Atherosclerosis is determined by both systemic risk-factors and local vascular mechanisms. The arterial remodeling in response to plaque development plays a key role in atherosclerosis. Compensatory expansive remodeling is an adaptive mechanism which maintains lumen patency as a plaque develops. In contrast, excessive expansive remodeling, signifying an enlargement in vascular and lumen volume as a result of local plaque build-up, is a consistent attribute of high-risk plaques. Local hemodynamic factors, in particular low endothelial shear stress (ESS), is an intensely pro-inflammatory and pro-atherogenic stimulus and largely accounts for the spatially diverse distribution of atherosclerotic plaques. However, plaque, remodeling and ESS have hitherto been investigated only in the cross-sectional arterial axis and their distribution in the longitudinal axis of individual plaques has not been characterized.
We performed a detailed and comprehensive description of the longitudinal spatial heterogeneity of plaque, arterial remodeling and ESS in human coronary plaques. Patients enrolled in the PREDICTION (Prediction of Progression of Coronary Artery Disease and Clinical Outcome Using Vascular Profiling of Shear Stress and Wall Morphology) study (n=219) underwent coronary angiography and intravascular ultrasound for 3-dimensional reconstruction and blood flow simulation in all major coronary arteries at the time of an acute coronary syndrome (1). A total of 371 plaques (maximum thickness ≥0.5mm, mean length 16.62±0.35 mm [range 9 to 30 mm]) were identified in 313 arteries and categorized in 3-mm subsegments. Plaque length was highly variable: the most frequently encountered plaque length was 9 mm (28.03%), but the majority of plaques were longer, ranging from 12 mm to 30 mm in various proportions.
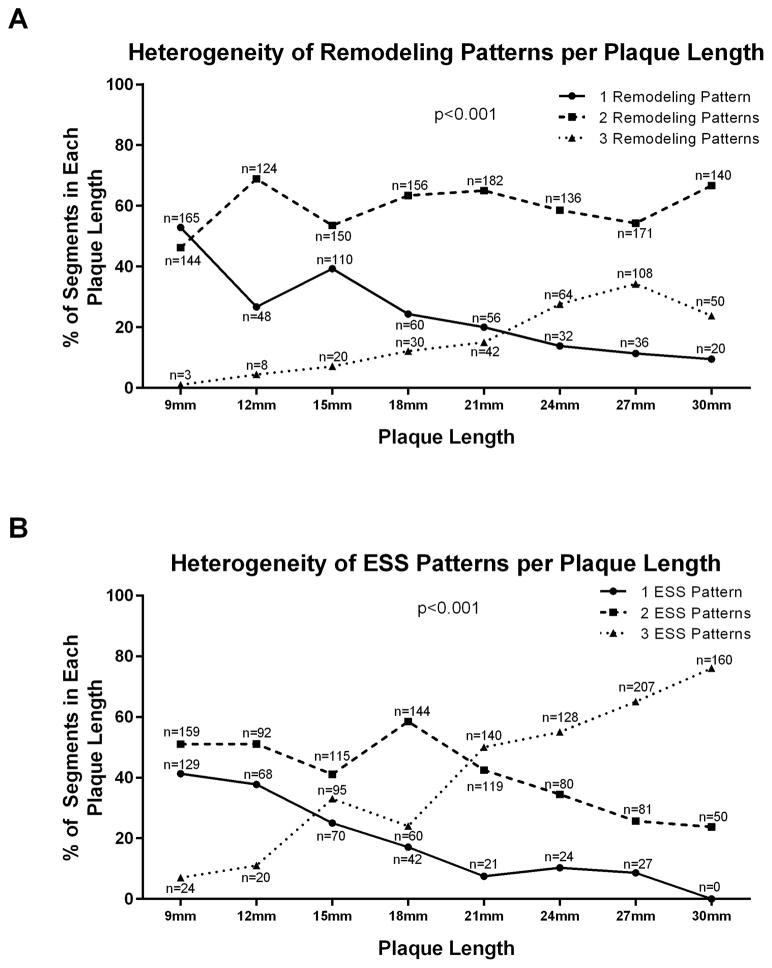
Compensatory remodeling was the most frequent remodeling type (1622 of 2055 subsegments, 78.9%). Excessive expansive remodeling occurred in 205 subsegments (10.0%) and constrictive remodeling in 228 subsegments (11.1%). A single remodeling pattern was seen in 117 plaques (31.5%) while 211 (56.9%) manifested two distinct remodeling patterns in different locations and 43 (11.6%) demonstrated all three remodeling patterns along the plaque length (p<0.001). The frequency of uniform remodeling progressively decreased as the plaques became longer, while a mixed remodeling profile with coexistence of all three remodeling patterns was more frequent as the plaque length increased (Figure 1A).
Figure 1. Heterogeneity of remodeling and ESS patterns per plaque length.
A. Heterogeneity of remodeling patterns per plaque length. B. Heterogeneity of ESS patterns per plaque length.
The distribution of ESS was also highly variable along each plaque. Only 90 plaques (24.3%) exhibited a uniform ESS profile, while 164 (44.2%) manifested two distinct ESS patterns in different locations within the plaque and 117 (31.5%) demonstrated low, moderate and high ESS patterns simultaneously along the plaque length. The frequency of uniform ESS progressively decreased, and the presence of mixed ESS profiles was more frequent, as the lesions became longer (Figure 1B).
Our analysis underscores the longitudinal variation of plaque morphology, remodeling and ESS which are evident in the majority of plaques, especially longer plaques. Intravascular ultrasound studies commonly focus on the single site of minimum luminal dimension along a lesion (2). This strategy inevitably underestimates the longitudinal variability of plaque constituents, vascular morphologies and local flow conditions which determine the natural history of each plaque. Longitudinal variability may account for the challenges encountered by clinical studies attempting to correlate plaque features with subsequent clinical events (3,4) because most plaques exhibit a greater degree of heterogeneity along their length and, therefore, at every single point in time, parts of the same plaque may exhibit different stages and trajectories of progression. This divergent evolution profile along a plaque does not allow an accurate risk-stratification based on the characteristics of a single specific location. The presence and potential implications of plaque morphology and remodeling heterogeneity have been previously noted (5) but that earlier study did not assess local ESS patterns along the length of each plaque. The present analysis, by incorporating hemodynamic and vascular assessments in a large population, may set the paradigm for a more accurate characterization of human atherosclerosis. The detailed assessment of arterial remodeling and ESS along the longitudinal aspect of lesions may enhance the early identification of plaques at highest risk for adverse outcomes.
Acknowledgments
We thank Michelle Lucier, Gail MacCallum, Nicholas Cefalo and Emily Bomba-Rienzo for technical evaluation of intravascular utrasound/angiographic images.
Abbreviations list
- ESS
endothelial shear stress
Footnotes
Funding Sources and Disclosures: This investigator-initiated study was supported by Boston Scientific Corp. We acknowledge the support of the George D. Behrakis Cardiovascular Research Program, the Hellenic Cardiological Society and the Schaubert Family. No conflicts of interest.
References
- 1.Stone PH, Saito S, Takahashi S, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172–81. doi: 10.1161/CIRCULATIONAHA.112.096438. [DOI] [PubMed] [Google Scholar]
- 2.Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound assessment of patterns of arterial remodeling in the absence of significant reference segment plaque burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:806–10. doi: 10.1016/s0735-1097(03)00842-8. [DOI] [PubMed] [Google Scholar]
- 3.Sanidas EA, Mintz GS, Maehara A, et al. Adverse cardiovascular events arising from atherosclerotic lesions with and without angiographic disease progression. JACC Cardiovasc Imaging. 2012;5:S95–S105. doi: 10.1016/j.jcmg.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 5.Box LC, Angiolillo DJ, Suzuki N, et al. Heterogeneity of atherosclerotic plaque characteristics in human coronary artery disease: a three-dimensional intravascular ultrasound study. Catheter Cardiovasc Interv. 2007;70:349–56. doi: 10.1002/ccd.21088. [DOI] [PubMed] [Google Scholar]