Abstract
Background:
Early upper gastrointestinal (UGI) cancer detection had led to organ-preserving endoscopic therapy. Endoscopy is a suitable method of early diagnosis of UGI malignancies. In Iran, exclusion of malignancy is the most important indication for endoscopy. This study is designed to see whether using alarm symptoms can predict the risk of cancer in patients.
Materials and Methods:
A total of 3414 patients referred to a tertiary gastrointestinal (GI) clinic in Isfahan, Iran, from 2009 to 2016 with dyspepsia, gastroesophageal reflux disease (GERD), and alarm symptoms, such as weight loss, dysphagia, GI bleeding, vomiting, positive familial history for cancer, and anorexia. Each patient had been underwent UGI endoscopy and patient data, including histology results, had been collected in the computer. We used logistic regression models to estimate the diagnostic accuracy of each alarm symptoms.
Results:
A total of 3414 patients with alarm symptoms entered in this study, of whom 72 (2.1%) had an UGI malignancy. According to the logistic regression model, dysphagia (P < 0.001) and weight loss (P < 0.001) were found to be significant positive predictive factors for malignancy. Furthermore, males were in a significantly higher risk of developing UGI malignancy. Through receiver operating characteristic curve and the area under the curve (AUC) with adequate overall calibration and model fit measures, dysphagia and weight loss as a related cancer predictor had a high diagnostic accuracy (accuracy = 0. 72, AUC = 0. 881). Using a combination of age, alarm symptoms will lead to high positive predictive value for cancer.
Conclusion:
We recommend to do an early endoscopy for any patient with UGI symptoms and to take multiple biopsies from any rudeness or suspicious lesion, especially for male gender older than 50, dysphagia, or weight loss.
Keywords: Alarm symptom, diagnostic accuracy, upper gastrointestinal malignancy
INTRODUCTION
Upper gastrointestinal (UGI) malignancy is one of the most common cancers and the second most common cause of cancer-related mortality worldwide.[1,2,3,4,5,6,7] Survival of UGI cancer is related to early-stage detection.[8] Detection of premalignant lesions has improved and early UGI cancer detection had led to organ-preserving endoscopic therapy and potentially reducing the number of end-stage UGI cancers and resulting in improved prognosis.[9] Incidence and prevalence of alarm symptoms are required to diagnose UGI malignancy. In addition, endoscopy is suitable, but costly method of early diagnosis of UGI malignancies, which are considered as the most common causes of cancer deaths.[10,11,12,13,14,15] There are a lot of indications for endoscopy such as evaluation of benign and malignant lesions; however, in Iran, exclusion of malignancy is the most important indication.[10]
Early referral for investigation and prompt endoscopic assessment will lead to decrease malignancy.[2] Therefore, it is important to select high-risk patients for endoscopy immediately to treat empirically low-risk patient. The diagnostic value of alarm features in predicting which patient has malignancy is, however, unclear.[16]
We have conducted a cross-sectional study to evaluate the diagnostic accuracy of alarm symptoms in UGI malignancies.
MATERIALS AND METHODS
This was a cross-sectional study that was conducted among the patients that were referred to Poursina Hakim gastrointestinal (GI) clinic, Isfahan, Iran, from the June 2009 to January 2016, with complaints of UGI symptoms. The patients with alarm symptoms such as weight loss (10% ≤ unintentional and during recent 6 months), dysphagia, GI bleeding (GIB) (any evidence of hematemesis, melena, hematochesia, anemia, and positive occult blood [OB+]), dyspepsia, vomiting, familial history of cancer, and anorexia were considered to be included in the present study.[12,13,14,15] The data according to alarm symptoms had been collected by a general physician and entered into the computer. The patients with previously detected UGI cancer, cirrhosis, anemia due to the chronic disease, dysphagia according to obvious causes, and the patients with intentional weight loss were excluded from the study.
All of the patients underwent endoscopic diagnostic procedure with Pentax EG 2440 EMP 3300 and biopsy sampling for any redness or suspicious lesions. The biopsy samples were interpreted by an expert pathologist who was completely blind to the alarm symptoms and endoscopic classification. The alarm symptoms of each patient were documented previously.
Among the 3414 patients who were visited in the Poursina Hakim clinic, a tertiary referral GI clinic in Isfahan, Iran, from June 2009 to Januarry2016 with UGI complaints, 72 cases had histology proven UGI malignancy and included in the case group and 3342 patients with normal pathologic findings were selected to be in the control group.
Data analysis
The logistic regression model was used to determine the diagnostic accuracy of age, sex, and alarm symptoms (dysphagia, gastroesophageal reflux disease [GERD], dyspepsia, GIB, weight loss, vomiting, anorexia, and familial history) for UGI. First, univariate logistic regression model was fitted on each alarm symptoms, and then, multivariate regression model with adjustment for the effects of other covariates was used. Variables that were significant in univariate models were entered into multivariate model. Selection of variables in the multivariate model was based on backward procedure. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for sex, age group, and each of alarm symptoms using logistic regression models. The area under the receiver operating characteristic (ROC) curve or area under the curve (AUC) was developed based on predicted probabilities of the final model. Youden's J statistic criteria (maximum [sensitivity − (1 − specificity)]) are used to find an optimal threshold point from ROC curve. Using pathology as the gold standard for diagnosis of UGI malignancies, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy based on this cutoff point, respectively. In evaluating external validation of model, a 10-fold cross-validation was carried out by randomly partitioning the datasets into ten equal subsamples. One subsample is used as the validation data for testing the model, and the remaining nine subsamples are used as training data. The cross-validation process is then repeated ten times (the folds). The ten results from the folds can then be averaged to produce a single estimation. All statistical analyses were conducted using SPSS statistical software (version 16) (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA).
RESULTS
Finally, 3414 patients completed the study for endoscopic evaluation with UGI symptoms. A total of 72 cases (2.1%) were diagnosed as UGI cancers by pathology.
The mean age of all patients and patients with cancer was 48.2 ± 21 and 65 ± 14 years, respectively.
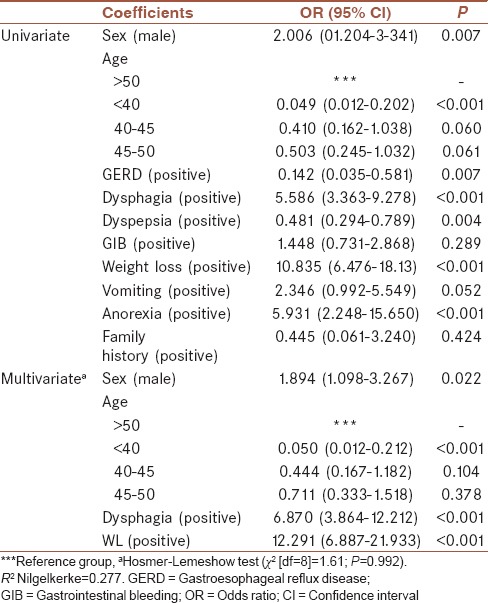
According to the Table 1, dyspepsia was the most (51.3%) and anorexia was the least (1.1%) common symptoms.
Table 1.
Distribution of alarm symptoms in all patients

Univariate and multivariate logistic regression models are shown in Table 2. According to the univariate model, age, sex, GERD, dysphagia, dyspepsia, weight loss, and anorexia were significantly related to UGI cancer. Hence, all of them were entered into multiple logistic regression models. Using multivariate logistic regression analysis, dysphagia (OR: 6.87) and weight loss (OR: 12.291) were found to be significant positive predictive factors for malignancy. Furthermore, males were in a significantly higher risk of developing UGI malignancies compared to females (OR: 1.894). Furthermore, patients with age <40 were approximately 120th as likely to have positive malignancy results compared to patients with age more than 50 (OR: 0.049).
Table 2.
Significant and estimated odds ratios of demographic characteristics and alarm symptoms based on univariate and multivariate logistic regression models
According to the Youden's J statistic, the optimal cutoff point was estimated to be 0.0164, at which the sensitivity and specificity of the test would be 88% and 72%, respectively [Tables 3 and 4]. The AUC (95% CI) of 0.881 (0.846–0.917) for predicted model was statistically significant (P < 0.001) [Table 3 and Figure 1]. Furthermore, the results of 10-fold cross-validation indicated that the estimated of AUC from predicted model was not largely different from the average AUC in validation set (0.871 [0.756–0.981], [P = 0.507]).
Table 3.
Diagnostic characteristics and validation of predicted model

Table 4.
The sensivity, spesifity, positive and negative predictive values for each alarm symptoms
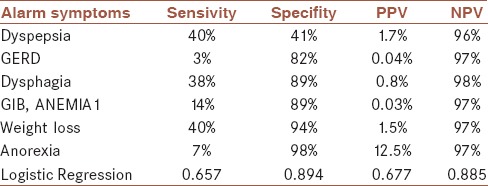
Figure 1.
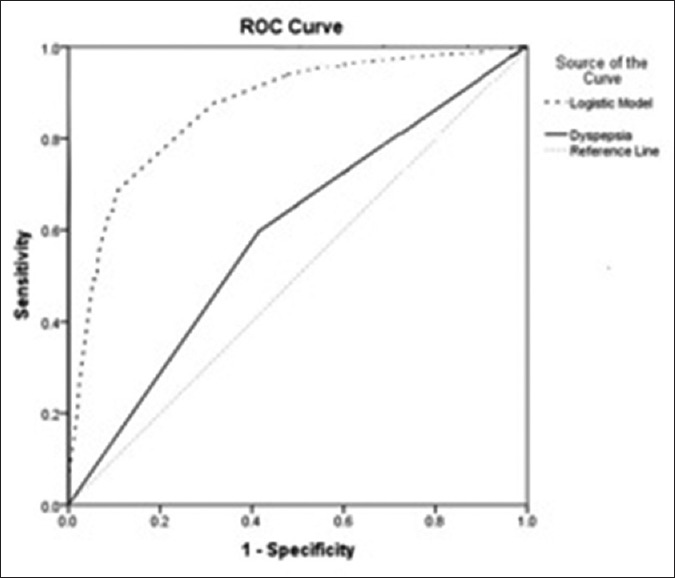
Receiver operating characteristic curve based on predictive models for upper gastrointestinal malignancy
DISCUSSION
Alarm features are symptoms associated with serious GI disease such as neoplasm or benign diseases such as peptic ulcer and GERD. The current guideline recommendation is that endoscopic evaluation of the high-risk patient should be based on age and alarm symptoms.[12,17]
Early referral for investigation and prompt endoscopic assessment will lead to decrease malignancy.[2] We evaluate the diagnostic value of alarm symptoms to clarify whether they can predict UGI malignancy.
According to adjusted model, weight loss, dysphagia, and age more than 50 were significantly associated with the probability of UGI cancers. Other alarm symptoms such as GERD, dyspepsia, GIB, vomiting, anorexia, and family history were associated with cancer in the unadjusted model, but there was no relation with adjusted model.
In recent years, several studies have shown the diagnostic accuracy of age and alarm symptoms in predicting UGI malignancy.[16,18,19,20,21,22,23,24,25,26]
Malekzadeh et al. studied alarm symptoms in patients with dyspepsia and found each single predictor had low sensitivity and specificity. In this study, none of the predictors showed the high diagnostic value. Furthermore, in this study, Helicobacter pylori infection was studied as a variable in dyspeptic patients. In their study, logistic regression model was used to evaluate the diagnostic value of each alarm symptoms, and a risk-prediction model was developed. A combination of age, alarm symptoms, and smoking lead to a risk-prediction model that differentiate high-risk and low-risk individuals with an area under the ROC curve.[10]
Kapoor et al. evaluated the diagnostic accuracy of alarm symptoms in a clinical prediction model for cancer and prospectively used this model in a cohort study. Using backward multivariable logistic regression analysis, their study showed that dysphagia (OR = 6.87), weight loss (OR = 12.291), and age <40 years (OR = 0.049) significantly were predictive factors for cancer, but the diagnostic value of other alarm features was limited. However, the predictive value of individual features for cancer varies widely. The clinical prediction model showed high sensitivity and high NPV but low specificity and low PPV to predict risk of UGI malignancies; however, in their study, the OR and diagnostic accuracy of each alarm symptom were not clear. Use of narrower referral indication for endoscopy leads to high sensitivity for cancer.[19]
Fransen et al. showed each individual alarm symptom through a meta-analysis, clarify limited diagnostic values, including sensitivity, specificity, and predictive value. The risk of UGI malignancy in any individual without alarm symptoms is very low. They recommended other variable such as age, gender, or smoking for better diagnosis of UGI malignancy is required. The stage of the cancers in this meta-analysis was not evaluated.[16]
Vakil et al. performing a meta-analysis and found that alarm symptoms have limited diagnostic value for predicting and detecting malignancy.[22]
Chen et al. study through a meta-analysis and construction of ROC curve and calculation of the area AUC showed that alarm features and age were of limited value in predicting malignancy. They calculated AUC and showed a low age threshold for endoscopy in Asia.[23]
Nearly all of these studies clarify relatively low diagnostic value for each alarm symptom while our study through the adjusted logistic regression model, ROC curve, and AUC showed high diagnostic value for alarm symptoms related to cancer. Our study has relatively large sample size, several cancer predictors, and performing an adjusted regression model with ROC and AUC. The prevalence of UGI cancer was 2.1% that was lesser than previous studies.[27,28,29,30,31,32,33]
Several limitations could be considered in our study. Despite the high prevalence of H. pylori infection in Iran and Asia, first, H. pylori and its relation to cancer as predictor was not evaluated. Second, cancer stage and surveillance as an outcome of malignancy and its relation to alarm symptoms were not evaluated. Third, risk prediction model was based on a development set and there was no cohort validation set. Fourth, other predictors such as gender, smoking, education, and economic situation were not evaluated, further studies that evaluated this parameter are recommended.
UGI malignancy prevalence was 2.1% that were lower than previous studies, so a comprehensive epidemiological study needed to evaluate the prevalence of UGI cancer in recent decades and their relation to risk factors and alarm symptoms. Furthermore, a clinical prediction model that validated in cohort study is required and a cohort study that evaluates relation of dyspepsia with other alarm symptoms is recommended.
CONCLUSION
Although alarm symptoms were shown to lead a moderate diagnostic accuracy, they were not the ideal indicators for detecting malignancy. In summary, dysphagia, weight loss, and older age demonstrated high diagnostic accuracy. Using age, sex, dysphagia, and weight loss, we were able to construct a useful risk-prediction model that distinguished between malignant and nonmalignant and adequate overall calibration and model fit measures. However, the decision on how to use this model will depend on cost-benefit analytic models that depend on several other factors.
We recommend to do an early endoscopy for any patient with UGI symptoms and to take multiple biopsies from any rudeness or suspicious lesions, especially for male gender older than 50, dysphagia, anorexia, or weight loss.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
We are thankful to the personnel of Poursina Hakim Research Institute, for providing the data. This study was supported by Isfahan University of Medical Sciences (research project number: 392195).
REFERENCES
- 1.Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121–5. doi: 10.1016/s1535-6108(04)00033-9. [DOI] [PubMed] [Google Scholar]
- 2.DU Guan FU. Epigenetic alterations in gastric cancer (review) Mol Med Rep. 2015;12:3223–30. doi: 10.3892/mmr.2015.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patru CL, Surlin V, Georgescu I, Patru E. Current issues in gastric cancer epidemiology. Rev Med Chir Soc Med Nat Iasi. 2013;117:199–204. [PubMed] [Google Scholar]
- 4.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: Cancer Incidence, Mortality, and Prevalence Worldwide, Version 1.0. Vol. 5. Lyon: IARC Press; 2001. [Google Scholar]
- 5.Copotoiu C, Sgarbura A, Popescu I. Malignant tumors of the stomach. Bucharest: Romanian Academy Publishing House; 2008. pp. 1351–65. [Google Scholar]
- 6.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, et al. Screening for gastric cancer in Asia: Current evidence and practice. Lancet Oncol. 2008;9:279–87. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen S, Larsen PV, Svendsen RP, Haastrup PF, Søndergaard J, Jarbøl DE. Alarm symptoms of upper gastrointestinal cancer and contact to general practice – A population-based study. Scand J Gastroenterol. 2015;50:1268–75. doi: 10.3109/00365521.2015.1033745. [DOI] [PubMed] [Google Scholar]
- 8.Veitch AM, Uedo N, Yao K, East JE. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol. 2015;12:660–7. doi: 10.1038/nrgastro.2015.128. [DOI] [PubMed] [Google Scholar]
- 9.Khademi H, Radmard AR, Malekzadeh F, Kamangar F, Nasseri-Moghaddam S, Johansson M, et al. Diagnostic accuracy of age and alarm symptoms for upper GI malignancy in patients with dyspepsia in a GI clinic: A 7-year cross-sectional study. PLoS One. 2012;7:e39173. doi: 10.1371/journal.pone.0039173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–80. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ, Vakil N. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–37. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 13.Bodger K, Eastwood PG, Manning SI, Daly MJ, Heatley RV. Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia guidelines (1996) Aliment Pharmacol Ther. 2000;14:413–20. doi: 10.1046/j.1365-2036.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 14.Eisen GM, Dominitz JA, Faigel DO, Goldstein JA, Kalloo AN, Petersen BT, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc. 2001;54:815–7. doi: 10.1016/s0016-5107(01)70083-1. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuyzen van Zanten SJ, Flook N, Chiba N, Armstrong D, Barkun A, Bradette M, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. Canadian Dyspepsia Working Group. CMAJ. 2000;162(12 Suppl):S3–23. [PMC free article] [PubMed] [Google Scholar]
- 16.Fransen GA, Janssen MJ, Muris JW, Laheij RJ, Jansen JB. Meta-analysis: The diagnostic value of alarm symptoms for upper gastrointestinal malignancy. Aliment Pharmacol Ther. 2004;20:1045–52. doi: 10.1111/j.1365-2036.2004.02251.x. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Moayyedi P. Current guidelines for dyspepsia management. Dig Dis. 2008;26:225–30. doi: 10.1159/000121351. [DOI] [PubMed] [Google Scholar]
- 18.Martin IG, Young S, Sue-Ling H, Johnston D. Delays in the diagnosis of oesophagogastric cancer: A consecutive case series. BMJ. 1997;314:467–70. doi: 10.1136/bmj.314.7079.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapoor N, Bassi A, Sturgess R, Bodger K. Predictive value of alarm features in a rapid access upper gastrointestinal cancer service. Gut. 2005;54:40–5. doi: 10.1136/gut.2004.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Numans ME, van der Graaf Y, de Wit NJ, de Melker RA. How useful is selection based on alarm symptoms in requesting gastroscopy? An evaluation of diagnostic determinants for gastro-oesophageal malignancy. Scand J Gastroenterol. 2001;36:437–43. doi: 10.1080/003655201300051379. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Li ZS, Zou DW, Wu RP, Yao YZ, Jin ZD, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: An endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722–8. doi: 10.1136/gut.2009.192401. [DOI] [PubMed] [Google Scholar]
- 22.Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: Systematic review and meta-analysis. Gastroenterology. 2006;131:390–401. doi: 10.1053/j.gastro.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Chen SL, Gwee KA, Lee JS, Miwa H, Suzuki H, Guo P, et al. Systematic review with meta-analysis: Prompt endoscopy as the initial management strategy for uninvestigated dyspepsia in Asia. Aliment Pharmacol Ther. 2015;41:239–52. doi: 10.1111/apt.13028. [DOI] [PubMed] [Google Scholar]
- 24.Voutilainen M, Mäntynen T, Mauranen K, Kunnamo I, Juhola M. Is it possible to reduce endoscopy workload using age, alarm symptoms and H. pylori as predictors of peptic ulcer and oesophagogastric cancers? Dig Liver Dis. 2005;37:526–32. doi: 10.1016/j.dld.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Hammer J, Eslick GD, Howell SC, Altiparmak E, Talley NJ. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666–72. doi: 10.1136/gut.2003.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace MB, Durkalski VL, Vaughan J, Palesch YY, Libby ED, Jowell PS, et al. Age and alarm symptoms do not predict endoscopic findings among patients with dyspepsia: A multicentre database study. Gut. 2001;49:29–34. doi: 10.1136/gut.49.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaei M, Pourfarzi F, Yazdanbod A, Chiniforush MM, Derakhshan MH, Mousavi SM, et al. Gastric cancer in Ardabil, Iran – a review and update on cancer registry data. Asian Pac J Cancer Prev. 2010;11:595–9. [PubMed] [Google Scholar]
- 28.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: Epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 29.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 30.Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6:359–63. [PubMed] [Google Scholar]
- 31.Mohagheghi MA, Mosavi-Jarrahi A, Malekzadeh R, Parkin M. Cancer incidence in Tehran metropolis: The first report from the Tehran population-based cancer registry, 1998-2001. Arch Iran Med. 2009;12:15–23. [PubMed] [Google Scholar]
- 32.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the Northwest of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derakhshan MH, Yazdanbod A, Sadjadi AR, Shokoohi B, McColl KE, Malekzadeh R. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut. 2004;53:1262–6. doi: 10.1136/gut.2003.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]


