Abstract
Those with type 1 diabetes (T1D) are more likely to suffer a fracture than age- and sex-matched individuals without diabetes, despite daily insulin therapy. In rodent studies examining the effect of bone- or glucose-targeting therapies on preventing the T1D-related decrease in bone strength, insulin co-therapy is often not included, despite the known importance of insulin signaling to bone mass accrual. Therefore, working toward a relevant pre-clinical model of diabetic bone disease, we assessed the effect of continuous subcutaneous insulin infusion (CSII) therapy at escalating doses on preserving bone and the effect of delayed CSII on rescuing the T1D-related bone deterioration in an established murine model of T1D. Osmotic minipumps were implanted in male DBA/2 J mice 2 weeks (prevention study) and 6 weeks (rescue study) after the first injection of streptozotocin (STZ) to deliver insulin at 0, 0.0625, 0.125, or 0.25 IU/day (prevention study; n = 4–5 per dose) and 0 or 0.25 IU/day (rescue study; n = 10 per group). CSII lasted 4 weeks in both studies, which also included age-matched, non-diabetic DBA/2 J mice (n = 8–12 per study). As the insulin dose increased, blood glucose decreased, body weight increased, a serum maker of bone resorption decreased, and a serum marker of bone formation increased such that each end-point characteristic was linearly correlated with dose. There were insulin dose-dependent relationships (femur diaphysis) with cross-sectional area of cortical bone and cortical thickness (micro-computed tomography) as well as structural strength (peak force endured by the mid-shaft during three-point bending). Likewise, trabecular bone volume fraction (BV/TV), thickness, and number (distal femur metaphysis) increased as the insulin dose increased. Delayed CSII improved glycated hemoglobin (HbA1c), but blood glucose levels remained relatively high (well above non-diabetic levels). Interestingly, it returned the resorption and formation markers to similar levels as those seen in non-T1D control mice. This apparent return after 4 weeks of CSII translated to a partial rescue of the structural strength of the femur mid-shaft. Delayed CSII also increased Tb.Th to levels seen in non-T1D controls but did not fully restore BV/TV. The use of exogenous insulin should be considered in pre-clinical studies investigating the effect of T1D on bone as insulin therapy maintains bone structure without necessarily lowering glucose below diabetic levels.
Keywords: Diabetes, Bone strength, Insulin, Trabecular architecture, Cortical structure
Highlights
-
•
Insulin infusion can preserve and partially restore skeletal integrity in type 1 diabetes.
-
•
Skeletal improvements observed with continuous insulin infusion are dose-dependent.
-
•
Markers of bone formation improve with continuous insulin therapy.
-
•
Continuous insulin infusion decreases markers of bone resorption.
1. Introduction
Individuals affected with type 1 diabetes (T1D) are 6 times more likely to suffer a fracture than the non-diabetic population (Janghorbani et al., 2007), and low areal bone mineral density (aBMD) is associated with poor glycemic control (Danielson et al., 2009). Even though T1D patients receive insulin via multiple daily injection therapy (MDI) or via continuous subcutaneous insulin infusion therapy (CSII) to achieve glycemic control, they have a greater fracture risk in adulthood compared to age- and sex-matched non-diabetic individuals (Weber and Schwartz, 2016). The reason for this elevated risk is not entirely explained by low aBMD (Vestergaard, 2007), the primary clinical measurement to predict fracture risk. Thus, there is a need for pre-clinical models that mimic the human condition in order to identify the deleterious changes to bone that explain how T1D increases fracture risk or decreases fracture resistance.
The administration of streptozotocin (STZ), which is cytotoxic to beta cells of the pancreas, is a well-established rodent model of T1D. The toxin causes a reduction in insulin production and subsequent hyperglycemia with the severity depending on the strain of the rodent (Rodrigues et al., 1997, Shimizu et al., 2012) and the dose of STZ (Ventura-Sobrevilla et al., 2011). Typically induced between 11-week to 14-week of age in either mice or rats, STZ-T1D impedes normal bone mass accrual resulting in lower structural strength of cortical bone and reduced trabecular bone within several weeks as compared to non-diabetic controls (Nyman et al., 2011, Silva et al., 2009). The resulting hypoinsulinemia/hyperglycemia in the STZ model also affects the material properties of cortical bone (fracture resistance independent of bone structure), but durations of T1D exceeding 8 weeks and 14 weeks are required before significant differences in material strength and toughness between diabetic and control rodents, respectively, are typically observed (Nyman, 2012).
Despite the known importance of insulin signaling in osteoblasts to bone mass accrual (Fulzele et al., 2010, Thrailkill et al., 2014), insulin co-therapy is not always included in pre-clinical studies investigating the effect of bone- or glucose-targeting therapies on preventing the T1D-related decrease in bone strength (Altan et al., 2007, Glorie et al., 2014, Maycas et al., 2016, Thrailkill et al., 2016, Zhang et al., 2014). In early rat studies, daily subcutaneous injections of insulin improved the structural strength of the femur mid-shaft (Dixit and Ekstrom, 1980) and femoral neck (Hou et al., 1993) when compared to untreated alloxan-induced T1D rats. Recent rat studies report daily subcutaneous injections of insulin nearly restored the STZ-related loss i) in peak force endured by the femur mid-shaft during three-point (3 pt) bending (Rao Sirasanagandla et al., 2014), ii) in total femur aBMD (Zhang et al., 2008), iii) in femoral neck and mid-shaft diameters (Abd El Aziz et al., 2015), iv) in femur mid-shaft aBMD (but not in tensile mechanical properties) (Erdal et al., 2012), and v) in peak bending stress (but not in peak force during 3 pt. bending) of the tibia mid-shaft (Bortolin et al., 2016). While the insulin dosing (~ 2 IU/day) tended to be similar among these studies, discrepancies in the protective effect on mechanical properties could be due to other experimental factors (e.g., age and strain of the rat, duration of T1D before therapy, dose of STZ, method of mechanical testing). There are at least two rat studies of T1D involving 4 weeks of CSII: one reporting an improvement in trabecular bone volume fraction with treatment compared to untreated rats (Hie et al., 2011) and one reporting no treatment effect on the osteointegration of miniscrews even when combined with intermittent parathyroid hormone (PTH) injections (Rybaczek et al., 2015).
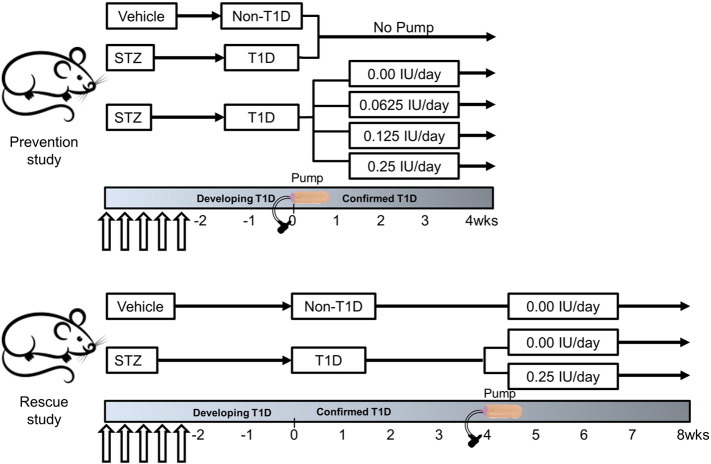
To the best of our knowledge, there are no rodent studies reporting how variable insulin therapy, delivered as escalating doses of insulin via CSII, impacts bone early in the course of T1D (i.e., first 4 weeks of established diabetes) or whether delayed insulin therapy could also be beneficial in promoting bone strength in T1D. Therefore, we first studied the dose-dependent relationship between insulin therapy and bone strength in mice by assessing the effect of continuous insulin delivery on cortical structure and trabecular architecture in the early phase of diabetic bone disease. Next, we studied the effect of starting CSII after ~ 4 weeks of persistent STZ-induced T1D on mouse bone. The two hypotheses then were: i) CSII early in the course of T1D could prevent the diabetes-induced deterioration of cortical bone structure and strength as well as the deterioration of trabecular bone microarchitecture in a dose-dependent fashion (prevention study) and ii) CSII could rescue the diabetes-induced deterioration of cortical bone structure and trabecular bone microarchitecture (Study 2: rescue study). In both studies, CSII lasted for 4 weeks (see Fig. 1 for specifics of each study design).
Fig. 1.
Overview of the two study designs. In the prevention study, insulin was administered via CSII at escalating doses early in T1D duration. Non-diabetic and diabetic mice were age-matched to the mice receiving the insulin pumps in order to establish the effect of diabetes on bone. In the rescue study, insulin therapy did not commence until 6 weeks after the first STZ injection (arrows).
2. Materials and methods
2.1. Study design
2.1.1. Prevention study
Over 5 days, 11-week old, male, DBA/2 J mice (The Jackson Laboratories, Bar Harbor, ME) received intraperitoneal (ip) injections of STZ at 40 mg/kg/day in 100 mM citrate buffer. After confirming persistent hyperglycemia (non-fasting blood glucose remaining above 250 mg/dl), 13-week, STZ-diabetic mice were treated with saline vehicle (0.0 dose) or insulin (Humulin R Insulin, Eli Lilly and Co., Indianapolis, IN) at 1 of 3 doses (0.0625, 0.125, and 0.25 IU/day; n = 4–5 per group) using an osmotic minipump (Model 2004, ALZET®, Cupertino, CA) for 4 weeks and then euthanized. Following the instructions from the manufacturer, the Alzet pump was implanted subcutaneously in the left anterodorsal region. Matched for age, additional male DBA/2 J mice were also administered ip injections of STZ at 40 mg/kg/day (T1D: n = 9 but 8 individual femurs available for analysis) or only buffer (100 mM citrate buffer) over 5 days (non-T1D: n = 10) and euthanized at 17-weeks without treatment (Fig. 1). Two mice died within 3 weeks of the STZ injection (STZ-T1D with no pump and STZ-T1D-i0.0625), and one mouse died within 2 weeks of receiving the Alzet pump (STZ-T1D-i0.0).
2.1.2. Rescue study
12-Week old, male, DBA/2 J mice (The Jackson Laboratories, Bar Harbor, ME) received ip injections of STZ (T1D) or citrate buffer (non-T1D, n = 12) as described for the prevention study. After 4 weeks of confirmed T1D (non-fasting blood glucose > 250 mg/dl per week), an Alzet minipump was implanted subcutaneously in the left anterodorsal region of each T1D mouse (18-weeks of age) to continuously deliver either saline (n = 11 instead of 12 because 1 died within 5 weeks) or 0.25 units/day of insulin (n = 10 instead of 12 because 2 died within 5 weeks). Matched for age, non-T1D mouse group also received continuous saline via the same minipump technique (Fig. 1). All mice were euthanized 4 weeks after pump implantation at 22-weeks of age.
For both studies, non-fasting glucose levels were monitored weekly with a glucometer (OneTouch® Ultra®2 Blood Glucose Monitoring System, Lifescan, Inc., Milpitas, CA). Following euthanasia, the left femurs were stored in phosphate buffered saline (PBS) at − 20 °C. All animal procedures followed a protocol approved by the University of Kentucky Institutional Animal Care and Use Committee or the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
2.2. Biochemical measurements
Procollagen type 1 N-terminal propeptide (P1NP) was measured in serum, from blood collected at sacrifice, using the Rat/Mouse P1NP Enzyme immunoassay (Immunodiagnostics Systems, Inc., Fountain Hills, AZ; #AC-33F1). C-terminal telopeptides of type I collagen (RatLAPs) were measured in serum at sacrifice using the RatLAPs ELISA (Immunodiagnostics Systems, Inc., Fountain Hills, AZ; #AC-06F1). HbA1c was measured on whole blood (Crystal Chem, Downers Grove, Ill. #80310).
2.3. Micro-computed tomography (μCT) analysis
Each femur was inserted in a 6 mm tube filled with PBS. Then, the mid-shaft and distal femur metaphysis were each scanned at an isotropic voxel size of 6 μm using a high-resolution μCT scanner (μCT50, Scanco Medical, Brϋttisellen, Switzerland). The scan settings were as follows: peak voltage of 70 kVp, current of 0.114 mA, integration time of 300 ms, and 1000 projections per full rotation onto a 1024 × 1024 pixel detector. A 0.1 mm thick aluminum filter was included, and the manufacturer's beam correction was applied. A phantom of hydroxyapatite (HA) was scanned weekly to verify the linear conversion of attenuation to mineral density (mgHA/cm3). Following reconstruction, 1.29 mm of the mid-shaft at the point of loading (described in the subsequent section) was evaluated using the algorithms provided by the manufacturer to determine cortical parameters. This involved applying a Gaussian noise filter (standard deviation of 0.2 and support of 1) and a global threshold of 864.3 mgHA/cm3 (prevention study) or 973.0 mgHA/cm3 (rescue study) to segment bone from water and air. Likewise, following reconstruction and auto-contouring (Nyman et al., 2011), 1.48 mm of the metaphysis at a set distance from the growth plate was evaluated to determine trabecular parameters using a noise filter (standard deviation of 0.2 and support of 2) and global threshold of 417.3 mgHA/cm3 (prevention study) or 381.1 mgHA/cm3 (rescue study).
2.4. Three-point bend testing
Following the same procedures described in our previous publications (Nyman et al., 2011, Thrailkill et al., 2014), each hydrated femur mid-shaft was loaded to failure at 3 mm/min in three-point bending with a span of 8 mm. The stiffness and structural strength were determined from the resulting force vs. displacement curve as the slope of the initial linear portion of the curve and the peak force endured by the bone, respectively. Using the moment of inertia (Imin), cross-sectional area of the cortex (Ct.Ar), and the distance between the centroid and the outer most point in the anterior-posterior direction (cmin) from the μCT evaluation of the femur mid-shaft, we estimated bending strength and span-adjusted toughness (Uppuganti et al., 2016).
2.5. Statistical analysis
The Mann-Whitney test was used to determine whether each bone property or measurement significantly differed between STZ-T1D and Non-T1D or between STZ-i0.25 vs. Non-T1D. To determine whether dose-dependent relationships existed while minimizing the number of animals per group, a linear regression between each property or measurement (bootstrapped with 500 replicates) and insulin dose was performed. For rescue study, the Kruskal-Wallis test was first used to determine whether differences in outcomes differed among the three experimental groups followed by Dunn's test to correct for multiple comparisons when the null hypothesis was false (p < 0.05). Results are reported as mean ± standard deviation (SD).
3. Results
3.1. Prevention study: effects of early continuous subcutaneous insulin infusion treatment as a function of dose
As expected for 4–5 weeks of T1D, untreated STZ-mice significantly weighed less and had higher non-fasting glucose levels (> 500 mg/dl) compared to non-diabetic mice (Table 1). Continuous subcutaneous insulin infusion therapy (CSII) dose dependently increased body weight of the diabetic mice while decreasing their glucose levels as evident by the significant slope (not equal to 0) from linear regressions between the measurements and insulin dose (Table 1). In addition, the P1NP marker of bone formation and RatLAPS (C-terminal telopeptides of type I collagen) marker of bone resorption were significantly lower and higher, respectively, for the diabetic than for non-diabetic mice (Table 1). Interestingly, the serum marker of bone formation increased while the serum marker of bone resorption decreased in a dose dependent manner in response to CSII (Table 1). These data suggest that absolute insulin dose might directly relate to improvements in bone formation as well as preventing bone resorption in T1D mice. These possibilities were explored further at the level of bone microarchitecture and biomechanical properties as follows.
Table 1.
Differences in selected mouse characteristics (mean ± SD) at sacrifice between non-diabetic and diabetic mice as well as the dose-response relationship between continuous insulin dose and each characteristic.
| Characteristic | No treatment |
STZ-T1D insulin dose (IU/day) |
Linear regression |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-T1D | STZ-T1D | p-Value | 0 | 0.0625 | 0.125 | 0.25 | Slopea (p-value) | R2 (%) | |
| Body weight (g) | 27.8 ± 4.1 | 20.9 ± 1.2 | < 0.001 | 22.7 ± 1.7 | 25.0 ± 1.0 | 24.9 ± 1.8 | 26.8 ± 0.9 | 14.8 (< 0.001) | 54.6 |
| Glucose (mg/dl) | 139 ± 19 | 535 ± 63 | < 0.001 | 595 ± 10 | 562 ± 28 | 449 ± 32 | 209 ± 119 | − 1626 (< 0.001) | 84.3 |
| RatLAPS (ng/ml) | 14.5 ± 5.3 | 35.4 ± 11.1 | 0.001 | 39.9 ± 18.6 | 46.6 ± 2.0 | 39.5 ± 13.6 | 25.4 ± 7.0 | − 71.0 (0.033) | 25.4 |
| P1NP (ng/ml) | 48.9 ± 4.6 | 18.6 ± 4.5 | < 0.001 | 26.6 ± 11.5 | 29.5 ± 5.5 | 39.1 ± 6.9 | 63.3 ± 23.7 | 155 (0.001) | 54.7 |
Slope is the change in characteristic per change in dose as determined by linear regression of each characteristic value vs. dose (i.e., not the mean value vs. dose).
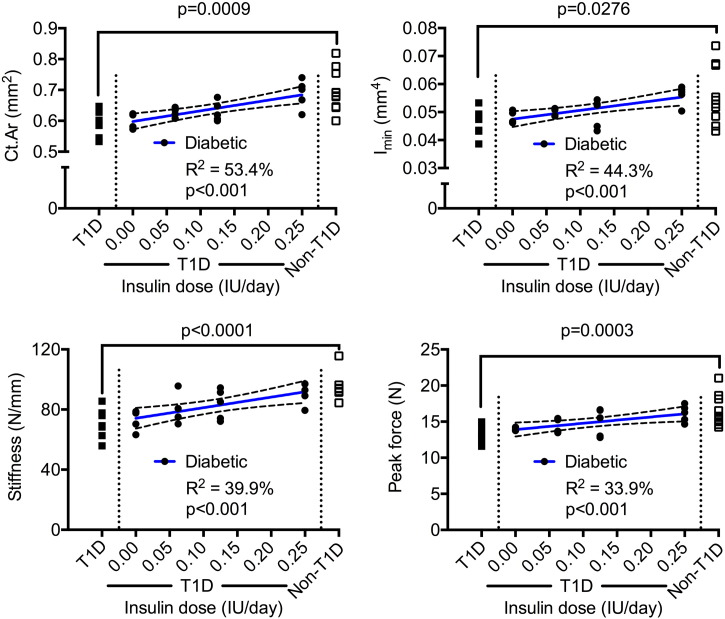
There was also a linear relationship between CSII and structural strength such that the cross-sectional area of the cortex (Ct.Ar), moment of inertia (Imin), and peak force increased as the dose increased (Fig. 2). Like structural strength, short-term T1D reduced the stiffness of the femur diaphysis when compared to non-diabetic controls, and CSII also appeared to maintain this bone stiffness (Fig. 2) as well as the periosteal perimeter (Ps.Pm) for diabetic mice (Table 2). While loss of insulin following STZ injections affects bone structure at the mid-shaft, it does not necessarily reduce the length of the femur in the short-term. There is however a weak, positive correlation between CSII dose and femur length (Table 2). The estimated material properties and tissue mineral density of cortical bone (Ct.TMD) did not vary among insulin doses, and diabetes for 4–5 weeks only decreased bending strength, not toughness (Table 2).
Fig. 2.
Dose-dependent relationships with cortical parameters of the femur mid-shaft. The bone structure and strength increased with an increase in insulin dose. The p-value above the bracket is for T1D vs. Non-T1D, while the p-value below the coefficient of determination (R2) is for the slope (not equal to zero when p < 0.05).
Table 2.
Differences in selected properties (mean ± SD) of the femur between non-diabetic and diabetic mice as well as the dose-response relationship between continuous insulin dose and each characteristic.
| Property | No treatment |
STZ-T1D insulin dose (IU/day) |
Linear regression |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-T1D | STZ-T1D | p-Value | 0 | 0.0625 | 0.125 | 0.25 | Slopea (p-value) | R2 (%) | |
| Lengthb (mm) | 14.0 ± 0.4 | 13.7 ± 0.3 | 0.146 | 13.7 ± 0.2 | 13.9 ± 0.2 | 13.9 ± 0.2 | 14.0 ± 0.1 | 0.95 (0.016) | 21.9 |
| Ps.Pm (mm) | 4.17 ± 0.25 | 4.00 ± 0.09 | 0.036 | 4.08 ± 0.10 | 4.16 ± 0.07 | 4.12 ± 0.08 | 4.31 ± 0.11 | 0.86 (0.001) | 43.7 |
| Es.Pm (mm) | 2.59 ± 0.19 | 2.67 ± 0.09 | 0.203 | 2.71 ± 0.06 | 2.73 ± 0.08 | 2.56 ± 0.09 | 2.68 ± 0.09 | = 0 (0.469) | 2.6 |
| Ct.Th (μm) | 199 ± 7 | 176 ± 9 | < 0.001 | 179 ± 2 | 182 ± 3 | 186 ± 7 | 193 ± 6 | 55 (< 0.001) | 54.5 |
| Ct.TMD (mgHA/cm3) | 1365 ± 8 | 1373 ± 10 | 0.087 | 1389 ± 5 | 1368 ± 18 | 1379 ± 7 | 1377 ± 10 | = 0 (0.500) | 2.5 |
| Modulus (GPa) | 16.6 ± 3.0 | 18.5 ± 2.8 | 0.180 | 16.1 ± 1.9 | 17.2 ± 2.2 | 17.9 ± 0.7 | 17.3 ± 0.7 | = 0 (0.260) | 6.8 |
| Bending strength (MPa) | 285 ± 19 | 262 ± 15 | 0.022 | 274 ± 17 | 272 ± 17 | 284 ± 19 | 278 ± 14 | = 0 (0.505) | 2.3 |
| Toughness (MJ/m3) | 2.12 ± 0.48 | 2.45 ± 0.91 | 0.310 | 1.96 ± 0.82 | 1.81 ± 0.29 | 2.00 ± 0.31 | 2.51 ± 0.67 | = 0 (0.122) | 17.5 |
Slope is the change in property per change in dose as determined by linear regression of each property value vs. dose.
Distance between the intercondyler groove and femoral neck as determined by calipers.
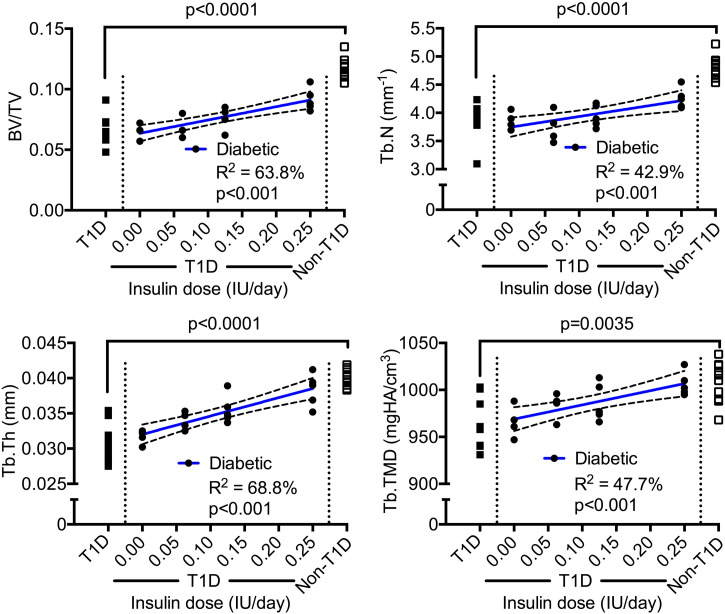
A dose-dependent relationship also existed between CSII and trabecular bone volume fraction (BV/TV) as well as trabecular tissue mineral density (Tb.TMD) (Fig. 3). As insulin dose increased, trabecular number (Tb.N) and trabecular thickness (Tb.Th) increased (Fig. 3). Whereas insulin at 0.25 IU/day (T1D-i0.25) appeared to prevent the diabetes-related decrease in Ct.Th (T1D-i0.25 vs. Non-T1D: p = 0.058), in Ct.Ar (T1D-i0.25 vs. Non-T1D: p > 0.999), and in Imin (T1D-i0.25 vs. Non-T1D: p = 0.514), it did not completely maintain BV/TV (T1D-i0.25 vs. Non-T1D: p = 0.0004) and Tb.N (T1D-i0.25 vs. Non-T1D: p = 0.001). Lastly, there was a direct linear correlation between insulin dose and Tb.TMD (Fig. 3), but not between dose and Ct.TMD (Table 2).
Fig. 3.
Dose-dependent relationships with trabecular parameters of the distal femur metaphysis. Trabecular bone volume fraction increased and architecture improved with an increase in insulin dose. The p-value above the bracket is for T1D vs. Non-T1D, while the p-value below the coefficient of determination (R2) is for the slope (not equal to zero when p < 0.05).
3.2. Rescue study: effect of delayed continuous subcutaneous insulin infusion treatment at 0.25 IU/day
The metabolic effects that were observed after 4 weeks of confirmed T1D also existed after 8 weeks of confirmed T1D such that vehicle-treated diabetic (T1D-Veh) animals had lower body weight, higher non-fasting blood glucose, higher RatLAPS, and lower P1NP compared to non-diabetic controls (ND-Veh in Table 3). Delayed CSII at 0.25 IU/day (T1D-i0.25) for 4 weeks did not significantly increase body weight nor decrease blood glucose to levels observed for ND-Veh mice (Table 3), but delayed CSII did significantly reduce glycated hemoglobin (HbA1c, a marker of diabetic severity) and significantly improved the bone resorption and formation markers in comparison to T1D-Veh mice (Table 3).
Table 3.
Differences in selected characteristics (mean ± SD) among vehicle-treated non-diabetic mice, vehicle-treated diabetic mice, and insulin-treated diabetic mice.
| Experimental groups | Adjusted p-values | |||||
|---|---|---|---|---|---|---|
| Characteristic | Non-Veh1 | T1D-Veh2 | T1D-i0.253 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 |
| Body weight (g) | 31.5 ± 2.6 | 25.4 ± 1.8 | 27.5 ± 1.7 | < 0.001 | 0.007 | 0.339 |
| Glucose (mg/dl)a | 150 ± 12 | 582 ± 59 | 489 ± 99 | < 0.001 | 0.003 | 0.407 |
| Glucose (mg/dl)b | 143 ± 13 | 581 ± 35 | 473 ± 59 | < 0.001 | 0.017 | 0.067 |
| HbA1c (%) | 4.3 ± 1.0 | 10.2 ± 1.0 | 7.5 ± 1.2 | < 0.001 | 0.037 | 0.034 |
| RatLAPS (ng/ml) | 10.7 ± 1.7 | 16.8 ± 5.1 | 10.6 ± 2.3 | 0.006 | > 0.999 | 0.002 |
| P1NP (ng/ml) | 39.2 ± 9.4 | 17.6 ± 3.3 | 29.3 ± 7.8 | < 0.001 | 0.317 | 0.008 |
Blood glucose levels at euthanasia. Ten of the 11 mice in the T1D-Veh group and 3 of 10 mice in the T1D-i0.25 group had glucose levels > 600 mg/dl, the maximum value that the glucometer can measure.
Average of blood glucose measurements when the minipumps were implanted (weeks 5–8).
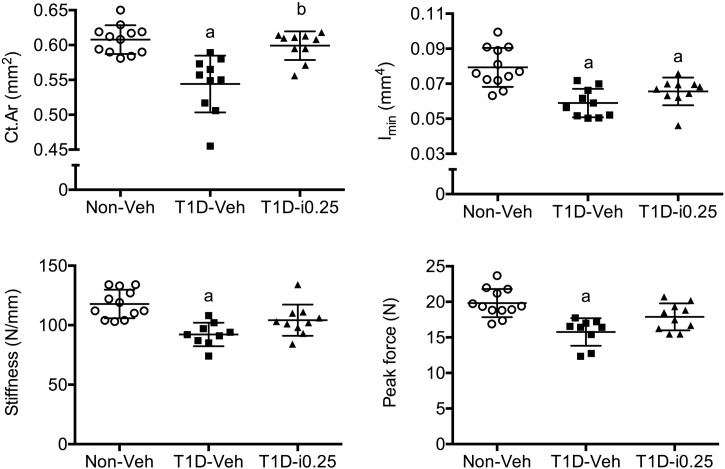
The CSII-related return of resorption and near return of formation markers to non-diabetic levels (Table 3) did not necessarily translate to a full rescue of structural stiffness and strength of the femur mid-shaft. While these properties were lower for the T1D-Veh mice than for ND-Veh mice, stiffness and peak force were not significantly different between T1D-i0.25 and ND-Veh mice indicating a partial rescue with insulin therapy (Fig. 4). Delayed CSII had a positive effect on cortical cross-sectional area but not on Imin and Ps.Pm (Fig. 4). As observed for 4 weeks of confirmed T1D (prevention study), 8 weeks of confirmed diabetes (rescue study) did not affect Ct.TMD and the estimated material properties of cortical bone (Table 4). There were differences in femur length and Ct.Th between Non-Veh and T1D-Veh mice, and delayed CSII partially rescued these deficits (Table 4).
Fig. 4.
Group differences in cortical bone properties (femur mid-shaft). Delayed insulin therapy via osmotic minipumps (CSII) rescued the T1D-induced loss in cortical cross-sectional area but not the loss in the moment of inertia. Even though the bone structure was not fully restored, delayed CSII partially improved whole-bone stiffness and strength. a: adjusted p-value < 0.05 vs. ND-Veh and b: adjusted p-value < 0.05 vs. T1D-Veh.
Table 4.
Differences in select properties (mean ± SD) of the femur among vehicle-treated non-diabetic mice, vehicle-treated diabetic mice, and insulin-treated diabetic mice.
| Property | Experimental groups |
Adjusted p-values |
||||
|---|---|---|---|---|---|---|
| Non-Veh1 | T1D-Veh2 | T1D-i0.253 | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |
| Length (mm) | 14.4 ± 0.3 | 13.8 ± 0.6 | 14.2 ± 0.3 | 0.009 | 0.273 | 0.671 |
| Ps.Pr (mm) | 4.56 ± 0.21 | 4.34 ± 0.27 | 4.36 ± 0.19 | 0.028 | 0.318 | > 0.999 |
| Es.Pr (mm) | 2.79 ± 0.25 | 2.87 ± 0.35 | 2.65 ± 0.18 | 0.192a | ||
| Ct.Th (μm) | 182 ± 14 | 163 ± 14 | 176 ± 13 | 0.009 | 0.532 | 0.363 |
| Ct.TMD (mgHA/cm3) | 1345 ± 22 | 1332 ± 20 | 1340 ± 22 | 0.274 | ||
| Modulus (GPa) | 16.0 ± 1.6 | 16.6 ± 2.1 | 17.0 ± 1.3 | 0.321 | ||
| Bending strength (MPa) | 269 ± 26 | 267 ± 28 | 281 ± 26 | 0.487 | ||
| Toughness (MJ/m3) | 1.80 ± 0.60 | 1.74 ± 0.61 | 1.51 ± 0.53 | 0.930 | ||
Single p-value comes from the Kruskal-Wallis test indicating no difference among the groups.
With respect to trabecular micro-architecture of the distal femur metaphysis, delayed CSII nearly restored Tb.Th and Ct.TMD to levels observed for ND-Veh mice (Fig. 5). Unlike the prevention study, there were no significant difference in Tb.N between 22-week old, non-diabetic and 22-week old diabetic mice in which saline was delivered via the minipumps between weeks 4 and 8 of confirmed T1D. Delayed CSII possibly had a negative effect on Tb.N, though there was no difference between T1D-Veh and T1D-i0.25 groups (Fig. 5). Overall, delayed CSII partially rescued BV/TV with no significant difference between ND-Veh and T1D-i0.25 but also no significant difference between T1D-Veh and T1D-i0.25 (Fig. 5).
Fig. 5.
Group differences in trabecular bone properties (distal femur metaphysis). Delayed insulin therapy via osmotic minipumps (CSII) significantly improved trabecular thickness nearly restoring Tb.Th to the level of non-T1D controls. It also had positive effect on trabecular tissue mineral density, but little effect on the trabecular bone volume fraction. a: adjusted p-value < 0.05 vs. ND-Veh and b: adjusted p-value < 0.05 vs. T1D-Veh.
4. Discussion
A pre-clinical rodent model of T1D for investigating diabetic bone disease should consider the impact that exogenous insulin therapy (continuous infusion or daily injections) has on the skeleton. Insulin is universally used to treat T1D patients; thus, any pre-clinical findings related to variations in insulin administration and glycemic control are relevant to understanding skeletal health in the T1D population. Notably, T1D treatment is distinct in this way from human type 2 diabetes treatment, wherein multiple alternative strategies to control blood glucose levels may be employed outside of insulin (which is often added only as an escalation to treat hyperglycemia). To better understand the relationship between insulin and bone strength in a mouse model of T1D, we investigated the effect of increasing doses of insulin, delivered by CSII, on cortical and trabecular bone following STZ-induced diabetes; and we found that insulin therapy not only dose-dependently controls blood glucose in DBA/2 J mice but also dose-dependently preserves cortical structure and trabecular architecture of the femur diaphysis and metaphysis, respectively. Thus, with the use of implantable osmotic minipumps, varying degrees of glycemic control and varying deficits in bone structure and architecture can be achieved by titrating the insulin dose in STZ-induced T1D mice, especially when CSII is initiated early in the course of T1D.
Although this study cannot decouple the effect of insulin from the effect of glucose on bone, 7 out of the 10 T1D mice receiving insulin at ≥ 0.125 IU/day continued to have consistent blood glucose readings > 200 mg/dl (the weekly average over the 4-week period in the prevention study was 357 ± 89 mg/dl) while structural strength (mean ± SD) of the femur mid-shaft was similar between these 7 mice (15.2 ± 1.8 N) and the 10 non-T1D mice (16.5 ± 2.3 N), which typically had blood glucose readings between ~ 140 mg/dl and ~ 150 mg/dl. This finding from the pre-clinical mouse model suggests that glycemic control alone may not fully account for the structural strength improvements observed with CSII, thereby suggesting treatment at an optimal insulin dose and mode of delivery may positively impact skeletal growth through additional mechanisms not dependent on blood glucose alone.
While insulin therapy is known to preserve bone strength and trabecular architecture in STZ rodents (Hie et al., 2011, Rao Sirasanagandla et al., 2014), whether insulin can restore bone structure and strength is unknown for diabetic rodents. Four weeks of CSII partially rescued cortical structure and structural strength when started 6 weeks after the first STZ injection (i.e., 4 weeks of established, uncontrolled diabetes). Thus, for those newly diagnosed with T1D or for those who are non-complaint or under-treated with insulin (poor control), optimizing insulin therapy using means such as CSII may prove beneficial in promoting peak bone mass and strength. Additional studies will be necessary to determine if optimizing further insulin therapy (using higher insulin doses and/or longer duration of insulin treatment) may have an even more profound effect on rescuing the skeletal integrity in T1D.
In the STZ model, diabetes deleteriously affects both trabecular and cortical bone, and CSII does not necessarily preserve both cortical structure and trabecular architecture to the same extent. CSII dose-dependently increased both Tb.Th (Fig. 3) and Ct.Th (Table 1). While this increase prevented a decrease in Ct.Ar and Imin of the femur diaphysis (Fig. 2), it did not translate to complete preservation of trabecular BV/TV of the distal femur metaphysis likely because the Tb.N remained low in the CSII-treated T1D mice (Fig. 3). This differential effect of CSII on trabecular and cortical bone could be due to differences in the expression or sensitivity of insulin receptors on bone cells between the two compartments. Also, in growing mice, the cortex undergoes modeling in which the cross-sectional area and moment of inertia of the cortex increases (Ferguson et al., 2003). Trabecular bone of the metaphysis on the other hand undergoes remodeling, thereby altering trabecular architecture of the metaphysis. Thus, the high dose of insulin and duration of CSII used in the prevention study herein were sufficient to maintain normal cortical modeling of the mid-shaft (periosteal perimeter was positively associated with insulin dose, Table 2), but were not sufficient to maintain normal trabecular remodeling. Of interest, the marker of resorption (RatLAPS) and the marker of formation (P1NP) were 1.75 times and 1.29 times higher, respectively, for T1D-i0.25 than for non-treated, non-T1D mice indicating resorption activity may still have outpaced formation activity in the insulin-treated T1D mice at the highest dose.
In the rescue study, CSII treatment of diabetic mice increased Tb.Th without affecting Tb.N (Fig. 5). Even though the resorption and formation markers returned to near non-diabetic levels at 0.25 IU/day (0.99 and 0.75 times ND-Veh levels, respectively), 4 weeks was not sufficient for new trabeculae to form between existing trabeculae. Curiously, there was no difference in Tb.N between non-diabetic and diabetic mice (vehicle treated) in the rescue study unlike the prevention study. The diabetes may have accelerated the loss in Tb.N, but by 22 weeks of age, the typical age-related decrease in Tb.N for the non-T1D mice possibly caught up with diabetic animals. With respect to cortical bone, diabetes did not affect endosteal perimeter in the prevention and rescue studies, but it did lower periosteal perimeter (Table 2, Table 4) suggesting delayed CSII started to increase periosteal bone formation in the diabetic mice.
The findings that insulin incrementally improves skeletal parameters both in the cortical and trabecular compartments is consistent with previous studies showing that the elimination of the insulin receptor in osteoprogenitors in male mice results in lower trabecular bone volume fraction and thinner trabeculae as well as thinner cortices and a smaller moment of inertia in the diaphysis, which manifests as decreased structural strength of the femur mid-shaft, independent of body mass (Fulzele et al., 2010, Thrailkill et al., 2014). Thus, insulin may directly improve bone formation through its effects on osteoblastic activity in both the trabecular and cortical compartments.
There are several limitations to the reported findings. While markers of resorption and formation provided insight into how CSII in the context of T1D affects bone metabolism, bone histology and dynamic histomorphometry would further establish how insulin therapy preserved or partially rescued bone structure and architecture. The sample size in the prevention study precluded detecting significant differences in the selected bone properties among the different groups of escalating insulin dose. Obviously, there is overlap in each property among the different insulin groups, but overall bone properties improve with an increase in insulin dose for T1D mice. The slope and R2 of these dose relationships depend on the dynamic range in the given property (i.e., how much the property differs between non-T1D and T1D mice), so we cannot conclude that insulin dosing has more of an effect on Tb.Th (R2 = 68.8%) than on Imin (R2 = 44.3%). Without longitudinal measurements (e.g., in vivo μCT), our ex vivo measurements are unable to quantify the relative change in a bone property over the treatment period as a function of dose, and they are less sensitive to detecting the rescue effect of delayed CSII. Ultimately though, assessment of bone strength requires destructive testing, and early and delayed CSII does appear beneficial to this biomechanical property. Finally, only male mice were used in these studies, thus limiting our conclusions to only one sex.
5. Conclusion
As the dose of insulin increased with 4 weeks of CSII therapy when administered soon after the onset of frank diabetes, cortical structure and strength as well as trabecular architecture improved, though trabecular number was not fully restored at the highest dose. This appeared to occur without fully lowering non-fasting blood glucose to non-diabetic levels. Delayed CSII for 4 weeks in the T1D model rescued certain structural properties, namely cross-sectional area of the cortex, and partially rescued structural strength. It also rescued trabecular thickness, but not trabecular bone volume fraction of the femur metaphysis, despite nearly restoring markers of bone resorption and bone formation to non-diabetic levels. Thus, these studies show that continuous insulin therapy early after diabetes onset can promote normal bone mass accrual in a dose-dependent fashion, while delayed treatment with insulin can partially improve strength and structure of bone. Ultimately, these studies provide pre-clinical evidence that either approach to optimize insulin dose, duration, and mode of administration may prove relevant to improving bone structure and quality and reduce fracture risk in humans with T1D.
Acknowledgements
Support for this work was provided by the Children's University Medical Group Fund of the Arkansas Children's Hospital Research Institute (KMT), the Arkansas Biosciences Institute (JLF), National Institutes of Health (1R01DK084045 to JLF and 1R01AR063157 to JSN), Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (1I01BX001018 to JSN). The micro-computed tomography scanner was supported by the National Center for Research Resources (1S10RR027631) and matching funds from the Vanderbilt Office of Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies.
References
- Abd El Aziz G.S., Ramadan W.S., El-Fark M.O., Saleh H.A.M. The beneficial roles of insulin and parathyroid hormones in the treatment of experimentally induced diabetic osteoporosis in female rats: bone mineral density, morphometric and histological studies. Folia Morphol. (Warsz) 2015 doi: 10.5603/FM.a2015.0129. [DOI] [PubMed] [Google Scholar]
- Altan M.F., Kanter M., Donmez S., Kartal M.E., Buyukbas S. Combination therapy of Nigella sativa and human parathyroid hormone on bone mass, biomechanical behavior and structure in streptozotocin-induced diabetic rats. Acta Histochem. 2007;109:304–314. doi: 10.1016/j.acthis.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bortolin R.H., Freire Neto F.P., Arcaro Filho C.A., Bezerra J.F., da Silva F.S., Ururahy M.A.G., da Costa Souza K.S., Lima V.M.G.D.M., Luchessi A.D., Lima F.P., Lia Fook M.V., da Silva B.J., Almeida M.D.G., Abreu B.J., de Rezende L.A., de Rezende A.A. Anabolic effect of insulin therapy on the bone: osteoprotegerin and osteocalcin up-regulation in streptozotocin-induced diabetic rats. Basic Clin. Pharmacol. Toxicol. 2016 doi: 10.1111/bcpt.12672. [DOI] [PubMed] [Google Scholar]
- Danielson K.K., Elliott M.E., LeCaire T., Binkley N., Palta M. Poor glycemic control is associated with low BMD detected in premenopausal women with type 1 diabetes. Osteoporos. Int. 2009;20:923–933. doi: 10.1007/s00198-008-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit P.K., Ekstrom R.A. Decreased breaking strength of diabetic rat bone and its improvement by insulin treatment. Calcif. Tissue Int. 1980;32:195–199. doi: 10.1007/BF02408541. [DOI] [PubMed] [Google Scholar]
- Erdal N., Gürgül S., Demirel C., Yildiz A. The effect of insulin therapy on biomechanical deterioration of bone in streptozotocin (STZ)-induced type 1 diabetes mellitus in rats. Diabetes Res. Clin. Pract. 2012;97:461–467. doi: 10.1016/j.diabres.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Ferguson V.L., Ayers R.A., Bateman T.A., Simske S.J. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–398. doi: 10.1016/s8756-3282(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Fulzele K., Riddle R.C., DiGirolamo D.J., Cao X., Wan C., Chen D., Faugere M.-C., Aja S., Hussain M.A., Brüning J.C., Clemens T.L. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorie L., Behets G.J., Baerts L., De Meester I., D'Haese P.C., Verhulst A. DPP IV inhibitor treatment attenuates bone loss and improves mechanical bone strength in male diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2014;307:E447–E455. doi: 10.1152/ajpendo.00217.2014. [DOI] [PubMed] [Google Scholar]
- Hie M., Iitsuka N., Otsu T., Tsukamoto I. Insulin-dependent diabetes mellitus decreases osteoblastogenesis associated with the inhibition of Wnt signaling through increased expression of Sost and Dkk1 and inhibition of Akt activation. Int. J. Mol. Med. 2011;28:455–462. doi: 10.3892/ijmm.2011.697. [DOI] [PubMed] [Google Scholar]
- Hou J.C., Zernicke R.F., Barnard R.J. Vol. 11. 1993. Effects of Severe Diabetes and Insulin on the Femoral Neck of the Immature Rat; pp. 263–271. [DOI] [PubMed] [Google Scholar]
- Janghorbani M., Van Dam R.M., Willett W.C., Hu F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- Maycas M., McAndrews K.A., Sato A.Y., Pellegrini G.G., Brown D.M., Allen M.R., Plotkin L.I., Gortazar A.R., Esbrit P., Bellido T. PTHrP-derived peptides restore bone mass and strength in diabetic mice: additive effect of mechanical loading. J. Bone Miner. Res. 2016 doi: 10.1002/jbmr.3007. [DOI] [PubMed] [Google Scholar]
- Nyman J.S. Effect of diabetes on the fracture resistance of bone. Clin. Rev. Bone Miner. Metab. 2012 [Google Scholar]
- Nyman J.S., Even J.L., Jo C.-H., Herbert E.G., Murry M.R., Cockrell G.E., Wahl E.C., Bunn R.C., Lumpkin C.K., Fowlkes J.L., Thrailkill K.M. Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone. 2011;48:733–740. doi: 10.1016/j.bone.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Sirasanagandla S., Ranganath Pai Karkala S., Potu B.K., Bhat K.M.R. Beneficial effect of Cissus quadrangularis Linn. on osteopenia associated with streptozotocin-induced type 1 diabetes mellitus in male Wistar rats. Adv. Pharmacol. Sci. 2014;2014 doi: 10.1155/2014/483051. (483051–10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues B., Cam M.C., Kong J., Goyal R.K., McNeill J.H. Strain differences in susceptibility to streptozotocin-induced diabetes: effects on hypertriglyceridemia and cardiomyopathy. Cardiovasc. Res. 1997;34:199–205. doi: 10.1016/s0008-6363(97)00045-x. [DOI] [PubMed] [Google Scholar]
- Rybaczek T., Tangl S., Dobsak T., Gruber R., Kuchler U. The effect of parathyroid hormone on osseointegration in insulin-treated diabetic rats. Implant. Dent. 2015;24:392–396. doi: 10.1097/ID.0000000000000288. [DOI] [PubMed] [Google Scholar]
- Shimizu R., Sakazaki F., Okuno T., Nakamuro K., Ueno H. Difference in glucose intolerance between C57BL/6J and ICR strain mice with streptozotocin/nicotinamide-induced diabetes. Biomed. Res. 2012;33:63–66. doi: 10.2220/biomedres.33.63. [DOI] [PubMed] [Google Scholar]
- Silva M.J., Brodt M.D., Lynch M.A., McKenzie J.A., Tanouye K.M., Nyman J.S., Wang X. Vol. 24. 2009. Type 1 Diabetes in Young Rats Leads to Progressive Trabecular Bone Loss, Cessation of Cortical Bone Growth, and Diminished Whole Bone Strength and Fatigue Life; pp. 1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrailkill K., Bunn R.C., Lumpkin C., Wahl E., Cockrell G., Morris L., Kahn C.R., Fowlkes J., Nyman J.S. Loss of insulin receptor in osteoprogenitor cells impairs structural strength of bone. J. Diabetes Res. 2014;2014:703589. doi: 10.1155/2014/703589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrailkill K.M., Clay Bunn R., Nyman J.S., Rettiganti M.R., Cockrell G.E., Wahl E.C., Uppuganti S., Lumpkin C.K., Fowlkes J.L. SGLT2 inhibitor therapy improves blood glucose but does not prevent diabetic bone disease in diabetic DBA/2J male mice. Bone. 2016;82:101–107. doi: 10.1016/j.bone.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppuganti S., Granke M., Makowski A.J., Does M.D., Nyman J.S. Age-related changes in the fracture resistance of male Fischer F344 rat bone. Bone. 2016;83:220–232. doi: 10.1016/j.bone.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Sobrevilla J., Boone-Villa V.D., Aguilar C.N., Román-Ramos R., Vega-Avila E., Campos-Sepúlveda E., Alarcón-Aguilar F. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc. West. Pharmacol. Soc. 2011;54:5–9. [PubMed] [Google Scholar]
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos. Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- Weber D.R., Schwartz G. Epidemiology of skeletal health in type 1 diabetes. Curr. Osteoporos. Rep. 2016:1–10. doi: 10.1007/s11914-016-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Fei Y., Zhang M., Wei D., Li M., Ding W., Yang J. Reversal of osteoporotic changes of mineral composition in femurs of diabetic rats by insulin. Biol. Trace Elem. Res. 2008;121:233–242. doi: 10.1007/s12011-007-8043-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Diao T.-Y., Wang L., Che C.-T., Wong M.-S. Protective effects of water fraction of Fructus Ligustri Lucidi extract against hypercalciuria and trabecular bone deterioration in experimentally type 1 diabetic mice. J. Ethnopharmacol. 2014;158(Pt A):239–245. doi: 10.1016/j.jep.2014.10.025. [DOI] [PubMed] [Google Scholar]