Abstract
Background:
Muscle strength is necessary for upper body normal function. Upper extremity function impairments have been reported in breast cancer (BC) survivors. It is not possible to know precisely if cancer adjuvant therapy such as radiation and chemotherapy had any effect on the unaffected arm. The aim of this study was to compare shoulder girdle strength among women with BC and similarly aged women without cancer.
Materials and Methods:
Thirty-three postmenopausal women (51 ± 6.46 years) with BC who underwent surgery, chemotherapy, and radiation therapy and 30 healthy postmenopausal women (53.26 ± 5.05 years) were selected. Muscle strength was measured using a handheld dynamometer for flexion, horizontal adduction, internal and external rotation, scapular abduction and upward rotation, scapular depression, and adduction. Data were analyzed by multivariate analysis of variance (P < 0.05).
Results:
The findings indicated significant differences between groups of 6 of the shoulder girdle strength measure (flexion (P = 0.003), internal rotation (P = 0.001), external rotation (P = 0.040), scapular abduction and upward rotation (P = 0.001), scapular depression and adduction (P = 0.025), and shoulder horizontal adduction (P = 0.00)). Patients showed significantly lower strength compared with healthy controls (flexion = 34.3%, abd = 64.2%, int.rot = 51.2%, ext.rot = 32.4%, hor.add = 58.06, and depression = 35.2%).
Conclusion:
The results indicate that the shoulder girdle strength in women with BC decreased compared with healthy women without BC. Therefore, during the treatment of patients with BC, designing of training programs and rehabilitation programs need to be performed on shoulder girdle strength factors in patients undergoing mastectomy with axillary surgery and radiation therapy.
Keywords: Breast neoplasm, chemotherapy, lymph nodes, menopause, radiotherapy, upper extremity function
INTRODUCTION
Muscle strength is an essential element of normal function of body muscle. Atrophy and progressive reduction in muscle volume can be seen in patients with breast cancer (BC) operation and adjuvant therapy.[1,2] In the modified radical mastectomy (MRM), the breast tissue, some of axillary lymph nodes and pectoral muscle fascia are completely removed which can cause severe breast deformity and upper arm disability.[3,4,5] However, muscle strength was not changed postoperatively in some studies.[6,7]
The aim of this study is to evaluate shoulder girdle strength in women with BC postoperatively and compared the results with healthy matched group of women.
MATERIALS AND METHODS
This study was a randomized control trial (IRCT2013052213432N1) with two groups of BC survivors (BCS) and healthy controls (control group [CON]). The study samples were included postmenopausal women with BC (Stage 0-III), who had spent surgical treatment, chemotherapy, and radiotherapy. By reviewing patient records, referring physicians and nurses, eligible subjects (800 patients) were selected to participate in the study. To select healthy individuals, records of patients referred to the prevention and screening centers studied and 700 healthy controls without a history of BC disease were also investigated.
Based our inclusion criteria, 33 postmenopausal women 45–65 years were selected, who had been diagnosed with stage 0-III BC and all initial treatment (surgery, chemotherapy and/or radiation) had completed at least 12 months before MRM and lymph node dissection. Subjects of this study were under hormone therapy (tamoxifen, 20 mg/day). Other inclusion criteria were no specific disease (diabetes, cardiovascular disease, MS, and thyroid disease), lack of menstrual periods in the past 6 months, no participation in regular exercise or physical activity program, no rehabilitation history of the upper thoracic spine and neck in the past 6 months. Among 700 healthy controls, 30 healthy postmenopausal women with the same age range as the CON (without cancer) were selected.
The research ethics committee approval was received from the Department of Sport Medicine, Kish International Campus, University of Tehran. Before registering and patient consent, the group confirmed the study. Medical consent was required from each participant's physician before beginning this study. In addition, all participants completed the consent form and demographic information.
Shoulder girdle strength measurement with a handheld dynamometer
Peak muscle force as kilogram was measured by means of a maximal voluntary isometric contraction using a handheld dynamometer (Lafayette Instrument®, Lafayette, IN). Concurrent validity of Digital Handheld Dynamometer with isokinetic dynamometer has been proved.[8] The dynamometer reliability with (intraclass correlation coefficient = 0.82–0.97) has been reported in several studies for the strength test.[9] Strength was assessed for shoulder flexor muscles, internal and external rotators muscles, pectoral muscles, back muscles, and scapula stabilizer muscles. The participants were asked to push with maximum effort without moving their arm, for a 5 s count. Thirty seconds rest occurred between each trial. A 1 min rest period occurred between each testing position. Scapula abduction and upward rotation and scapula depression and adduction were performed according to Kendall[10] and the test positions of humeral flexion, scaption, and adduction were performed according to Hislop and Montgomery.[11] All assessments were done with same staff, at A.M.
Data analysis
Mean and standard deviation was used to describe the data, and multivariate analysis of variance was used to examine differences between the two healthy and patient groups (P < 0.05). Data analysis was performed using the software SPSS 21 (IBM SPSS Statistics software, version 21).
RESULTS
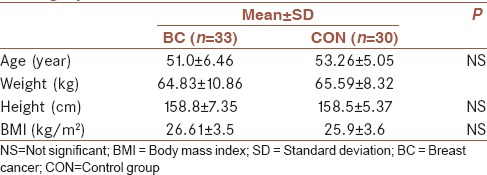
Subjects demographics characteristics are shown in Table 1. Study participants were postmenopausal and had similar demographic characteristics (sex, age, and body mass index). BC patients according to the type of previous surgery, chemotherapy, and radiotherapy, metastasis or recurrence of the disease were similar.
Table 1.
Participant (patients and healthy women) demographic data
Significant differences were found between the groups on the dependent measures, Wilks's = 0.73 (F1,61 = 3.40, P = 0.006) indicate decreased upper extremity strength in the BCS group when compared to the control (CON) group.
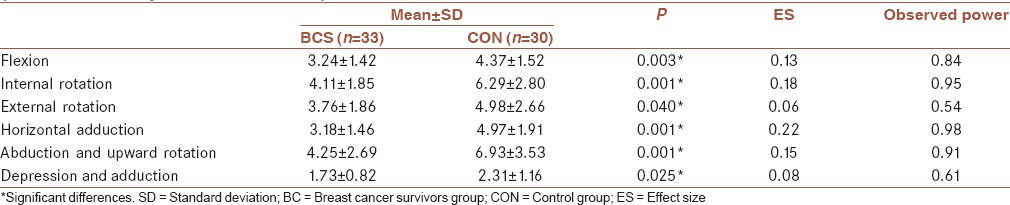
All six of the shoulder strength measures were different between groups and are displayed in Table 2; flexion (F1,61 = 9.25, P = 0.003), abduction (F1,61 = 11.51, P = 0.001), internal rotation (F1,61 = 13.49, P = 0.001), external rotation (F1,61 = 4.41, P = 0.040), horizontal adduction (F1,61 = 17.56, P = 0.000), and depression (F1,61 = 5.27, P = 0.25). The findings indicate that the shoulder girdle strength in patients with BC is significantly lower than in healthy controls.
Table 2.
Comparison of shoulder girdle strength between breast cancer survivors and healthy matched group (multivariate analysis of variance test)
DISCUSSION
In the present study, differences between BCS and healthy age, matched, and gender controls were studied for upper extremity strength. The results show the impact of BC and its treatment on the shoulder girdle strength in patients with BC. The findings show that, there is significant difference in strength between BCS and healthy group.
Approximately, one-third of BCS have persistent losses in strength more than 1 year after treatment.[12] Several studies found decreased upper body strength in BCS. Subjectively, it is has been reported that BCS have decreased muscle strength on the affected extremity, however, very few studies have quantified these actual deficits.[13] Shamley et al. found decreased activity in upper trapezius, rhomboid and serratus anterior muscle, in compared to affected limb with unaffected in BCS, particularly when lowering the arm.[14] Their study has shown decreased EMG activity for muscles that are not in the field of surgery and radiotherapy.[14]
In the present study, the strength in flexion, internal rotation, external rotation, shoulder horizontal adduction, scapular abduction, and adduction, respectively, (25.85%, 53.04%, 32.44%, 56.28%, 63.05%, 33.52%) decreased, in the BCS when compared to healthy women with no history of BC. External rotation strength in female patients is lower than healthy individuals with same age in other studies.[15] Although measurement methods differed slightly (flexion resistance at the epicondyle instead of distally at the ulnar styloid process),[16] and rotation positioning at 45° abduction instead of 90° abduction).[15] Whether these deficits can be definitively linked to a decline in upper extremity function is less clear. Overall, BCS demonstrate lower levels than those of women without BC.
While there was no difference with grip strength at 6 months, 1 year, and 2 years after surgery.[17] Some researchers have found 40% of BCS 2.7 years after surgery[18] and 76% of BCS Within 6-18 months after surgery[19] had a decrease in their grip strength. Part of the difference in these findings may be due to the method used to measure strength.
Since hand grip specifically not examine the shoulder complex muscles, not selected for this study and the hand-held dynamometer was used to assess more accurately. A few studies have evaluated strength with this utilities.[1,20,21,22] Consistent with the present study results, Harrington and et al., 6 months after treatment (surgery, chemotherapy and/or radiotherapy), found significant decreases in muscle strength for scapular abduction and upward rotation, depression and adduction, shoulder flexion, internal and external rotation, scaption, and horizontal adduction in BCS.[1] Similar results were obtained in shoulder strength for internal and external rotation[21] and shoulder flexors at flexion 90°,[20] with similar measurement method, at least 12 months before. A similar measurement method,[1,20,21] the type of surgery (mastectomy and axillary dissection), as well as unilateral surgery[20] and 12 months after surgery,[20,21] can cause consistency the results of the present study with results of previous studies. The results of Lee et al., using the same method, suggests that, among women who had undergone surgery, during radiation therapy to the chest and breast, with the exception of the armpit, normal strength of arm was maintained during 6 weeks of radiation and the flexion, abduction, and external rotation strength at elevation 90° was decreased.[22] The difference between our results and the other's findings can be due to the type of surgery and the time of surgery.
Reduction in flexion, adduction and internal rotation strength[1,17,20,21] are understandable because the main muscles responsible for these movements, the pectorals and latissimus dorsi, are innervated by nerves that could be damaged during the surgery.[5] Serratus anterior muscles are also responsible for scapula protraction and upward rotation and arm elevation make possible. The weakness of abduction and upward rotation strength can occur due to long thoracic nerve injury.[1] With respect to variation in the treatment of BC patients, different effects are expected. Harrington[1] and Fisher,[21] in the same studies, were observed a significant reduction in strength of people with cancer. In these studies, 66.7% and all test subjects, respectively, had undergone mastectomy. In studied by Lee et al.[22] and Hayes,[19] 7/78% and 74% of subjects, respectively, had undergone breast conservative surgery and thus the normal strength of arm was maintained;[22] and 41.6% and 52.7% of patients had shown, respectively, decrease and increase in strength during the 6 months after surgery.[19] The difference in shoulder girdle strength between mentioned studied, and current study findings may be due to the type of operation that is applied on patients with BC. Since all subjects in our study underwent mastectomy, it appears the more invasive the BC surgery, the greater the impact on upper extremity disability, although further research is needed to explore this topic.[1]
The group that had axillary radiation treatment, compared with nonirradiated group, had a greater loss of shoulder strength in flexion, external rotation, and abduction.[17] In another study, irradiated patients reported significantly reduction in flexion and abduction except external rotation, whereas, nonirradiated patients exhibited a significant reduction only in shoulder strength for flexion.[23] Blomqvist et al. believe radiotherapy is the major cause for the reduction of shoulder strength in patients who underwent mastectomy surgery and axillary dissection.[23] Strength was decreased in the BC patients in the recent study, all of whom had completed radiation therapy, is in agreement with the finding of the current study. On the other hand, according to Lee et al., radiation therapy, which is not in the armpit area, may be does not impair shoulder function. However, because of the lack of complete information about the exact position of radiotherapy in our study and some studies, it is not possible to compare the results. There is some recommendation that shoulder disability following radiotherapy may be latent, with reports of impairment starting at 3.9 years after radiotherapy.[24] Perhaps for this reason, Lee et al. found no significant differences in strength following radiotherapy. It seems that our findings about BC complications and lower strength in patients confirmed Sugden's study,[24] because of 3 years (mean) distance than BC treatment.
The current study had some strengths and limitations; strengths are (1) comparison between healthy and BCS, (2) use of Hand Held Dynamometer as a reliable and valid strength measurements tools, (3) use of patients with same surgery type (MRM) and limitations are (1) small sample size, (2) wide age range, (3) no control of daily fatigue.
CONCLUSION
The results indicate the shoulder girdle strength in women with BC decreased compared with healthy women without cancer. Therefore, during the treatment of patients with BC, designing of training programs and rehabilitation programs need to be performed on special shoulder girdle strength factors in patients undergoing mastectomy with axillary surgery and radiation therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This project was performed at Isfahan University of Medical Sciences (the BC Research Center of Seyedoshohada Hospital). We appreciate the patients who participated in this study. This project was approved in University of Tehran (project number: 3568113) and was performed in University of Isfahan without collaboration between universities.
REFERENCES
- 1.Harrington S, Padua D, Battaglini C, Michener LA, Giuliani C, Myers J, et al. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. Journal of Cancer Survivorship. 2011;5:167–74. doi: 10.1007/s11764-010-0168-0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Sharma C, Tickoo S. Effect of radiotherapy following modified radical mastectomy on hand grip strength and pinch grip strength in breast cancer females. University Medical Journal. 2016;1:35–7. [Google Scholar]
- 3.Gyedu A, Kepenekci I, Alic B, Akyar S. Evaluation of muscle atrophy after axillary lymph node dissection. Acta Chir Belg. 2009;109:209–15. doi: 10.1080/00015458.2009.11680407. [DOI] [PubMed] [Google Scholar]
- 4.Harmer V. Breast Cancer Nursing Care and Management: John Wiley & Sons. 2011:113. [Google Scholar]
- 5.Wong V. A Cross-Sectional Study of Chronic Impairments and Activity Limitations in Women at Least Six Months Post-Operative for Breast Cancer: An Exploratory Study: University of Ottawa. 2014 [Google Scholar]
- 6.Karki A, Simonen R, Malkia E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. Journal of Rehabilitation Medicine. 2005;37:180–8. doi: 10.1080/16501970410024181. [DOI] [PubMed] [Google Scholar]
- 7.Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Medical hypotheses. 2011;77:481–7. doi: 10.1016/j.mehy.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Care & Research. 2009;61:623–32. doi: 10.1002/art.24396. [DOI] [PubMed] [Google Scholar]
- 9.Kolber Mj, Cleland ja. Strength testing using hand-held dynamometry. Physical therapy reviews. 2005;10:99–112. [Google Scholar]
- 10.Kendall F, McCreary E, Provance P. Muscle testing and function. 4th. Baltimore, Maryland: Williams and Wilkins; 1993. [Google Scholar]
- 11.Hislop HJ, Montgomery J. Muscle testing, techniques of manual examination. 7th. Philadelphia: Saunders; 2002. [Google Scholar]
- 12.Schmitz KH, Speck RM, Rye SA, DiSipio T, Hayes SC. Prevalence of breast cancer treatment sequelae over 6 years of follow-up. Cancer. 2012;118(S8):2217–25. doi: 10.1002/cncr.27474. [DOI] [PubMed] [Google Scholar]
- 13.Merchant C, Chapman T, Kilbreath S, Refshauge K, Krupa K. Decreased muscle strength following management of breast cancer. Disability & Rehabilitation. 2008;30:1098–105. doi: 10.1080/09638280701478512. [DOI] [PubMed] [Google Scholar]
- 14.Shamley DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast cancer research and treatment. 2007;106:19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 15.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Archives of physical medicine and rehabilitation. 1997;78:26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 16.Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Physical therapy. 1996;76:248–59. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- 17.Johansson K, Ingvar C, Albertsson M, Ekdahl C. Arm Lymphoedema, Shoulder Mobility and Muscle Strength after Breast Cancer Treatment. A Prospective 2-year Study. Advances in Physiotherapy. 2001;3:55–66. [Google Scholar]
- 18.Rietman J, Dijkstra P, Geertzen J, Baas P, De Vries J, Dolsma W, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Annals of surgical oncology. 2004;11:1018–24. doi: 10.1245/ASO.2004.03.512. [DOI] [PubMed] [Google Scholar]
- 19.Hayes SC, Rye S, Battistutta D, DiSipio T, Newman B. Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health Qual Life Outcomes. 2010;8:92. doi: 10.1186/1477-7525-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosbie J, Kilbreath SL, Dylke E, Refshauge KM, Nicholson LL, Beith JM, et al. Effects of mastectomy on shoulder and spinal kinematics during bilateral upper-limb movement. Physical therapy. 2010;90:679–92. doi: 10.2522/ptj.20090104. [DOI] [PubMed] [Google Scholar]
- 21.Fisher MI. A Comparison of Upper Extremity Function Between Female Breast Cancer Survivors and Healthy Controls: Typical Self-Report of Function, Motion, Strength and Muscular Endurance. Lexington, Kentucky: University of Kentucky; 2013. [Google Scholar]
- 22.Lee T, Kilbreath S, Refshauge K, Pendlebury S, Beith J, Lee M. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast cancer research and treatment. 2007;102:313–21. doi: 10.1007/s10549-006-9339-0. [DOI] [PubMed] [Google Scholar]
- 23.Blomqvist L, Stark B, Engler N, Malm M. Evaluation of arm and shoulder mobility and strength after modified radical mastectomy and radiotherapy. Acta oncologica. 2004;43:280–3. doi: 10.1080/02841860410026170. [DOI] [PubMed] [Google Scholar]
- 24.Sugden E, Rezvani M, Harrison J, Hughes L. Shoulder movement after the treatment of early stage breast cancer. Clinical Oncology. 1998;10:173–81. doi: 10.1016/s0936-6555(98)80063-0. [DOI] [PubMed] [Google Scholar]


