Abstract
Background:
There is interest about the role of platelet (PLT) number and function in nonalcoholic fatty liver disease (NAFLD). NAFLD patients have abnormalities of PLT number and function, especially mean platelet volume (MPV) which is known as a novel biomarker for atherosclerosis. We decided to compare PLT number and function between NAFLD and healthy participants.
Materials and Methods:
In this case–control study, two groups of patients (65 cases with NAFLD and 65 cases without NAFLD) were included consecutively. The diagnosis of NAFLD was made using ultrasound examination of the liver. Venous blood samples were taken, and the required laboratory markers including PLT number and function (MPV, platelet distribution width [PDW]), prothrombin time (PT), partial thromboplastin time (PTT), lipid profile, hepatic transaminases, ferritin, and fasting blood sugar were assayed.
Results:
Mean (± standard deviation [SD]) MPV in NAFLD group (10.29 ± 0.95 fL) was significantly higher than in control group (9.56 ± 1.18 fL); P < 0.001. No significant difference was observed regarding mean (± SD) PLT count between NAFLD (271.20 ± 52.11 × 103/mm3) and healthy participants (262.86 ± 75.81 × 103/mm3) (P = 0.46). Mean (± SD) PDW values were not significantly different between NAFLD and control groups. Logistic regression showed that NAFLD was positively associated with higher MPV (odds ratio [OR] =1.9, 95% confidence interval [CI] =1.20–3.02) and body mass index (OR = 1.5, 95% CI = 1.05–2.15) values. However, PT (OR = 0.14, 95% CI = 0.02–0.82) and PTT (OR = 0.72, 95% CI = 0.58–0.88) had negative association with NAFLD.
Conclusion:
Higher MPV was found to be significantly associated with NAFLD. However, such significant association was not detected regarding PLT count or PDW. As MPV is a reported risk factor for atherosclerosis, this marker may be useful in follow-up of patients with NAFLD. These findings provide basis for further studies to address this marker in long-term follow-up of NAFLD patients.
Keywords: Blood platelets, mean platelet volume, nonalcoholic fatty liver disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of steatosis (fat accumulation) in hepatocytes in a patient who does not consume alcohol. Many patients may be asymptomatic, and the disease may be diagnosed accidentally when liver imaging (for example by ultrasound) is done for other purposes.[1]
Laboratory tests have been the focus of research studies recently to better understand the abnormalities occur in such patients. Various laboratory markers are being studied including lipid profile, resistin and ghrelin,[2] hepatic transaminases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]),[3] platelet (PLT) number and its function markers such as mean platelet volume (MPV)[4] and platelet distribution width (PDW), and many other markers.
All the efforts made in this discipline are to define more accurately the role that noninvasive laboratory tests can have in diagnosis, screening, or follow-up of NAFLD patients.[6] A laboratory marker which has gained attention in NAFLD is PLT count and its function. Several studies have addressed this laboratory marker, especially in alcoholic liver disease, liver cirrhosis, and prediction of fibrosis severity (grade) in NAFLD and nonalcoholic steatohepatitis (NASH).[7,8,9,10,11] The utility of PLT count stems from the observations in liver cirrhosis patients and pathophysiologic changes which occur including splenomegaly and sequestration of PLTs which end in thrombocytopenia. Moreover, PLT count has been included in some scoring systems such as NAFLD fibrosis score.[12] The fact that PLT count is decreased in liver diseases has long been known. In a study, it was reported that patients with moderate-to-severe NAFLD had higher PLT counts than milder form of NAFLD.[13] In contrast, another study reported a significant linear decrease in PLT count as the histological severity of fibrosis in NAFLD worsened.[10]
MPV is a biomarker of PLT activation and function. It has been reported that MPV has prognostic value for cardiovascular diseases and a risk factor for atherothrombosis[14] and raised levels of MPV are associated with acute myocardial infarction (MI) and higher mortality rate thereafter.[15] Larger PLTs compared to small PLTs are more active enzymatically and produce more thromboxane A2 with higher possibility of vascular damage and thrombosis formation.[4] MPV has also been noted to have association with metabolic diseases.[16] Therefore, much interest is observed in the literature about the level of MPV in NAFLD. There is controversy about this topic. A previous study reported that MPV is higher in NAFLD compared to control group.[7] However, there are studies that did not show such difference regarding MPV between healthy controls, simple steatosis, borderline NASH, and definitive NASH.[17] Another study also showed that MPV is not significantly different among healthy participants, alcoholic liver disease, NAFLD, and alcoholics.[8]
Here, we decided to compare PLT count and its function between NAFLD and healthy controls. We think that the results presented here would improve the current evidence about the role that PLT has in NAFLD.
MATERIALS AND METHODS
Study population and research design
In this case–control study conducted in February to September 2015 in Vali-e-Asr Hospital, Birjand, Iran, the study population consisted of patients who presented to the internal medicine clinic of our university hospital. There were two groups. One group had NAFLD based on ultrasound examination and the other group was healthy controls. The sample size was calculated based on the study by Ozhan et al. about mean values of high-density lipoprotein (HDL) in patients with NAFLD.[7] At confidence level of 95% and power of 80%, the estimated sample size was calculated as 61 patients in each group. The sampling method was convenient. The inclusion criteria were the age range of 18–45 years. Exclusion criteria were history of MI or stroke in the last month, infectious diseases, malignancy, chronic liver disease, hepatitis C, rapid weight loss (more than 10% of the body weight in the past 3 months) or rapid weight gain (more than 10% of the body weight in the past 3 months), connective tissue or autoimmune diseases, inflammatory bowel disease, alcoholics, hematologic diseases, taking medicines that interact with normal PLT functions (such as aspirin) or cause NAFLD (amiodarone, valproic acid, antiretroviral drugs, methotrexate, and tetracyclines), or medicines that are used for management of NAFLD (Vitamin E, metformin, thiazolidinediones, or herbal medicines/supplements). The controls were recruited from patients who presented for getting routine checkups.
Data collection
The demographic data gathered included age, gender, height, weight, and waist circumference. Body mass index (BMI) was calculated, and blood pressure (BP) readings were documented by a board-certified internist. Hypertension was defined as systolic BP (SBP) of ≥140 mmHg and/or diastolic BP (DBP) of ≥90 mmHg.
Five CC venous blood sample was obtained from the brachial vein and sent to the laboratory. The laboratory markers assayed were PLT count, MPV, PDW, prothrombin time (PT), partial thromboplastin time (PTT), lipid profile (HDL, low-density lipoprotein [LDL], total cholesterol, and triglyceride), hepatic transaminases (ALT and AST), fasting blood sugar (FBS), and ferritin.
NAFLD was divided into three grades based on ultrasound examination.[18]
Statistical analyses
The data were gathered and entered into the SPSS software for Windows (version 21.0) (IBM Corp., Armonk, NY, USA). Descriptive indices such as frequency, percentage, mean, and its standard deviation (SD) were used to express data. One-sample Kolmogorov–Smirnov (K–S) test was used to evaluate whether the distribution of continuous variables including SBP, DBP, BMI, PLT, MPV, PDW, PT, and PTT was normal or not. BMI, weight, PLT, and LDL in each group and MPV and PDW in control group had normal distribution (K–S test, P > 0.05). Other variables had nonnormal distribution. For comparison of mean values of BMI, weight, PLT, and LDL between the two studied groups, we used Student's t-test. Mann–Whitney U-test was used to compare nonnormal variables between the two groups. For comparison of qualitative variables between the studied groups, Chi-square test was used. For comparison of mean values of PLT between control, NAFLD Grade I, and NAFLD Grades II and III, one-way analysis of variance was used. Kruskal–Wallis test was used for comparison of MPV, PDW, PT, and PTT between the three mentioned groups. Finally, to control the effect of confounding variables and to determine the effect of the variables on NAFLD occurrence; a multiple logistic regression model (stepwise method) was developed. The variables included in the model were PLT, MPV, PDW, PT, PTT, age, BMI, weight, and gender. Significance level for all analyses was set at 0.05.
Ethics
The study protocol was approved by the Ethics Committee of our medical university (No. 987). The study objectives were explained for the patients before participation, and if agreed, written informed consent was obtained from them.
RESULTS
There were totally 130 patients (65 patients in NAFLD group and 65 in control group). Mean (± SD) age of the patients was 37.62 (±5.66) years. There were 28 males (44.4%) in NAFLD group and 37 males (55.2%) in control group (P = 0.29). Table 1 presents a comparison of age, BP, weight, BMI, and waist circumference between the study groups. As observed age, waist circumference, weight, and BMI were significantly higher in this group compared to healthy control group. More patients in NAFLD group (11 cases, 16.9%) had hypertension compared to control group (4 cases, 6.2%); P = 0.04. In NAFLD group, Grades I, II, and III were diagnosed, respectively, in 33 patients (50.8%), 30 patients (46.2%), and 2 patients (3.1%).
Table 1.
Comparison of age and body mass index, weight, and waist circumference between nonalcoholic fatty liver disease patients and healthy controls
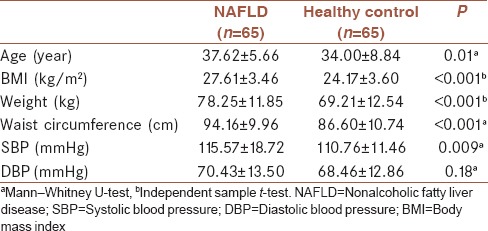
Mean (± SD) MPV in NAFLD group (10.29 ± 0.95 fL) was significantly higher than in control group (9.56 ± 1.18 fL); P < 0.001. Table 2 presents a comparison of PLT count and its function between the two study groups. As seen, PLT count and PDW were not different between the two groups. PT and PTT were both significantly lower in NAFLD group. In addition, comparison of the mentioned variables between three groups (control, NAFLD Grade I, and NAFKD Grades II and III) is presented in Table 2. Kruskal–Wallis test showed that MPV value was significantly different among NAFLD Grades I–III and control group (P = 0.001). Mann–Whitney test showed that MPV value was significantly different between NAFLD Grade I and control group (P = 0.004) and between NAFLD Grades II and III and control group (P = 0.001). Regarding PTT, this value was significantly different between Grade I NAFLD and control group (P = 0.001) and between Grades II and III NAFLD and control group (P = 0.006). Regarding PT, this value was significantly different between Grades II and III NAFLD and control group (P = 0.02). No statistically significant difference was detected regarding PLT and PDW between Grade I NAFLD and control group as well as between Grades II and III NAFLD and control group and PT between Grade I NAFLD and control group.
Table 2.
Comparison of platelet count and its function between nonalcoholic fatty liver disease patients and healthy controls as well as between control group and nonalcoholic fatty liver disease Grade I and Grades II and III
PLT count of more than 200,000/dL was seen in 60 patients in NAFLD group (92.3%) and in 56 patients of healthy group (86.2%); P = 0.39.
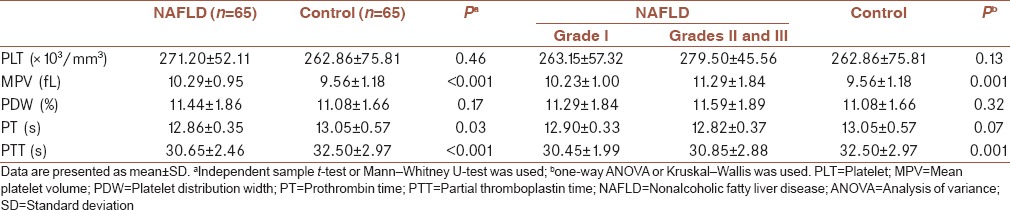
Table 3 presents comparisons made in regarding PLT count and its function markers separately in males and females. As shown in NAFLD group, PLT count was significantly lower in males than in females. The difference observed regarding MPV between NAFLD and control groups still existed based on gender. In both males and females, MPV values were significantly higher in NAFLD group compared to healthy participants.
Table 3.
Platelet count and its function markers based on gender in nonalcoholic fatty liver disease patients and healthy controls
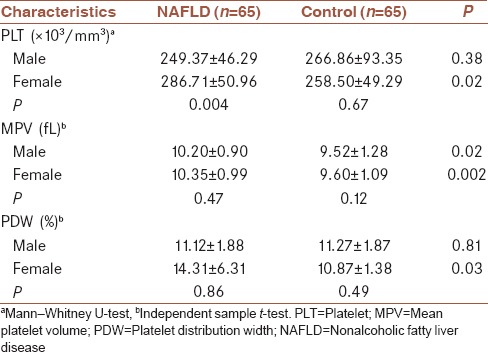
Table 4 presents lipid profile comparison between the two groups. As seen, serum total cholesterol and triglyceride levels were significantly higher in NAFLD group. However, no difference existed regarding serum LDL and HDL levels.
Table 4.
Comparison of lipid profile between nonalcoholic fatty liver disease patients and healthy controls
Mean (SD) AST (26.09 ± 11.2 vs. 23.4 ± 12.2 U/L; P = 0.2), FBS (103.3 ± 31.7 vs. 96.6 ± 10.8 mg/dL; P = 0.1), and ferritin (114.4 ± 115.8 vs. 104.1 ± 101.6 ng/mL; P = 0.5) levels were not statistically different between NAFLD and non-NAFLD groups, respectively. On the other hand, ALT was significantly higher in NAFLD group (37.3 ± 21.3 U/L) than in non-NAFLD group (27.06 ± 24.6 U/L); P = 0.01.
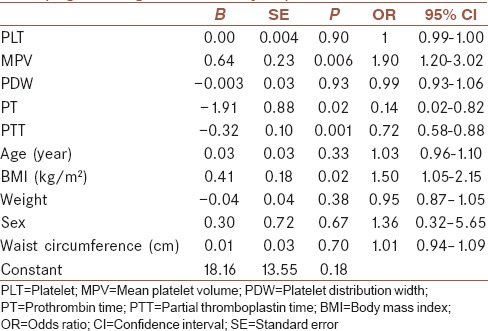
Using logistic regression analysis, BMI (odds ratio [OR] = 1.50, confidence interval [CI] = 1.05–2.15), MPV (OR = 1.90, CI = 1.20–3.02), PT (OR = 0.14, CI = 0.02–0.82), and PTT (OR = 0.72, CI = 0.58–0.88) were found to be significantly associated with the risk of NAFLD occurrence [Table 5]. MPV and BMI had a significant positive association with NAFLD. However, PT and PTT had a negative association with NAFLD. In other words, with higher values of PT and PTT, the risk of NAFLD occurrence reduced.
Table 5.
Independent association of variables with fatty liver (logistic regression analysis)
DISCUSSION
As the basis for PLT count reduction in liver disease is portal hypertension and resultant splenomegaly which causes PLT destruction,[11] it is assumed that the patients here did not have liver injury so severe to produce portal hypertension though measuring spleen size and determining portal hypertension was not performed here. This finding is in contrast to a former study which reported that PLT count was significantly lower in NAFLD group (average of 237.9 × 103/dL) in comparison to control group (average of 266 × 103/dL).[8] The reason of this discrepancy seems to be due to the presence of eight patients (out of 105 cases with NAFLD) with biopsy-proven NASH and heterogeneous group in terms of gender (89 males and 16 females) in the mentioned study.[8] Herein, as the severity of NAFLD worsened, an increase in PLT count was found though this trend was not statistically significant. There are studies which tried to find any possible correlation between PLT count and the severity of liver fibrosis in NAFLD. Another ultrasonography-based study also reported similar results to ours as with the increase in steatosis grade, PLT count increased, but they also reported that a significant difference existed between mild steatosis versus moderate/severe steatosis. However, this difference did not exist anymore in males when analyses were performed considering gender.[13] In a large cohort of 1048 biopsy-proven NAFLD patients, a negative correlation was reported between PLT count and histological grade of NAFLD as follows (mean PLT count): stage 0: 24.8 × 104/μL; Stage 1: 23.7 × 104/μL; Stage 2: 22.0 × 104/μL; Stage 3: 18.9 × 104/μL; and Stage 4: 12.4 × 104/μL; P < 0.0001. However, when the same authors re-analyzed their data of the same sample of 1048 patients, they reported that those with severe steatosis (Grades III and IV) were removed from the analyses; the authors reported that PLT count did not correlate with steatosis grade (23.5 × 104/μL in Grade I, 23.6 × 104/μL in Grade II, and 24.2 × 104/μL in Grade III, P = 0.2029).[9] Another finding which should be considered is the role of gender. When PLT count comparisons between the groups were done in the gender subgroups, it was found that PLT count was significantly higher in female patients with NAFLD in comparison to female controls. In our opinion, at the moment, considering the evidence in the literature and the presented findings here, the role of PLT count in determining NAFLD fibrosis severity yet to be studied more and clarified before advised as a routine use in clinical practice.
According to the obtained findings, MPV was a significant marker which was higher in NAFLD patients. MPV has been noted in several studies as a novel inflammatory marker and important risk factor for cardiovascular diseases.[19] MPV, which is the volume of the average circulating PLT, is measured easily by automated hematology analyzer in routine complete blood cell analysis.[20] It is considered as an indicator of PLT size and function. Since PLTs have a role in thrombosis formation and evidence pointing to association between PLT count and liver injury,[21] some studies have been done to elucidate the role of MPV in NAFLD. In particular, larger PLTs contain more granules which result in increased activation of PLTs.[7] In a previous study on seventy patients with NAFLD, it was noted that MPV was significantly higher than control healthy participants.[7] This study also showed that MPV was positively associated with AST and ALT level and negatively associated with PLT count. In another report including 100 patients who underwent liver biopsy, a significant stepwise increase in MPV was observed from normal biopsy (9.5 fL) to simple steatosis (10.2 fL) and NASH (11.3 fL).[4] However, there are reports that did not find considerable difference regarding MPV values between healthy individuals and patients with NAFLD.[22] In a study on 51 patients with NAFLD (9 patients with simple steatosis, 24 with borderline NASH, and 22 with definitive NASH), no significant difference existed regarding MPV between these patients and healthy controls. This study also demonstrated the inverse relationship between MPV and PLT count.[18] Since MPV has been introduced as an inflammatory marker, it is necessary to control any confounding factor which can affect MPV level. Here, we did not include patients with recent cardiovascular or cerebrovascular events and excluded those who had any autoimmune or connective tissue diseases. It seems that the role of MPV in NAFLD is still controversial, and with ongoing studies about MPV in various diseases, we should consider all contributing factors that can change MPV values. If MPV, possibly in the near future, is recognized as an established factor in NAFLD, it could be used as a noninvasive, simple test to follow the patients. Although MPV and PLT count were significantly higher in female patients with NAFLD compared to control groups, such difference was not observed in male patients. There is no similar evidence in the literature that such difference exists only in females. This finding should be addressed in future studies.
PDW is also a marker for PLT activation. PLTs change their shape to obtain a larger surface which results in increased PDW.[23] PDW is a marker which has been shown to be higher in patients with alcoholic liver cirrhosis.[5] This marker was not significantly different in our study between the two groups. Similarly, the above-mentioned study[5] did not find difference regarding PDW between healthy controls (11.99) and NAFLD (11.76). Although some differences existed between the groups regarding PT and PTT values, both were within normal ranges. As stated earlier, most patients studied here did not have so severe liver disease to expect changes in PT and PTT values. In a previous similar study on NAFLD patients and healthy participants to assess hemostatic alterations, it was shown that PT and activated PTT were normal in both groups.[24]
Triglyceride and total cholesterol are cardiovascular diseases risk factors which were found to be significantly higher in NAFLD group. Dyslipidemia is a common finding in NAFLD patients. In NAFLD, because of insulin resistance as one of the major factors, the balance between synthesis and accumulation impairs and results in high triglyceride level.[25] This finding was observed in our patients. Although atherogenic dyslipidemia is the most common form of dyslipidemia in NAFLD patients,[26] we did not find significant difference regarding LDL, the major atherosclerotic dyslipidemic factor, between NAFLD and control groups. Several factors including insulin resistance, alterations in intracellular cholesterol transport, unbalanced cellular cholesterol homeostasis, increased cholesterol de-esterification and attenuation of cholesterol export, and bile acid synthesis pathways have been mentioned as the basis for dyslipidemia observed in NAFLD.[27]
As fat accumulates in hepatocytes, it is expected to see abnormalities in hepatic transaminases. Here, ALT was significantly higher in NAFLD, but AST was not different. Normal AST and ALT levels do not rule out NAFLD and generally have been suggested to be insensitive factors in follow-up of NAFLD patients to determine improvement or worsening of condition.[3] AST and ALT have been studied mostly to differentiate alcoholic versus nonalcoholic fatty liver changes.
It is not clear that what percentages of patients with NAFLD have abnormal hepatic transaminases as it is not uncommon to suspect NAFLD when abnormal hepatic transaminases are noted.[28] Patients with NAFLD in whom AST and ALT are raised, their levels usually increase two to five times the upper limit of normal and AST to ALT ratio is usually >1.[28]
Another marker studied here was ferritin. There are reports that ferritin and iron are increased in NAFLD.[29] There is also evidence suggesting association between increased ferritin and insulin resistance in NAFLD. In a previous study, a serum ferritin concentration <1.5 times of the upper limit of normal was associated with higher NAFLD activity score.[30] However, according to our findings, even though ferritin concentration was higher in NAFLD, it was not statistically significant.
A limitation of the study was not an equal number of samples in Grades I, II, and III NAFLD so that patients included here mostly had Grade I or II NAFLD.
CONCLUSION
MPV, but not PLT count or PDW, seems to be a noticeable laboratory marker which increases significantly in NAFLD patients. As MPV is reported to be a potential risk factor for atherosclerosis, this marker may be useful in follow-up of patients with NAFLD.
Suggestions
Further studies are required to determine the evolution of changes in MPV, PT, and PTT in long-term follow-up of patients with NAFLD. In our opinion, such studies will add to our knowledge about the possible prognostic value of these laboratory markers. Furthermore, other studies can determine the potential effect of exercise, weight loss, and medicines used for NAFLD on these factors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Karandish M, Tamimi M, Shayesteh AA, Haghighizadeh MH, Jalali MT. The effect of magnesium supplementation and weight loss on liver enzymes in patients with nonalcoholic fatty liver disease. J Res Med Sci. 2013;18:573–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Eminler AT, Aygun C, Konduk T, Kocaman O, Senturk O, Celebi A, et al. The relationship between resistin and ghrelin levels with fibrosis in nonalcoholic fatty liver disease. J Res Med Sci. 2014;19:1058–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Charatcharoenwitthaya P, Lindor KD, Angulo P. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:1925–31. doi: 10.1007/s10620-012-2098-3. [DOI] [PubMed] [Google Scholar]
- 4.Alkhouri N, Kistangari G, Campbell C, Lopez R, Zein NN, Feldstein AE. Mean platelet volume as a marker of increased cardiovascular risk in patients with nonalcoholic steatohepatitis. Hepatology. 2012;55:331. doi: 10.1002/hep.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WS, Kim TY. Alcoholic fatty liver disease and alcoholic liver cirrhosis may be differentiated with mean platelet volume and platelet distribution width. Platelets. 2010;21:584–5. doi: 10.3109/09537104.2010.500423. [DOI] [PubMed] [Google Scholar]
- 6.Alisi A, Nobili V. Sensitive non-invasive circulating markers in paediatric non-alcoholic fatty liver disease. Pediatr Obes. 2012;7:89–91. doi: 10.1111/j.2047-6310.2012.00055.x. [DOI] [PubMed] [Google Scholar]
- 7.Ozhan H, Aydin M, Yazici M, Yazgan O, Basar C, Gungor A, et al. Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010;21:29–32. doi: 10.3109/09537100903391023. [DOI] [PubMed] [Google Scholar]
- 8.Das SK, Mukherjee S, Vasudevan DM, Balakrishnan V. Comparison of haematological parameters in patients with non-alcoholic fatty liver disease and alcoholic liver disease. Singapore Med J. 2011;52:175–81. [PubMed] [Google Scholar]
- 9.Imajo K, Yoneda M, Nakajima A. Are platelets count useful for detecting the grade of steatosis? Hepat Mon. 2015;15:e28957. doi: 10.5812/hepatmon.15(5)2015.28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:1300–6. doi: 10.1007/s00535-011-0436-4. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Kirikoshi H, Yoneda M, Mawatari H, Fujita K, Nozaki Y, et al. Measurement of spleen volume is useful for distinguishing between simple steatosis and early-stage non-alcoholic steatohepatitis. Hepatol Res. 2010;40:693–700. doi: 10.1111/j.1872-034X.2010.00643.x. [DOI] [PubMed] [Google Scholar]
- 12.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 13.Garjani A, Safaeiyan A, Khoshbaten M. Association between platelet count as a noninvasive marker and ultrasonographic grading in patients with nonalcoholic Fatty liver disease. Hepat Mon. 2015;15:e24449. doi: 10.5812/hepatmon.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celikbilek M, Gürsoy S, Deniz K, Karaman A, Zararsiz G, Yurci A. Mean platelet volume in biopsy-proven non-alcoholic fatty liver disease. Platelets. 2013;24:194–9. doi: 10.3109/09537104.2012.688898. [DOI] [PubMed] [Google Scholar]
- 15.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coban E, Afacan B. The effect of rosuvastatin treatment on the mean platelet volume in patients with uncontrolled primary dyslipidemia with hypolipidemic diet treatment. Platelets. 2008;19:111–4. doi: 10.1080/09537100701230444. [DOI] [PubMed] [Google Scholar]
- 17.Kocabay G, Karabay CY, Kalayci A, Colak Y. Mean platelet volume in patients with non-alcoholic fatty liver disease: Is mean platelet volume ready as a surrogate marker? Clin Chem Lab Med. 2014;52:e249–52. doi: 10.1515/cclm-2014-0303. [DOI] [PubMed] [Google Scholar]
- 18.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320–3. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 19.Colkesen Y, Muderrisoglu H. The role of mean platelet volume in predicting thrombotic events. Clin Chem Lab Med. 2012;50:631–4. doi: 10.1515/CCLM.2011.806. [DOI] [PubMed] [Google Scholar]
- 20.Schoorl M, Schoorl M, van Pelt J, Bartels PC. Application of innovative hemocytometric parameters and algorithms for improvement of microcytic anemia discrimination. Hematol Rep. 2015;7:5843. doi: 10.4081/hr.2015.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao W, Zhao C, Shen C, Wang Y. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS One. 2013;8:e82092. doi: 10.1371/journal.pone.0082092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilciler G, Genc H, Tapan S, Ors F, Kara M, Karadurmus N, et al. Mean platelet volume and its relationship with carotid atherosclerosis in subjects with non-alcoholic fatty liver disease. Ups J Med Sci. 2010;115:253–9. doi: 10.3109/03009734.2010.500062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: A simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Kargili A, Cipil H, Karakurt F, Kasapoglu B, Koca C, Aydin M, et al. Hemostatic alterations in fatty liver disease. Blood Coagul Fibrinolysis. 2010;21:325–7. doi: 10.1097/mbc.0b013e328337b3f8. [DOI] [PubMed] [Google Scholar]
- 25.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434–41. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang QQ, Lu LG. Nonalcoholic fatty liver disease: Dyslipidemia, risk for cardiovascular complications, and treatment strategy. J Clin Transl Hepatol. 2015;3:78–84. doi: 10.14218/JCTH.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arguello G, Balboa E, Arrese M, Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015;1852:1765–78. doi: 10.1016/j.bbadis.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 28.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–33. doi: 10.1016/j.cld.2004.04.004. viii. [DOI] [PubMed] [Google Scholar]
- 29.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: Evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–8. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 30.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]


