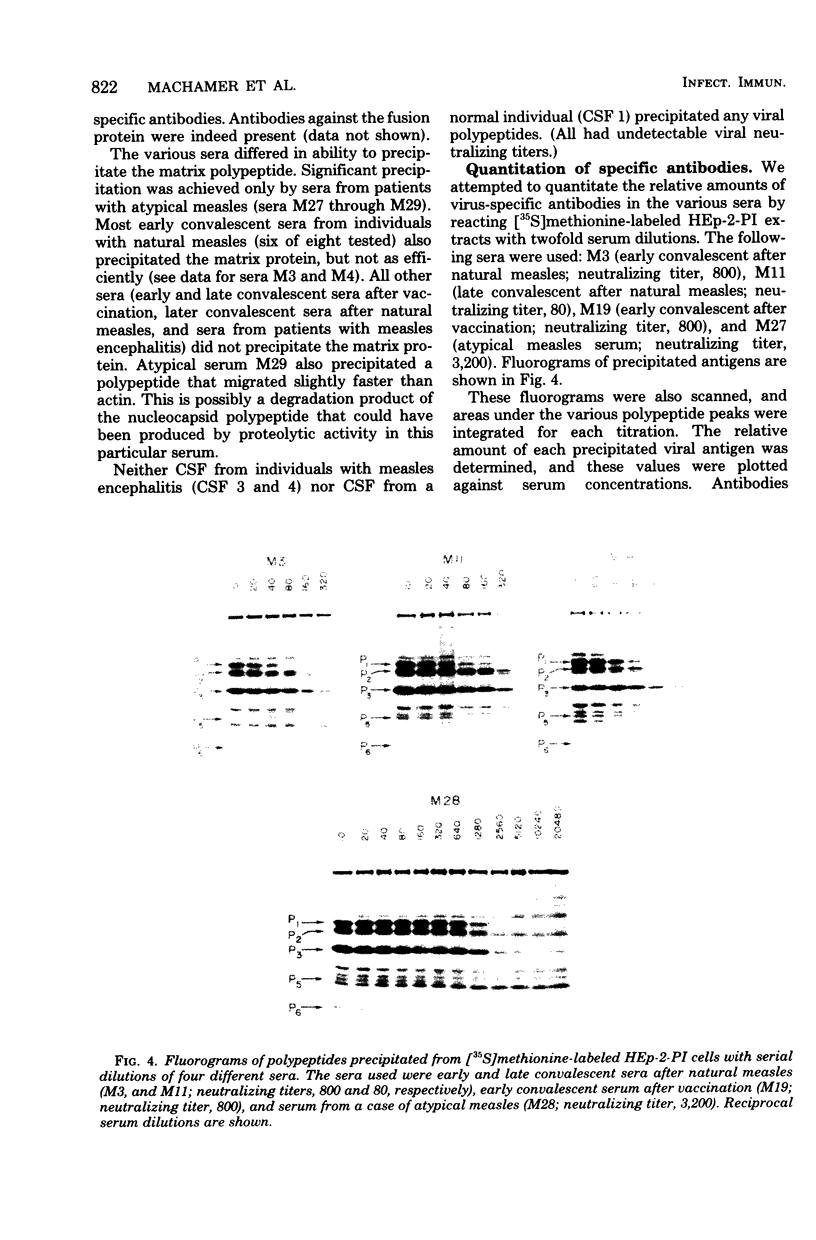
Sera from patients exposed to measles virus were investigated for the presence of antibodies against each of the viral antigens. All sera with measurable neutralizing titers contained antibodies against the two surface proteins (the glycoprotein and fusion protein), the nucleocapsid protein, and one of the internal proteins (P2). However, only sera from individuals with clinical symptoms of measles infection (natural measles and atypical measles) contained antibodies against the measles virus matrix protein. Levels of matrix-specific antibodies were highest in patients with atypical measles infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddison W. E., Doherty P. C., Webster R. G. Antibody to influenza virus matrix protein detects a common antigen on the surface of cells infected with type A influenza viruses. J Exp Med. 1977 Sep 1;146(3):690–697. doi: 10.1084/jem.146.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P., Collins J. J., Leis J. P., Moennig V., Schäfer W., Atkinson P. H. Role of carbohydrate in determining the immunochemical properties of the major glycoprotein (gp71) of Friend murine leukemia virus. J Virol. 1975 Dec;16(6):1453–1463. doi: 10.1128/jvi.16.6.1453-1463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Minden P., Sharpton T. R., McClatchy J. K., Farr R. S. Precipitation of radiolabeled antigen-antibody complexes with protein A-containing Staphylococcus aureus. J Immunol. 1977 Jul;119(1):193–198. [PubMed] [Google Scholar]
- Chen P., Farrar J. J., Oppenheim J. J., Mergenhagen S. E. Mechanism of adjuvant activity of dental plaque: in vitro activation of residual helper T-cell precursors in T-cell-deficient murine spleen cell cultures. Infect Immun. 1977 Sep;17(3):567–571. doi: 10.1128/iai.17.3.567-571.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Effros R. B., Bennink J. Heterogeneity of the cytotoxic response of thymus-derived lymphocytes after immunization with influenza viruses. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1209–1213. doi: 10.1073/pnas.74.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., Bussell R. H. Glycoproteins of measles virus under reducing and nonreducing conditions. J Virol. 1978 Feb;25(2):687–692. doi: 10.1128/jvi.25.2.687-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E. C., Wright L. L., Zweerink H. J. Staphylococcal protein A-sepharose columns and the characterization of measles virus-specific polypeptides in persistently infected cells. Anal Biochem. 1978 Nov;91(1):276–282. doi: 10.1016/0003-2697(78)90841-2. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Perrin L. H., Oldstone M. B. Measurement of virus antigens on the surface of HeLa cells persistently infected with wild type and vaccine strains of measles virus by radioimmune assay. J Gen Virol. 1976 Mar;30(3):329–337. doi: 10.1099/0022-1317-30-3-329. [DOI] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Frommel D. Definition of staphylococcal protein A reactivity for human immunoglobulin G fragments. Immunochemistry. 1970 Jan;7(1):124–127. doi: 10.1016/0019-2791(70)90036-4. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J. Immunopathology of measles. Proc R Soc Med. 1974 Nov;67(11):1120–1122. doi: 10.1177/003591577406701115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Schneider I., Schafer W. Properties of mouse leukemia viruses. VII. The major viral glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974 Sep;61(1):100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- Moore P. M., Hayes E. C., Miller S. E., Wright L. L., Machamer C. E., Zweerink H. J. Measles virus nucleocapsids: large-scale purification and use in radioimmunoassays. Infect Immun. 1978 Jun;20(3):842–846. doi: 10.1128/iai.20.3.842-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Salmi A. A., Norrby E., Panelius M. Identification of different measles virus-specific antibodies in the serum and cerebrospinal fluid from patients with subacute sclerosing pancencephalitis and multiple sclerosis. Infect Immun. 1972 Sep;6(3):248–254. doi: 10.1128/iai.6.3.248-254.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Intracellular synthesis of measles virus-specified polypeptides. J Virol. 1978 Jan;25(1):285–297. doi: 10.1128/jvi.25.1.285-297.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Holland J. Target antigens for H-2-restricted vesicular stomatitis virus-specific cytotoxic T cells. J Immunol. 1978 Aug;121(2):744–748. [PubMed] [Google Scholar]
- Zweerink H. J., Askonas B. A., Millican D., Courtneidge S. A., Skehel J. J. Cytotoxic T cells to type A influenza virus; viral hemagglutinin induces A-strain specificity while infected cells confer cross-reactive cytotoxicity. Eur J Immunol. 1977 Sep;7(9):630–635. doi: 10.1002/eji.1830070910. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]