Abstract
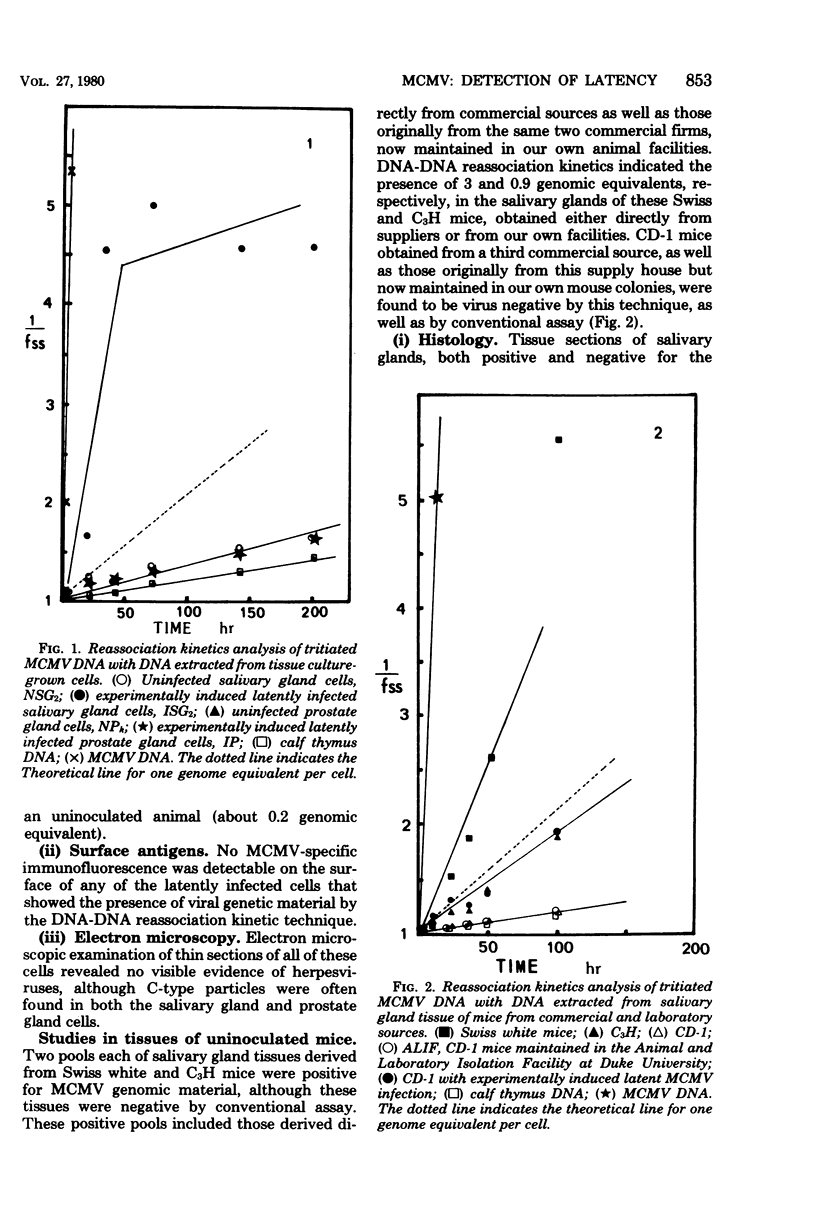
The technique of nucleic acid hybridization was used to detect the presence of murine cytomegalovirus (MCMV)-specific deoxyribonucleic acid (DNA) in cell cultures and salivary gland tissues. The presence of approximately 4.5 and 0.2 genome equivalents per cell of MCMV-specific DNA was identified in cultures of salivary (ISG2) and prostate gland (IP) cells, respectively. These cells, derived from animals with experimentally induced latent infections, were negative for virus-specific antigens by immunofluorescence and on electron microscopy revealed no visible evidence of the presence of herpesviruses. A cell line derived from the salivary gland of an uninoculated animal (NSG2) was also found to possess MCMV-specific DNA (0.2 genome equivalents per cell). For this reason, salivary gland tissues from uninoculated animals supplied as "specific pathogen-free" mice by three commercial sources were tested upon arrival for the presence of MCMC-specific DNA. MCMV-specific DNA was detectable in pooled salivary gland extracts from uninoculated animals derived from two commercial sources. All of these animals were seronegative and virus negative by conventional infectivity assays.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Lang D. J. Detection of latent cytomegalovirus in murine salivary and prostate explant cultures and cells. Infect Immun. 1977 Feb;15(2):568–575. doi: 10.1128/iai.15.2.568-574.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Lang D. J. Transmission and activation of cytomegalovirus with blood transfusion: a mouse model. J Infect Dis. 1977 May;135(5):841–845. doi: 10.1093/infdis/135.5.841. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C., Shanley J. D., Stevens J. G. Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J Gen Virol. 1977 Nov;37(2):419–423. doi: 10.1099/0022-1317-37-2-419. [DOI] [PubMed] [Google Scholar]
- Mayo D. R., Armstrong J. A., Ho M. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature. 1977 Jun 23;267(5613):721–723. doi: 10.1038/267721a0. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wu B. C., Dowling J. N., Armstrong J. A., Ho M. Enhancement of mouse cytomegalovirus infection during host-versus-graft reaction. Science. 1975 Oct 3;190(4209):56–58. doi: 10.1126/science.170676. [DOI] [PubMed] [Google Scholar]


