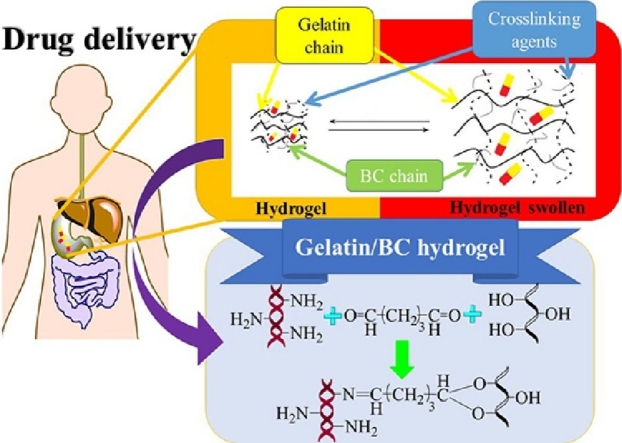
Graphical abstract
Keywords: Bacterial cellulose, Gelatin, Hydrogel, Drug delivery system
Highlights
-
•
Gelatin and bacterial cellulose based hydrogel composite was successfully prepared as drug delivery system.
-
•
Utilization of glutaraldehyde was employed as crosslinking agent for hydrogel formation.
-
•
Green hydrogel presented the excellent swelling ratio.
Abstract
Bacterial cellulose and gelatin were successfully used to develop a hydrogel composite material. Hydrogel was synthesized by copolymerization between bacterial cellulose and gelatin. Scanning electron microscopy (SEM) images showed that the bacterial cellulose chain was uniform in size and shape. Glutaraldehyde was employed as a crosslinking agent. H-bonds were formed via the reaction between the amine and hydroxyl groups, which were the functional groups of the gelatin and bacterial cellulose, respectively. The hydrogel composite presented excellent properties in terms of its thermal stability, chemical resistance, and mechanical properties. Moreover, the swelling ratio of the hydrogel network, in water, was estimated to be 400–600%. Importantly, the hydrogel composite developed during this study is considered a good candidate for drug-delivery systems.
1. Introduction
In recent years, naturally derived polymers, including proteins and polysaccharides, have been widely utilized as biomaterials. Numerous strategic methods have been extensively developed for many medical-technology applications. For instance, one of the most attractive applications involved the use of hydrogels. Hydrogel materials have been commonly employed in many fields, such as wastewater treatment, chemical sensors, and medical technology [1], [2]. Hydrogels have great potential because they can absorb a large amount of water or biological fluid, and offer high porosity as well as a soft consistency. Hydrogels are known as reversible gels if molecular entanglements, such as ionic, H-bonding, or hydrophobic forces, play a key role in forming the network [3], [4]. These entanglements are often reversible and can be dissolved by changing the environmental conditions, such as the pH, ionic strength of the solution, or temperature. Therefore, hydrogels offer numerous advantages and can be used for the controlled release of pharmaceuticals [5].
To date, with the growth of the global population, hydrogels have been increasingly developed for use in pharmaceutical technology [6], [7]. The design of hydrogels using bio-based materials is considered one of the most effective routes for sustainable development. With regard to the synthesis of bio-based materials for hydrogel applications, gelatin is considered the most effective bio-based polymer because it offers many advantages, such as non-toxicity, high water absorption, biodegradability, and biocompatibility. It is notable that these properties render gelatin as an excellent candidate with regard to drug-delivery vehicles. Hydrogels in drug-delivery systems can be used to deliver drugs over a specific time period via a controllable release mechanism. The use of hydrogels offers many advantages, such as reduced drug dosages, costs, and side effects. It is important to note that the use of hydrogels in drug-delivery systems relies on their swelling ability. The swelling mechanism occurs due to an increase in the distance between crosslinked polymer chains, which allows drug molecules to be released and absorbed into the bloodstream [8], [9], [10], [11], [12], [13]. Researchers have focused on identifying materials that are non-toxic, environmentally friendly, and highly biocompatible. In previous studies, natural materials, such as polysaccharides, have been employed as reinforcement materials during the production of hydrogels [14], [15], [16], [17], [18]. Bacterial cellulose is considered to be one of the most effective reinforcement materials. Bacterial cellulose has a similar structure to that of cellulose, with ultrafine fibers. The most effective source of bacterial cellulose is considered to be Acetobacter xylinum: its cellulose has β-1,4-glycosidic bonds between two glucose molecules [19], [20], [21], [22]. Previous studies have stated that bacterial cellulose could be used in hydrogel composites for healthcare research because of their excellent biocompatibility and biodegradability. Bacterial cellulose has many versatile advantages. The Young’s modulus of a single fibril can be as high as 114 GPa [23]. Bacterial cellulose also exhibits other attractive features, such as a high degree of crystallinity (89%) [24], high degree of polymerization (14,400) [25], and a high specific surface area (37 m2/g) [26]. Moreover, bacterial cellulose also offers a large surface area, high aspect ratio, and low bulk density, as well as hydrophilicity. It is important to note that the existence of a small amount of bacterial cellulose in gelatin-based hydrogels can offer significant enhancement with regard to their tensile strength and dimensional stability when employed under externally applied forces.
In this paper, we present the design of a bacterial cellulose and gelatin-based composite hydrogel. The effect of glutaraldehyde as a crosslinking agent was investigated. Preliminary experiments with regard to drug-delivery systems have been performed.
2. Experimental
2.1. Materials
Bacterial cellulose was successfully extracted from the nata de coco product (Chaokoh coconut gel in syrup, Ampol Food Processing Ltd., Nakornpathom, Thailand). This is an indigenous dessert, in which the main component is bacterial cellulose. Bacterial cellulose extracted from nata de coco was characterized and reported in previous work [27]. Its characteristics match those of bacterial cellulose extracted from A. xylinum cultures. Food-grade gelatin and glutaraldehyde were purchased from Sigma Aldrich, Co. Ltd. They were employed as a matrix material and crosslinking agent, respectively. All chemical reagents were used as received without further purification.
2.2. Methods
2.2.1. Bacterial cellulose extraction and purification
Bacterial cellulose was extracted from nata de coco, which was rinsed with distilled water to remove excess sugar and blended in a laboratory blender to obtain nata de coco pellicles. These pellicles were treated with 0.1 M NaOH at 80 °C for 1 h to remove any remaining microorganisms, medium components, and soluble polysaccharides. The purified bacterial cellulose was then thoroughly washed with distilled water until a neutral pH was achieved.
2.2.2. Bacterial cellulose and gelatin hydrogel composite preparation
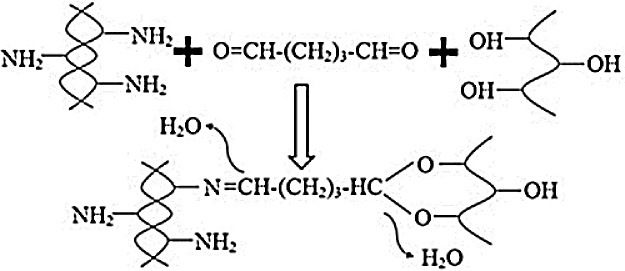
A bacterial cellulose and gelatin-based hydrogel composite were successfully synthesized owing to the reaction between bacterial cellulose and gelatin. To achieve this, 10 wt% of gelatin was completely dissolved in water, and then a bacterial cellulose suspension was poured into the gelatin solution. Glutaraldehyde was employed as a crosslinking agent. The reaction was performed at 55 °C for 4 h. The chemical reaction that occurred between the bacterial cellulose and gelatin, with 1 wt% of glutaraldehyde, is exhibited in Fig. 1. Subsequently, the hydrogel composite was washed with deionized water to remove any unreacted chemicals and stored at 4 °C. The properties of the as-synthesized hydrogel were characterized using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), atomic force microscopy (AFM), and Brunauer–Emmett–Teller (BET) analysis.
Fig. 1.
Chemical reaction between bacterial cellulose and gelatin with glutaraldehyde.
In this experiment, neat gelatin was also studied for comparison. The ratios of gelatin and bacterial cellulose were determined as 25:1, 50:1, 100:1, 200:1, 300:1, 400:1, respectively.
The gelatin and bacterial cellulose-based hydrogel composites were evaluated based on their swelling capability. The water swelling ratio and equilibrium water content of the gelatin and bacterial cellulose hydrogel composite were determined using a gravimetric technique at ambient temperature. The hydrogel composite was immersed into deionized water. The investigation periods were determined as 24 h and 48 h. Prior to measurement, the hydrogel composite was dried in an oven at 45 °C until a constant weight was achieved. The swelling ratio was calculated using the following equation:
| Swelling ratio = (W swollen − W dry)/W dry × 100% |
2.2.3. Drug-loading and release experiments
Loading experiments, where methylene blue (MB, used as a model drug) was loaded into the hydrogel composites, were conducted using the swelling-diffusion method. The molecular structure of methylene blue has a positive change, similar to doxorubicin and hydroxyurea. They were commonly used as anticancer drug [28], [29]. First, the hydrogel composites were dried in an oven at 45 °C until a constant weight was achieved, and then allowed to swell in a MB aqueous solution (5 mg/mL) at 37 °C for 48 h. The swollen hydrogels were rinsed with deionized water and dried again to obtain drug-loaded hydrogels. The concentration of the drug remaining in the loading solution was determined using a UV–vis spectrophotometer (UV-2600, Shimadzu, Japan) at 658 nm. The drug entrapment efficiency (EE) of the hydrogel composites was calculated using the following equation [30]:
| EE (%) = [(Wo − Wf)/Wo] × 100, |
where Wo is the total amount of MB in the solution prior to loading, and Wf is the total amount of MB in the solution following loading.
In the case of the drug-release experiments, the drug-loaded hydrogel composites were immersed into 50 mL of deionized water at 37 °C for 48 h under conditions of constant vibration (70 rpm). At predetermined time intervals, aliquots (0.5 mL) of the release medium were removed, and an identical volume of fresh medium was added. The concentration of the drug in the release medium was quantified via spectrophotometry (UV-2600, Shimadzu, Japan) at 658 nm. Each release experiment was performed in triplicate. The cumulative percentage release was calculated as follows [30]:
| Cumulative percentage release = Wt/Wl × 100, |
where Wt is the amount of MB released from the hydrogel at time t, and Wl is the amount of MB loaded onto the hydrogel.
2.3. Characterization techniques
2.3.1. Fourier transform infrared spectroscopy
FTIR was performed using a Bruker Vector 22 mid-IR spectroscope (Bruker, Germany). All the FTIR absorption spectra were recorded over the wavenumber range of 4500 cm−1 to 500 cm−1 at a resolution of 8 cm−1, with 1024 scans, using a deuterated triglycine sulfate (DTGS) detector. A straight line between the two lowest points in the respective spectra region was selected as a baseline. The bacterial cellulose and gelatin-based hydrogel composites were cast onto glass slides prior to investigation.
2.3.2. Field emission scanning electron microscopy
The morphological properties of the bacterial cellulose and gelatin-based hydrogel composites were investigated using a field-emission scanning electron microscope (FE-SEM, Hitachi, S-4800) at an acceleration voltage of 2 kV. Prior to investigation, the samples were stored in desiccators to avoid exposure to humidity. The hydrogel composites were prepared using a freeze-drying technique to remove any existing water. Each sample was placed on a carbon tape and sputtered with gold particles prior to analysis.
2.3.3. Atomic force microscopy
AFM was performed using a Digital Instruments Nanoscope III Scanning Probe Microscope (Digital Instruments, CA, USA) under ambient conditions (22 °C, 45–55% relative humidity) over areas measuring 10 μm × 10 μm. The bacterial cellulose and gelatin based-hydrogel composites were prepared in the form of a thin, flat sheet. The instrument was equipped with a silicon nitride tip and operated in the lateral contact mode. The measurements were repeated five times for comparable topological analysis.
2.3.4. BET analysis
The specific surface area, pore diameter, and pore volume of each bacterial cellulose and gelatin-based hydrogel were investigated. Prior to investigation, each hydrogel was subjected to a freeze-drying process. The investigation was conducted using nitrogen at 77 K in an Autosorb-1 gas sorption system (Quantasorb Jr.). The samples were degassed at 200 °C under reduced pressure prior to each measurement.
2.3.5. Thermogravimetric analysis
The thermal degradation behavior of each bacterial cellulose and gelatin-hydrogel composite was characterized using thermogravimetric analysis (TGA, TGA Q500, TA Instruments). To achieve this, 20 mg of the sample was heated from room temperature to 700 °C, under a N2 atmosphere, using a heating rate and flow rate of 5 °C/min and 70 mL/min, respectively
2.3.6. Differential scanning calorimetry
The bacterial cellulose and gelatin-based hydrogel composites were analyzed using differential scanning calorimetry (DSC), which was performed between room temperature and 700 °C, with a heating rate of 10 °C/min, using a TA-10000 DSC (TA Instruments, DE, USA). The glass transition temperature, melting temperature, and specific heat capacity were determined from the resultant heat flow curve.
2.3.7. UV–vis spectroscopy
A UV–vis NIR spectrophotometer (Spectrophotometer, Synergy H1 microplate reader, BioTek®), equipped with a transmittance accessory, was used to record the electronic spectra of the samples over wavelengths of 200–1000 nm. This allowed the absorbance spectra of the samples to be studied. The accessory comprised a 110-nm-diameter integrating sphere and an in-built high-performance photomultiplier. The core-shell material was investigated. Each sample was placed in a sample cell specifically designed for this instrument. The base line was recorded and calibrated using a polytetrafluoroethylene (PTFE) reference cell.
3. Results and discussion
3.1. Characterization of the bacterial cellulose and gelatin-based hydrogel composite
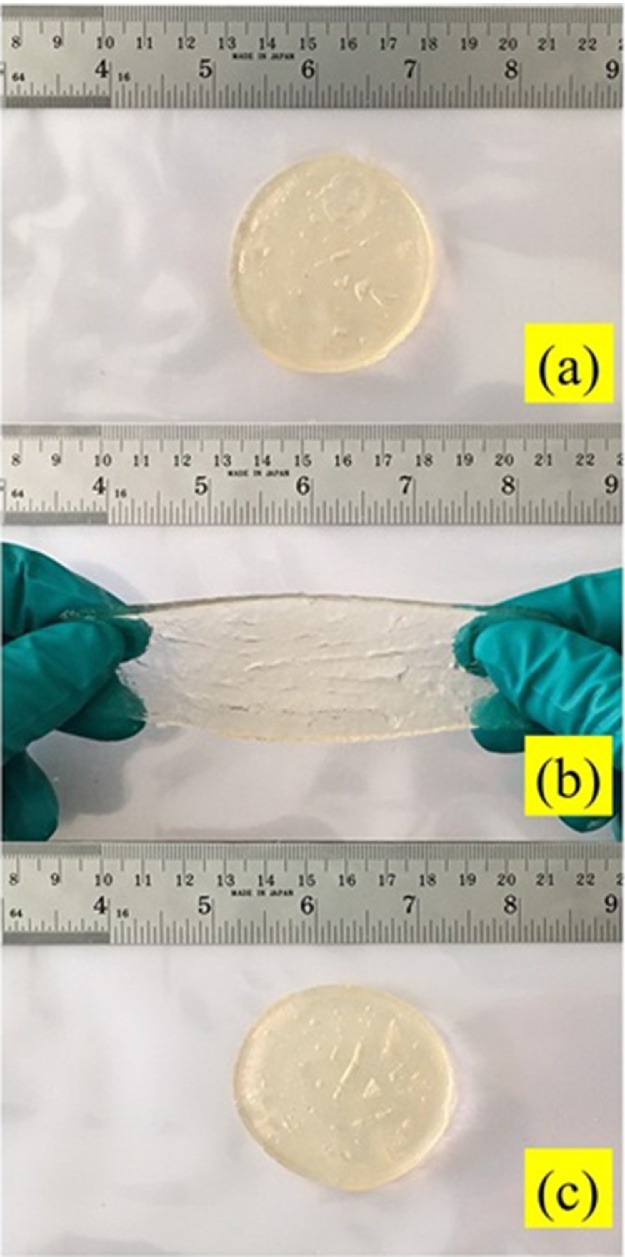
A hydrogel composite consisting of bacterial cellulose and gelatin was successfully synthesized. Glutaraldehyde was employed as a crosslinker. It is noteworthy that the hydrogel was malleable and easily shaped at ambient temperature without any external stimuli. The hydrogel composite possessed a semi-interpenetrating polymer network (IPN). The bacterial-cellulose network was filled with gelatin, and glutaraldehyde functioned as a crosslinker. Fig. 2 exhibits a photograph of the bacterial cellulose-based hydrogel.
Fig. 2.
Photograph of bacterial cellulose-based hydrogel composite (a) Original form, (b) Stretchable form, (c) Reformable form.
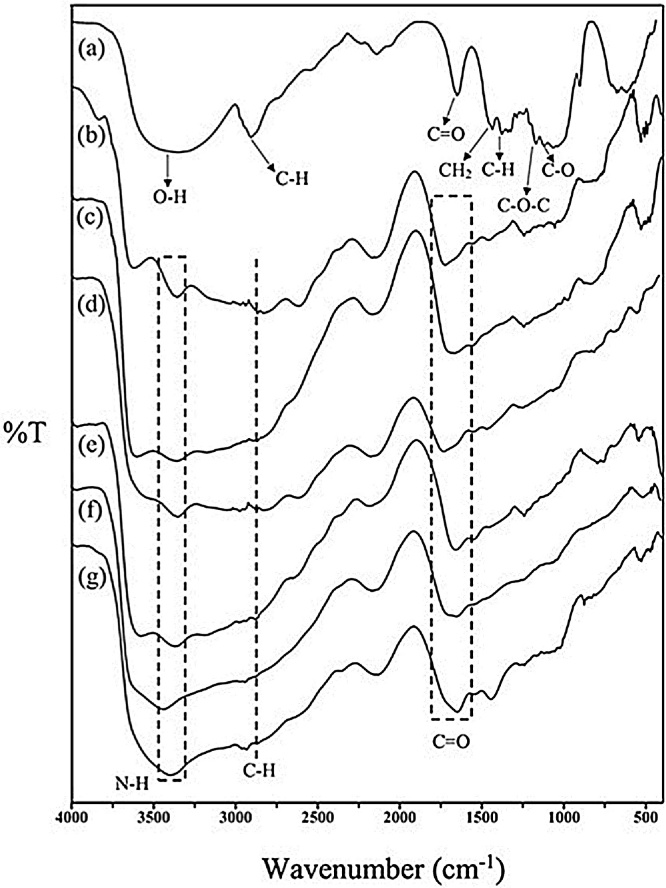
Fig. 3 exhibits the FTIR spectra of the bacterial cellulose and gelatin hydrogel composites. The FTIR spectral data were used to confirm the crosslinking of the gelatin chain, and to study the changes that occurred in the functional groups of the gelatin following the reaction with the bacterial cellulose and glutaraldehyde. It is important to note that only the neat gelatin presented absorption peaks at 3290 cm−1 and 2960 cm−1. These peaks were ascribed to N—H stretching and aliphatic C—H stretching, respectively, as suggested by Rokhade et el. [31]. Moreover, the bacterial cellulose and gelatin-based hydrogel composite exhibited broad peaks at 3400 cm−1 and 1600 cm−1, which were ascribed to the stretching vibration of N—H, and C O stretching, respectively. The characteristic peaks at 1600 cm−1 and 1100 cm−1 were attributed to N—H deformation and C—N stretching, respectively. These characteristic peaks were identical for all the hydrogel composites with glutaraldehyde. These results are very similar to those of a previous study [32]. However, the existence of the peak at 3600 cm−1 was ascribed to many reaction mechanisms, such as H-bond formation in the bacterial cellulose and gelatin network. Glutaraldehyde was used as a crosslinking agent for the bacterial cellulose and gelatin-based hydrogel composite. It is a dialdehyde that reacts with an amine group in the gelatin to form a Schiff base. As suggested by Li et al., it is successfully synthesized by the reaction between an amine group and a carbonyl group via nucleophilic addition, resulting in the generation of an imine [33]. As the amount of gelatin increased, the characteristic peak position at 1660 cm−1 was shifted slightly higher. This was ascribed to asymmetric COO stretching, owed to the reaction between the gelatin and glutaraldehyde. However, with the addition of bacterial cellulose, no further significant peaks were observed. This may be owed to the small amount of bacterial cellulose in the hydrogel composite. The proposed mechanism for the formation of the hydrogel composite is exhibited in Fig. 1.
Fig. 3.
FTIR spectra of the gelatin and bacterial cellulose hydrogel composites (a) neat gelatin; and with gelatin-to-bacterial cellulose ratios of (b) 25:1, (c) 50:1, (d) 100:1, (e) 200:1, (f) 300:1, (g) 400:1.
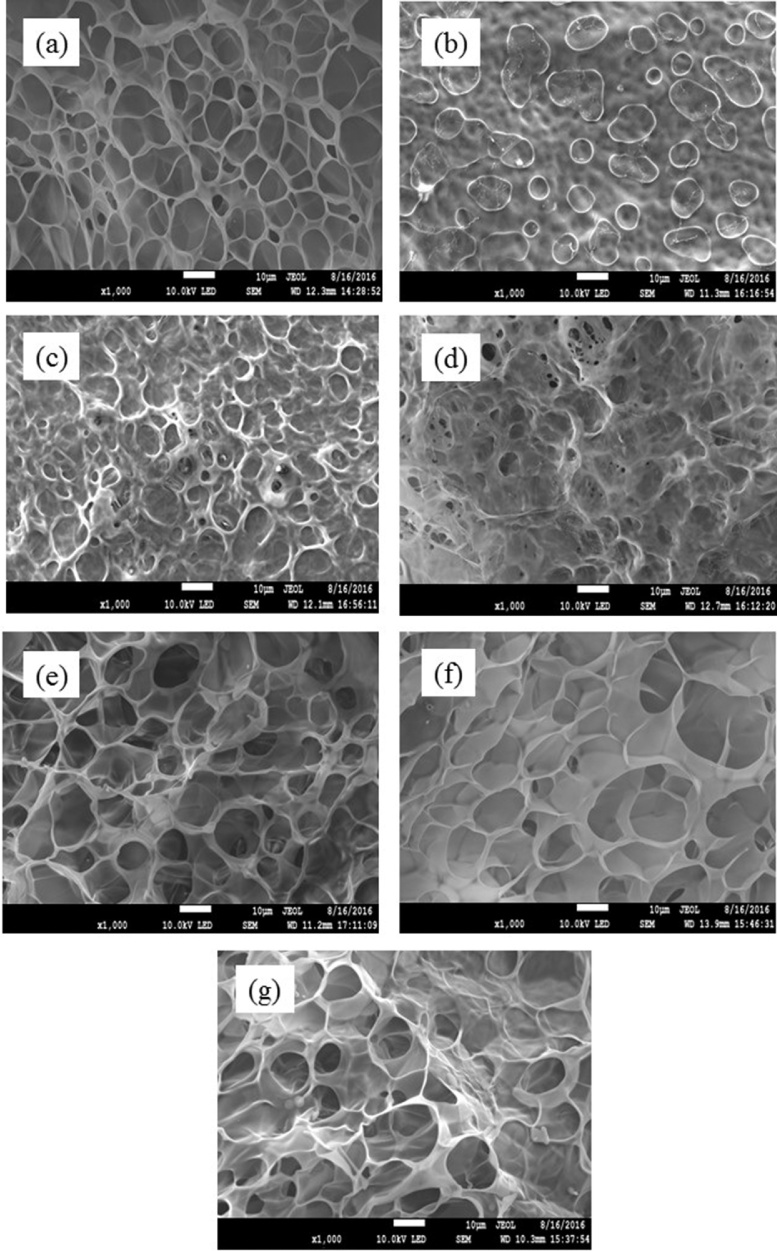
The morphological properties of the bacterial cellulose and gelatin hydrogel composites were evaluated using SEM, as shown in Fig. 4. The figure shows the closed-cell form of the porous network present throughout the hydrogel composite. A porous structure formed following the removal of the solvent. The pores were of various sizes, and considered to have a spherical shape. However, it is noteworthy that as the amount of gelatin in the hydrogel composite decreased, the size of the pores slightly decreased. This was owed to the reaction between the bacterial cellulose and gelatin. The excess bacterial cellulose content of the hydrogel composite presented as a matrix; therefore, there was an inadequate reaction with the gelatin, and less water, as a by-product, was consequently detected. Moreover, the image also presents the morphological properties of the bacterial cellulose fibers. The gelatin presented as a network, and bacterial cellulose was then inserted between the network. The properties of the hydrogel composite depend on the amounts of bacterial cellulose and gelatin present. The quantities of materials present, as well as the crosslinking agent, play an important role with regard to the chemical reaction and the production of by-products.
Fig. 4.
Morphological properties of gelatin and bacterial cellulose hydrogel composites (a) neat gelatin; and with gelatin-to-bacterial cellulose ratios of (b) 25:1, (c) 50:1, (d) 100:1, (e) 200:1, (f) 300:1, (g) 400:1.
Table 1 exhibits the results of the BET analysis performed on the freeze-dried hydrogel composites. Technical data was reported based on the specific surface area, pore diameter, and pore volume, respectively. It is noteworthy that with the addition of bacterial cellulose, the specific surface area of the composites significantly reduced from 200 m2/g to 40 m2/g. The type of porosity also changed from macroporous to mesoporous. The addition of bacterial cellulose resulted in H-bond formation between the amino groups (N—H) and hydroxyl groups (O—H). The hydrogel composite becomes stronger following the reaction between the bacterial cellulose and gelatin. Water was removed via the freeze-drying technique, and subsequently the hydrogel composite became dry. The results of this investigation are closely linked with those of the SEM analysis. Moreover, the pore volumes and pore diameters were investigated. The data regarding the pore diameters and pore volumes of the hydrogel composite are similar to that of the neat gelatin. It is strongly recommended that gelatin and bacterial cellulose-based hydrogel composites intended for drug-delivery systems should be evaluated; the effect of their specific surface area on the drug absorption/desorption mechanism should be investigated. This may involve mechanisms associated with the controlled release of drugs in any relative solvent.
Table 1.
BET analysis of gelatin and bacterial cellulose hydrogel composites.
| Hydrogel composite | Specific surface area (m2/g) | Pore diameter (nm) | Pore volume (cc/g) |
|---|---|---|---|
| Neat gelatin | 204 | 3.8 | 0.28 |
| 25:1 | 35 | 3.4 | 0.05 |
| 50:1 | 22 | 3.8 | 0.04 |
| 100:1 | 42 | 3.4 | 0.06 |
| 200:1 | 55 | 3.4 | 0.07 |
| 300:1 | 54 | 3.4 | 0.07 |
| 400:1 | 41 | 3.4 | 0.06 |
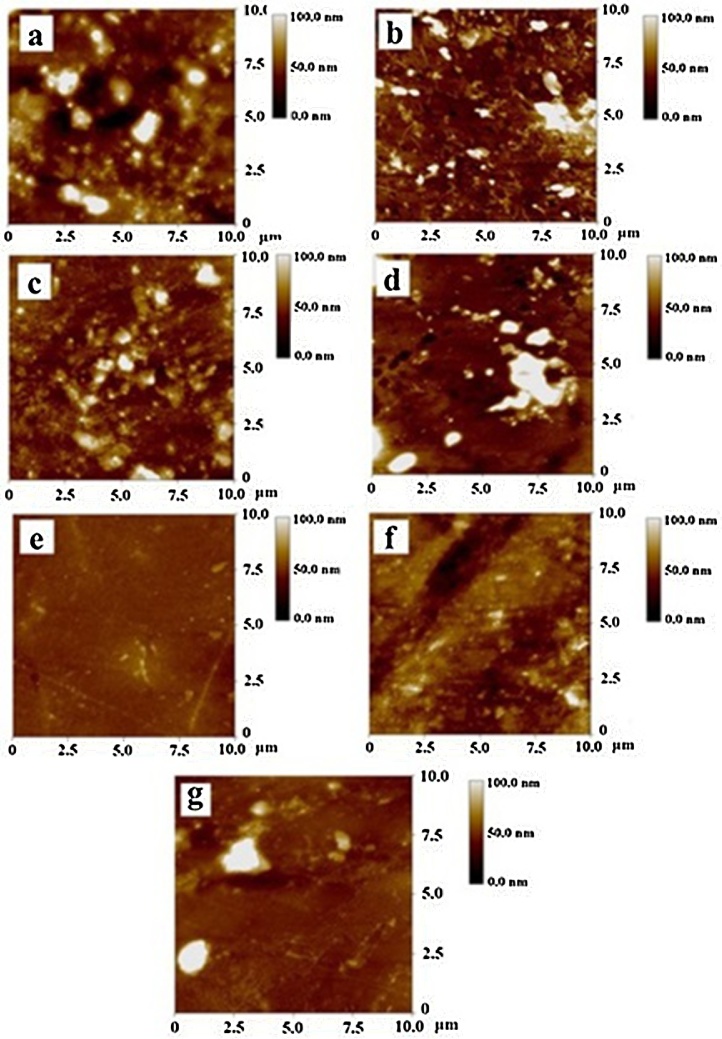
With regard to the utilization of the gelatin and bacterial cellulose composite hydrogel materials for drug-delivery systems, the surface roughness of the material is a key issue. Munz et al. [34] suggested that the roughness of such hydrogels plays an important role with regard to biomedical engineering applications, such the controlled release of water-soluble drugs, encapsulation of cells, tissue engineering, and adhesives. With regard to the controlled release of drugs from a hydrogel composite, the surface roughness of the material should be considered as well as its stability under acid and alkaline buffers. It is important to note that the stability of a hydrogel depends on its roughness. A hydrogel surface with a high degree of roughness can enable the dissociation of drug molecules. Fig. 5 exhibits an AFM image captured in lateral contact mode. In this work, five AFM images were captured at various areas of the gelatin and bacterial cellulose composite, all of which revealed similar topologies. The AFM scan size (10 μm × 10 μm) implies the uniformity of the surface roughness. It is notable that the degree of roughness of the bacterial cellulose was very high. However, in the case of the gelatin and bacterial cellulose-based composite, the bacterial cellulose entered the cavities of the gelatin network. It is evident that the smoothness of the bacterial cellulose significantly improved. The degree of roughness slightly decreased, from micron- to nano-scale level. Moreover, it was obvious that the morphological properties of the composite determined from the AFM investigation were identical to those of the SEM investigation. Bacterial cellulose nano-fibrils were observed; they had various sizes and orientations.
Fig. 5.
Topological properties of gelatin and bacterial cellulose hydrogel composite; (a) neat gelatin; and with gelatin-to-bacterial cellulose ratios of (b) 25:1, (c) 50:1, (d) 100:1, (e) 200:1, (f) 300:1, (g) 400:1.
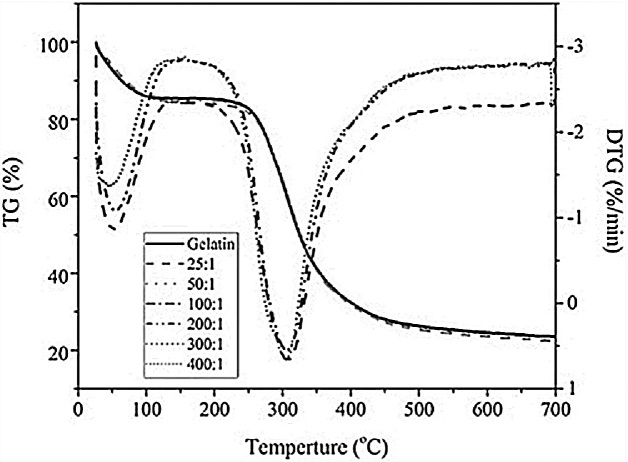
The thermal and kinetic behavior of bacterial cellulose and gelatin-based hydrogel composites is of importance with regard to their stability and controlled release mechanism. Rocha-Garcia et al. [35] suggested that the thermal and kinetic behavior of hydrogels depends on the thermo-responsive mechanism of the crosslinked hydrogel network. The TG and DTA curves of the gelatin and bacterial cellulose hydrogel composite are presented in Fig. 6. The change in the weight loss can be categorized into three different regions. From room temperature to 200 °C, the weight loss was owed to water and solvent evaporation. From 200 °C to 500 °C, the change in the weight loss was due to organic decomposition. The polymer backbone can break over this temperature region. Both the gelatin and bacterial cellulose decomposed at elevated temperature. The pores within the material can be regarded as pathways for CO2 and H2O removal. This phenomenon is associated with the results of the SEM observation and BET experiment. Above 500 °C, the thermal decomposition was complete. Only 20 wt% of char remained. It is suggested that gelatin and bacterial cellulose hydrogel composites for drug-delivery systems should be used at temperatures lower than 200 °C.
Fig. 6.
Thermal degradation behavior of the gelatin and bacterial cellulose hydrogel composites.
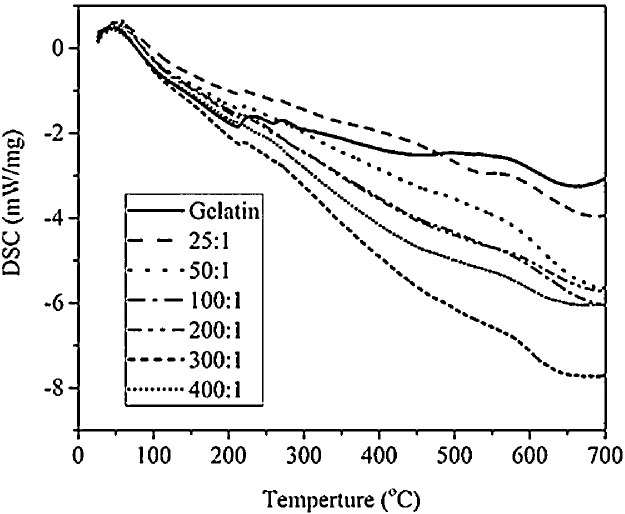
DSC was used to determine the glass transition temperature and melting temperature of the gelatin and bacterial cellulose-based hydrogel composite. Fig. 7 exhibits the DSC thermo-graph and relevant specific-heat parameters, respectively. Within the temperature range of 30 °C–400 °C, there are two peaks, which correspond to the glass transition temperature and melting temperature, respectively. The glass transition temperature of neat gelatin, provided for comparison, is 40 °C. However, the glass transition temperature of the hydrogel composite is slightly above that, at 60 °C. The small amount of bacterial cellulose that was incorporated within the system did not affect the glass transition temperature. The incorporation of the bacterial cellulose did not hinder the molecular motion of the gelatin from the glassy state to the rubbery state. These results are in agreement with those of de Oliveira et al. [36]. The bacterial cellulose was successfully embedded into the gelatin matrix, no evidence of its thermal properties was observed; however, there was a slight increase in the melting temperature. Since bacterial cellulose has a high degradation temperature, this may have increased the melting temperature of the gelatin matrix.
Fig. 7.
Differential scanning calorimetry results obtained for the gelatin and bacterial cellulose hydrogel composites.
3.2. Investigation of bacterial cellulose and gelatin-based hydrogel composites with regard to drug-delivery systems
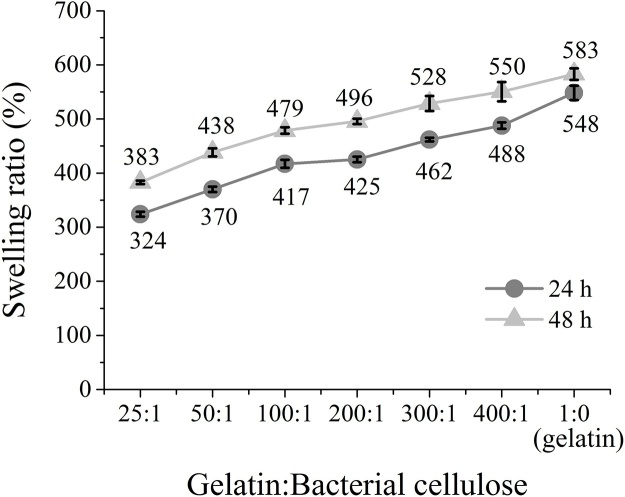
With regard to the use of hydrogels in drug-delivery systems, the swelling capacity of the bacterial cellulose and gelatin hydrogel composites was investigated. The swelling ratio of a hydrogel composite depends on the crosslinking density of the polymeric network, the hydrophilicity of the polymer, and its concentration. During swelling, the solution permeates the hydrogel composite. The swelling was therefore restricted by the crosslinking of the network. Fig. 8 exhibits the swelling ratios of the bacterial cellulose and gelatin hydrogel composites. The investigation was conducted in deionized water over periods of 24 and 48 h, respectively. It is noteworthy that in the case of all the hydrogel composites, the swelling ratio, at 48 h, was estimated to be 400–600%. There is no significant change in the swelling characteristics. Compared with that of neat gelatin, the swelling ratio was still high; this was because no network formed owing to the reaction between the bacterial cellulose and gelatin. With the incorporation of bacterial cellulose, the free space in the gelatin network was filled. This subsequently resulted in the formation of a more rigid hydrogel structure, which hindered the penetration of water molecules. Hence, the degree of water absorption and permeation decreased, which led to a reduction in the swelling ratio. Moreover, considering the incorporation of the bacterial cellulose, it is important to note that bacterial cellulose offers high hydrophobicity and supramolecular interaction; therefore, the hybrid hydrogels are less likely to swell, and should reach equilibrium in a relatively short time. The formation of the hydrogel was therefore complete. These results are similar to those of a previous study by Ooi et al. [16].
Fig. 8.
Swelling ratios of gelatin and bacterial cellulose hydrogel composites.
In addition to the neat gelatin hydrogel, the bacterial cellulose and gelatin-based hydrogel composite with a gelatin-to-bacterial cellulose ratio of 200:1 was selected for the drug loading and release studies because of its relatively high swelling ratio in deionized water (approximately 500%), and relatively high porosity and specific surface area (55 m2/g). The drug EE of the bacterial cellulose and gelatin-based hydrogel composite was 45%, which was lower than that of the neat gelatin hydrogel (60%). This observation was similar to those of the swelling test and BET analysis. The neat gelatin hydrogel had a high specific surface area and pore volume; therefore, its swelling ratio was greater, and consequently a greater quantity of the drug solution was adsorbed into the gelatin network. This phenomenon is directly related to the porosity characteristics and swelling behavior of the material. Therefore, the drug EE of a hydrogel is dependent on its porosity as well as its swelling ratio.
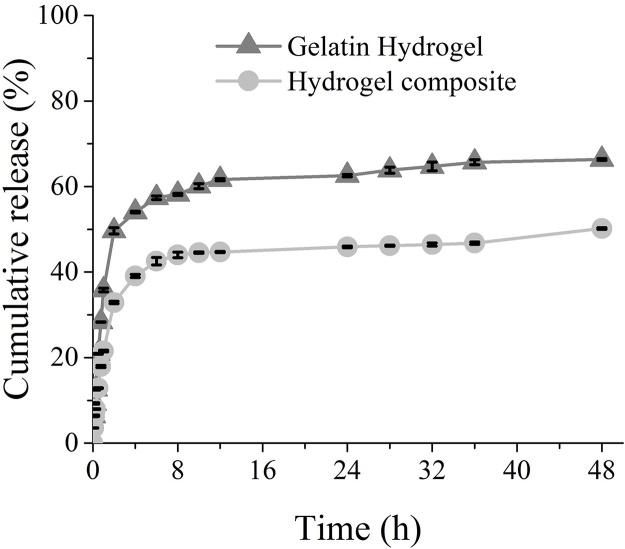
Drug-release experiments were conducted to study the drug-release rate of the neat gelatin hydrogel and bacterial cellulose and gelatin-based hydrogel composite in deionized water at 37 °C for 48 h. The gelatin and bacterial cellulose-based hydrogel composite with a gelatin-to-bacterial cellulose ratio of 200:1 was investigated. It was observed that both hydrogels had a remarkable effect on the controlled drug release. The rate of release of MB from both hydrogels was higher during the initial 2 h following the immersion of the drug-loaded hydrogels in the release medium. This burst effect was likely to be owing to the presence of some MB on the surface of the hydrogels. The dramatic difference in the concentration gradient between the release medium and hydrogel surface during the initial stage can be attributed to the driving force of the drug release. At a later stage, the release rates decreased because of the gradual diffusion of the drug from the hydrogels. As shown in Fig. 9, both the neat gelatin hydrogel and the bacterial cellulose and gelatin-based hydrogel composite could provide sustainable and stable drug release for more than 40 h. The cumulative percentage release of MB from the gelatin hydrogel (66.34% ± 0.21%) was greater than that from the bacterial cellulose and gelatin-based hydrogel composite (50.19% ± 0.2%). These drug-release profiles were consistent with the data obtained from the porosity and swelling studies. The gelatin hydrogel, with a higher swelling ratio and greater porosity, resulted in a higher cumulative percentage of drug release compared with that of the bacterial cellulose and gelatin-based hydrogel composite. However, gelatin has major disadvantages, such as its poor mechanical and thermal stabilities [37], [38], which consequently limit its biomedical applications. The incorporation of cellulose into a gelatin network can improve both the mechanical and thermal stabilities of gelatin hydrogels [39]. In this study, the bacterial cellulose, which filled the gelatin hydrogel, resulted in a denser structure, which therefore led to a slower, more sustained drug-release rate. This would be beneficial for the maintenance of plasma drug concentrations; thus, achieving desired treatment effects. Thus, bacterial cellulose and gelatin-based hydrogel composites are considered excellent candidates for controlled drug-delivery systems.
Fig. 9.
Drug release profiles of the neat gelatin hydrogel, and bacterial cellulose and gelatin-based hydrogel composite (with a gelatin-to-bacterial cellulose ratio of 200:1) in deionized water at 37 °C.
4. Conclusions
Gelatin and bacterial cellulose-based hydrogel composites were successfully prepared. Gelatin was inserted into the cavities of the bacterial cellulose network. It exhibited excellent compatibility with bacterial cellulose. Glutaraldehyde was employed as a crosslinking agent between the hydroxyl groups of the bacterial cellulose and the amine groups of the gelatin. The hydrogel composites presented excellent benefits in terms of their thermal stability, chemical resistance, and mechanical properties. Moreover, the swelling ratio of the hydrogel network, in water, was estimated to be 400–600%. These hydrogel composites are considered good candidates for drug-delivery systems.
Conflict of interest
No conflict of interest for this manuscript.
Acknowledgements
The authors would like to acknowledge the financial support provided by Thammasat University under the TU New Research Scholar, Contract No. 28/2557. The authors gratefully acknowledge the Center of Scientific Equipment for Advanced Research at Thammasat University (TU-CSEAR) for support related to the field-emission scanning electron microscopy.
References
- 1.Gyles D.A., et al. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017;88:373–392. [Google Scholar]
- 2.Kamoun E.A., Kenawy E.-R.S., Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017;8(3):217–233. doi: 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan M., et al. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Mater. Sci. Eng.: C. 2017;71:67–74. doi: 10.1016/j.msec.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 4.Singh B., Sharma V. Crosslinking of poly(vinylpyrrolidone)/acrylic acid with tragacanth gum for hydrogels formation for use in drug delivery applications. Carbohydr. Polym. 2017;157:185–195. doi: 10.1016/j.carbpol.2016.09.086. [DOI] [PubMed] [Google Scholar]
- 5.Xu X., et al. Construction and characterization of a pure protein hydrogel for drug delivery application. Int. J. Biol. Macromol. 2017;95:294–298. doi: 10.1016/j.ijbiomac.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 7.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1(12) doi: 10.1038/natrevmats.2016.71. p. 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhtar M.F., Hanif M., Ranjha N.M. Methods of synthesis of hydrogels … A review. Saudi Pharm. J. 2016;24(5):554–559. doi: 10.1016/j.jsps.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L., et al. Evaluation of genipin-crosslinked chitosan hydrogels as a potential carrier for silver sulfadiazine nanocrystals. Colloids Surf. B: Biointerfaces. 2016;148:343–353. doi: 10.1016/j.colsurfb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Haq M.A., Su Y., Wang D. Mechanical properties of PNIPAM based hydrogels: a review. Mater. Sci. Eng.: C. 2017;70(Part 1):842–855. doi: 10.1016/j.msec.2016.09.081. [DOI] [PubMed] [Google Scholar]
- 11.Khan M., Lo I.M.C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: recent progress, challenges, and perspectives. Water Res. 2016;106:259–271. doi: 10.1016/j.watres.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Lai W.-F., He Z.-D. Design and fabrication of hydrogel-based nanoparticulate systems for in vivo drug delivery. J. Control. Release. 2016;243:269–282. doi: 10.1016/j.jconrel.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Serafin Z., et al. Follow-up of cerebral aneurysm embolization with hydrogel embolic system: systematic review and meta-analysis. Eur. J. Radiol. 2015;84(10):1954–1963. doi: 10.1016/j.ejrad.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 14.García-Astrain C., et al. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016;149:94–101. doi: 10.1016/j.carbpol.2016.04.091. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H., Tovar-Carrillo K., Kobayashi T. Ultrasound stimulated release of mimosa medicine from cellulose hydrogel matrix. Ultrason. Sonochem. 2016;32:398–406. doi: 10.1016/j.ultsonch.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Ooi S.Y., Ahmad I., Amin M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crops Prod. 2016;93:227–234. [Google Scholar]
- 17.Yuan N., et al. Superior hybrid hydrogels of polyacrylamide enhanced by bacterial cellulose nanofiber clusters. Mater. Sci. Eng.: C. 2016;67:221–230. doi: 10.1016/j.msec.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 18.Zare-Akbari Z., et al. PH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int. J. Biol. Macromol. 2016;93(Part A):1317–1327. doi: 10.1016/j.ijbiomac.2016.09.110. [DOI] [PubMed] [Google Scholar]
- 19.Foresti M.L., Vázquez A., Boury B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: a review of recent advances. Carbohydr. Polym. 2017;157:447–467. doi: 10.1016/j.carbpol.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Peng S., et al. Flexible polypyrrole/copper sulfide/bacterial cellulose nanofibrous composite membranes as supercapacitor electrodes. Carbohydr. Polym. 2017;157:344–352. doi: 10.1016/j.carbpol.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Sanchis M.J., et al. Monitoring molecular dynamics of bacterial cellulose composites reinforced with graphene oxide by carboxymethyl cellulose addition. Carbohydr. Polym. 2017;157:353–360. doi: 10.1016/j.carbpol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Sheykhnazari S., et al. Bacterial cellulose composites loaded with SiO2 nanoparticles: dynamic-mechanical and thermal properties. Int. J. Biol. Macromol. 2016;93(Part A):672–677. doi: 10.1016/j.ijbiomac.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh Y.-C., et al. An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose. 2008;15(4):507–513. [Google Scholar]
- 24.Czaja W., Romanovicz D., Brown R.M. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose. 2004;11(3):403–411. [Google Scholar]
- 25.Watanabe K., et al. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose. 1998;5(3):187–200. [Google Scholar]
- 26.Kim D.-Y., Nishiyama Y., Kuga S. Surface acetylation of bacterial cellulose. Cellulose. 2002;9(3):361–367. [Google Scholar]
- 27.Juntaro J. University of London; UK: 2009. Environmentally Friendly Hierarchical Composites, in Department of Chemical Engineering and Chemical Technology. [Google Scholar]
- 28.Shabalala S., et al. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017;180:160–170. doi: 10.1016/j.lfs.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Nazha A., et al. Second line therapies in polycythemia vera: what is the optimal strategy after hydroxyurea failure? Crit. Rev. Oncol. Hematol. 2016;105:112–117. doi: 10.1016/j.critrevonc.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Amin M.C.I.M., et al. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012;88(2):465–473. [Google Scholar]
- 31.Rokhade A.P., et al. Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr. Polym. 2006;65(3):243–252. [Google Scholar]
- 32.Gorgieva S., Girandon L., Kokol V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng.: C. 2017;73:478–489. doi: 10.1016/j.msec.2016.12.092. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., et al. In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydr. Polym. 2014;110:87–94. doi: 10.1016/j.carbpol.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 34.Munz M. Microstructure and roughness of photopolymerized poly(ethylene glycol) diacrylate hydrogel as measured by atomic force microscopy in amplitude and frequency modulation mode. Appl. Surf. Sci. 2013;279:300–309. [Google Scholar]
- 35.Rocha-García D., et al. Thermal and kinetic evaluation of biodegradable thermo-sensitive gelatin/poly(ethylene glycol) diamine crosslinked citric acid hydrogels for controlled release of tramadol. Eur. Polym. J. 2017;89:42–56. [Google Scholar]
- 36.Oliveira J.P.d., et al. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017;221:153–160. doi: 10.1016/j.foodchem.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.Y., Shim J., Lee H.G. Mechanical properties of gellan and gelatin composite films. Carbohydr. Polym. 2004;56(2):251–254. [Google Scholar]
- 38.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem. Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 39.Yin O.S., Ahmad I., Amin M.C.I.M. Effect of cellulose nanocrystals content and pH on swelling behaviour of gelatin based hydrogel. Sains Malaysiana. 2015;44(6):793–799. [Google Scholar]