Abstract
Background
Although intensity-modulated radiotherapy (IMRT) is a standard of care for many head and neck cancers, its use for carotid-sparing (CS) therapy in early-stage laryngeal carcinoma is controversial.
Methods
330 consecutive patients with early-stage laryngeal carcinoma were treated from 1/1989 to 5/2011, including 282 CRT and 48 CS-IMRT patients. The median follow-up was 43 (CS-IMRT) and 66 (CRT) months.
Results
There was no difference in local failure rates comparing patients undergoing CS-IMRT with CRT, with 3-year local control rates of 88% vs. 89%, respectively (p=0.938). Using a 1cm circumferential margin, the average dose to the left and right carotid arteries was 48.3 and 47.9 Gy, respectively. 88% of locoregional recurrences involved the ipsilateral true vocal cord, including all local recurrences in the IMRT group.
Conclusions
These results warrant further prospective evaluation of CS-IMRT for early-stage glottic larynx cancer.
Keywords: Early stage laryngeal carcinoma, glottic cancer, IMRT, carotid sparing
INTRODUCTION
Conventional radiotherapy (CRT) delivered with opposed-lateral beams is a mainstay of therapy for early-stage (T1-2N0) glottic carcinoma and achieves excellent rates of larynx preservation and cancer eradication. Long-term local control is achieved in approximately 90% and 75% of patients with T1 and T2 tumors, respectively, and cancer-specific survival rates over 95% have been reported.1-4 Given these results and that nearly all patients die of causes unrelated to their laryngeal cancer, further improvement in cancer outcome via technological innovation is unlikely. Rather, the greatest opportunity for advancing therapy is preventing long-term toxicity while maintaining excellent oncologic control, similar to other malignancies with long-term survival.5-7
Although early-stage glottic larynx cancers are treated with relatively small radiation fields, longitudinal segments of both common carotid arteries receive radiation doses essentially equivalent to the prescription. Studies have shown increased risk of carotid artery stenosis and stroke for patients receiving radiation for head and neck cancer.8-13 Additionally, a recent Surveillance, Epidemiology and End Results (SEER) database study reported that T1N0 glottic larynx cancer patients treated with external beam radiation were significantly more likely to experience a fatal cerebrovascular accident than those treated with surgery.14 As tobacco use is the primary risk factor for developing laryngeal cancer, this population already has increased risk of cerebrovascular morbidity and mortality, and thus techniques minimizing additional cerebrovascular risk are appealing.
Given this background, several institutions have attempted to reduce radiation dose to the carotid arteries with intensity-modulated radiotherapy (IMRT).14-16 IMRT is capable of delivering highly conformal dose distributions with sharp dose fall-offs, thereby sparing surrounding normal tissue. Thus, IMRT may be able to decrease the dose to the carotid arteries while preserving the excellent oncologic outcomes of CRT. Indeed, several dosimetric studies have shown significantly decreased carotid artery radiation with IMRT compared with CRT for early-stage laryngeal cancer.14-16
Nevertheless, the role of IMRT in early-stage larynx cancer is controversial. Increased conformality also increases the risk of marginal misses through contouring errors and organ motion. Further, there have been anecdotal reports of radiation failures from patients undergoing IMRT.17,18 To investigate these concerns, we assembled a cohort of patients treated with both IMRT and CRT to determine if there was any difference in local control, salvage laryngectomy rates, or survival with either approach.
MATERIAL AND METHODS
Patients
From January 1989 to May 2011, all patients with clinical stage T1 or T2 squamous cell carcinoma of the glottic larynx and clinically negative cervical lymph nodes who underwent definitive radiation at our center were identified from an institutional database. Institutional review board approval was granted prior to data collection.
Staging
All patients underwent evaluation with history and physical exam, including indirect or flexible fiberoptic laryngoscopy. All suspicious lesions were biopsied, and pathology slides were reviewed by an expert in head and neck pathology at our institution. Routine staging computed tomography (CT) of the neck was not performed prior to simulation unless there were indeterminate cervical lymph nodes on clinical exam or extra-glottic spread was suspected.
Radiation techniques
All patients were treated to the larynx alone without irradiation of elective lymph node volumes. CRT consisted of opposed lateral fields typically spanning 5×5 cm. Borders were the bottom of the hyoid bone superiorly, the bottom of the cricoid inferiorly, the anterior edge of the vertebral bodies posteriorly, and 1 cm of flash anteriorly. For T2 tumors, the field borders for CRT could be expanded to a 6 × 6cm box, inferiorly extending to include the first tracheal ring, and ensuring that the field borders extended at least 2cm above and below gross disease. Simulation for CRT was either fluoroscopy or CT based. All IMRT patients underwent CT-based simulation. A GTV based on clinical exam was contoured to ensure coverage. The clinical target volume was defined as the entire larynx, including the anterior and posterior commissures, and arytenoids, from the top of the thyroid cartilage to the bottom of the cricoid. The planning target volume (PTV) consisted of a 1.0 cm circumferential expansion of the clinical target volume. The PTV extended to the top of thyroid cartilage superiorly and the bottom of the cricoid inferiorly. Generally, four 6 MV anterior coplanar photon beams were used. The carotid arteries were contoured on slices containing the PTV. Prescriptions were normalized so that ≥95% of the PTV received 100% of the prescription dose (i.e., PTV D95% = 100%). Additional dose constraints included limiting the maximum dose in the PTV to 105% of the prescription and a soft constraint limiting the mean carotid artery dose to 52 Gy. Coverage was prioritized over carotid artery dose. For both CRT and IMRT, patients with anterior commissure involvement were generally treated with daily bolus as well as anterior flash of the radiation field to ensure coverage of this region.
Follow-up
In general, patients were followed every 3 months for the first 2 years for history, physical exam, and indirect or flexible laryngoscopy, then every 4 months for years 3 and 4, every 6 months for year 5, and then annually. Most patients alternated visits between a radiation oncologist and a head and neck surgeon at our institution. For locoregional patterns of failure, site of first failure was based on clinical exam, laryngoscopy, and pathology report.
Statistical methods
Follow-up was calculated from the start of radiotherapy. All local failures were biopsy-proven disease occurring in the supraglottic, glottic, subglottic larynx, and/or adjacent soft tissue post-radiation. Actuarial estimated survival probabilities were calculated utilizing the standard Kaplan-Meier method, and p values were determined using a log-rank test. Hazard ratios (HRs) and 95% confidence intervals were calculated using a Cox proportional hazards model. All statistics were performed using SPSS version 21 and R version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline clinical characteristics are shown in Table 1. Forty eight patients were treated with IMRT and 282 received conventional external beam radiotherapy. The median radiation dose was 63 Gy and 66 Gy for IMRT and CRT, respectively. Overall, 125 patients (38%) were treated with fraction sizes of 2.25 Gy, all 48 undergoing IMRT and 75 (27%) undergoing CRT (p<0.001). There were slightly more T2 tumors in the IMRT group than the CRT group, but this did not reach statistical significance (27% vs 20%, p = 0.462). Median follow-up was 44 months and 66 months for surviving IMRT and CRT patients, respectively.
TABLE 1.
Clinical characteristics.
| IMRT | % | Conventional | % | Total | ||
|---|---|---|---|---|---|---|
| Number of patients | 48 | 282 | 330 | |||
| Median follow-up surviving (months) | 44 | 66 | 59 | |||
| Time Period | P < .001 | |||||
| 1989-1995 | 0 | 0% | 59 | 20.9% | 59 | |
| 1996-2005 | 0 | 0% | 169 | 59.9% | 169 | |
| 2006-2011 | 48 | 100% | 54 | 19.1% | 102 | |
| Age | P = .237 | |||||
| Median (years) | 65 | 68 | 68 | |||
| ≤65 | 25 | 52.1% | 121 | 42.9% | 146 | |
| >65 | 23 | 47.9% | 161 | 57.1% | 184 | |
| Gender | P = .028 | |||||
| Male | 37 | 77.1% | 250 | 88.7% | 287 | |
| Female | 11 | 22.9% | 32 | 11.3% | 43 | |
| T-stage | P = .462 | |||||
| Tis | 3 | 6.3% | 13 | 4.6% | 16 | |
| T1 | 32 | 66.7% | 212 | 75.2% | 244 | |
| T2 | 13 | 27.1% | 57 | 20.2% | 70 | |
| T2 Subcategories | ||||||
| Supraglottic extension | 6 | 12.5% | 14 | 5.0% | 20 | |
| Subglottic extension Impaired Cord | 4 | 8.3% | 31 | 11.0% | 35 | |
| Mobility | 3 | 6.3% | 12 | 4.3% | 15 | |
| Radiation dose | P < .001 | |||||
| Median dose (Gy) | 63 | 66 | 66 | |||
| 65.25 Gy or less | 47 | 97.9% | 85 | 30.1% | 132 | |
| 66 Gy or more | 1 | 2.1% | 197 | 69.9% | 198 | |
| Fraction size | P < .001 | |||||
| 200 cGy or less | 0 | 0.0% | 206 | 73.0% | 206 | |
| 225 cGy | 48 | 100.0% | 77 | 27.3% | 125 | |
| Smoking | P = .603 | |||||
| Yes | 43 | 89.6% | 245 | 86.9% | 288 | |
| No | 5 | 10.4% | 37 | 13.1% | 42 |
Abbreviation: Gy, Gray; IMRT, intensity-modulated radiotherapy.
Dosimetric characteristics of our patients undergoing IMRT are summarized in Table 2. The median PTV maximum dose was 105%. The mean of the average doses to the left and right carotid artery segments in the region of the PTV were 48.3 and 47.7 Gy, respectively. As shown, the magnitude of carotid sparing varied widely amongst the cohort, with some patients achieving mean carotid doses as low as 20.5 Gy, and others receiving mean carotid doses closer to the prescription. There was a high degree of correlation between the mean doses received to by left and right carotid arteries (correlation coefficient R2 = 0.907)
Table 2.
Dosimetric parameters for the 48 patients undergoing carotid sparing intensity modulated radiotherapy.
| PTV Dmax (Relative) | |
|
| |
| Median (range) | 105% (103%-111%) |
| Mean | 105% |
|
| |
| Left Carotid Dose | |
|
| |
| Median Dmean (range) | 49.9 Gy (21.7-60.5 Gy) |
| Mean | 48.3 Gy |
| Median V40Gy (range) | 78.4% (46.7%-96.6%) |
| 3.4cc (1.4-7.8cc) | |
| Median V50Gy (range) | 64.1% (21.9-93.4%) |
| 2.8cc (1.0-6.7cc) | |
|
| |
| Right Carotid Dose | |
|
| |
| Median Dmean (range) | 47.9 Gy (20.5-61.5 Gy) |
| Mean | 47.7 Gy |
| Median V40Gy (range) | 75.4% (30.9%-96.3%) |
| 3.9cc (1.4-8.7cc) | |
| Median V50Gy (range) | 60% (10.7%-93.7%) |
| 2.9cc (1.1-6.3cc) | |
Abbreviations: cc, cubic centimeters; DMax, maximum dose; DMean, average dose; PTV, planning target volume; V40Gy/V50Gy, the volume of the carotid artery receiving at least 40Gy/50Gy.
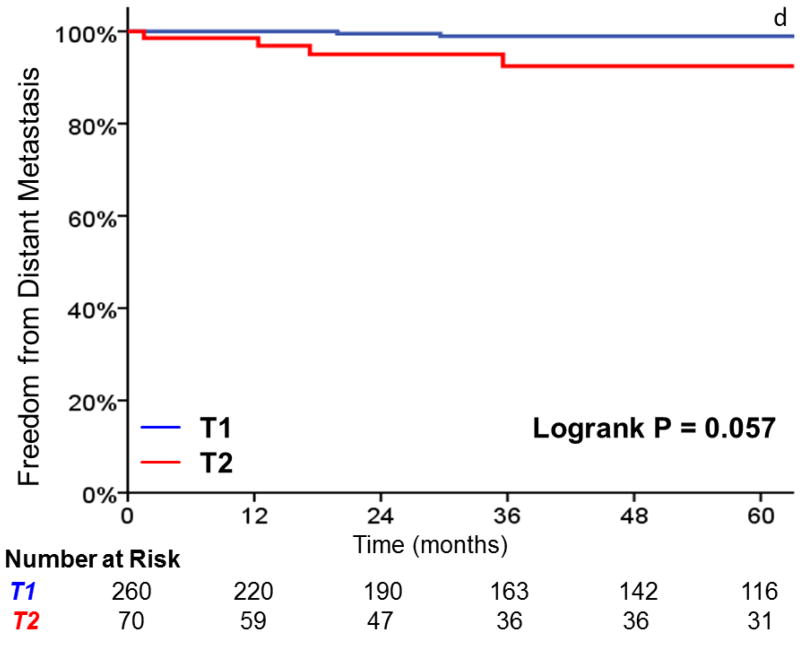
Three-year local control was 91% for T1 tumors, vs 80% for T2 tumors (HR, 2.38; 95%CI, 1.26 to 4.49; p=0.008) (Figure 1). Additionally, laryngectomy-free survival was significantly better in T1 vs T2 tumors (HR, 1.81; 95%CI, 1.16 to 2.83; p = .009). Three-year laryngectomy-free survival was 87% and 74% for T1 and T2 tumors, respectively. Although there was a trend towards increased rates of distant metastasis (1% vs. 8% at 3 years, p = .057) with T2 tumors, this did not reach statistical significance.
FIGURE 1.
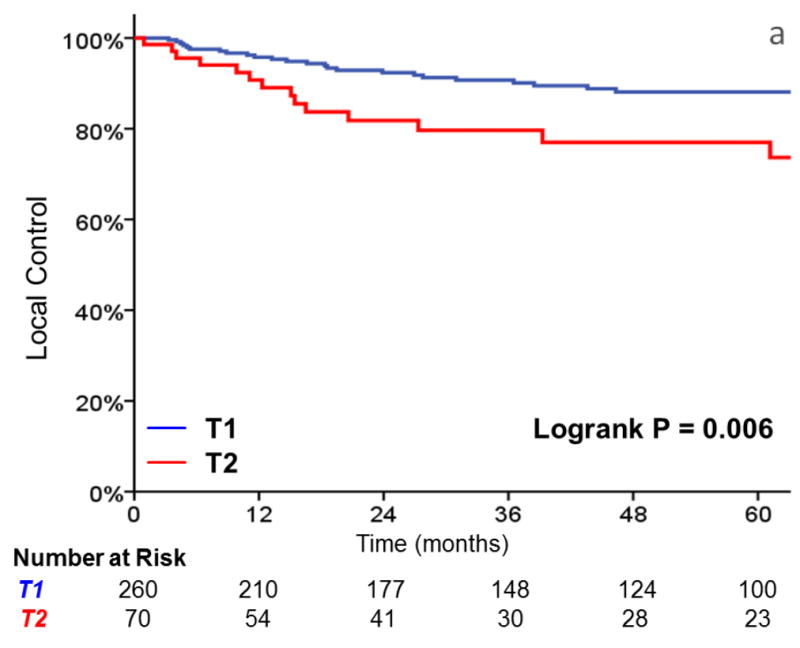
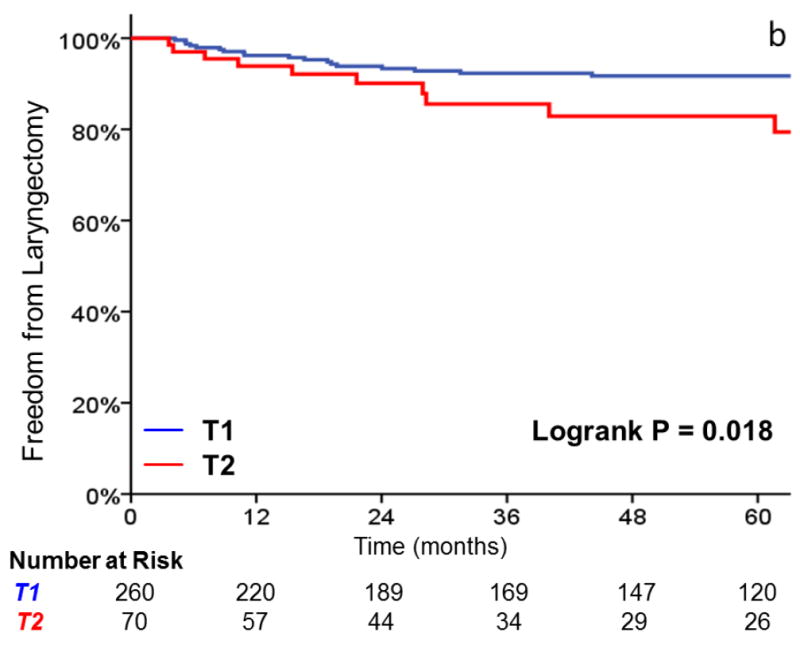
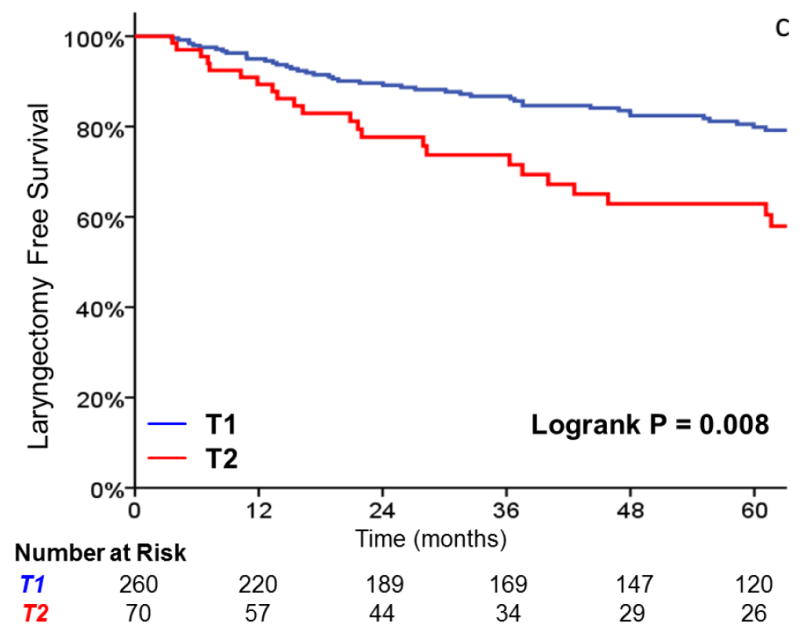
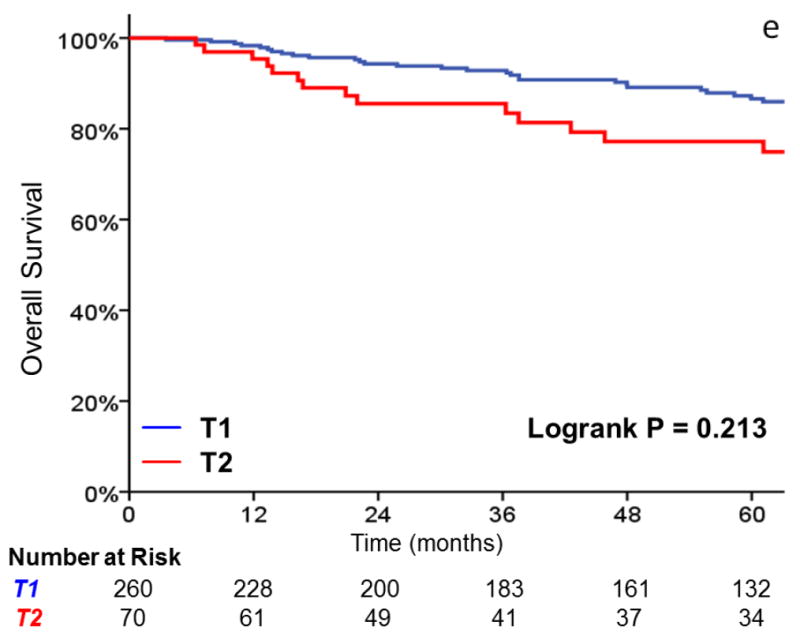
A comparison of A) local control, B) larynx preservation rates, C) laryngectomy-free survival, D) distant metastasis, and E) overall survival in patients with T1 vs T2 glottic carcinoma undergoing definitive radiotherapy.
As shown in Table 3, there was no significant difference in local failure rates comparing IMRT with CRT (HR, 0.96; 95%CI, 0.38 to 2.147; p = .938). The 3-year actuarial local control rate was 88% for IMRT and 89% for CRT (Figure 2). After adjusting for tumor stage, there was still no difference in local failure (HR, 0.90; 95%CI, 0.35 to 2.30; p = .821). Laryngectomy-free survival (HR, 1.39; 95%CI, 0.73 to 2.65; p = .318), distant metastasis (HR, 2.47; 95%CI, 0.48 to 12.65; p = .247), and overall survival (HR, 1.85; 95% CI, 0.89 to 3.86; p = .10) were not significantly different. Patients with T2 tumors (p = .008) and non-smokers (p = .027) had decreased local control in univariate analysis. Increasing age and tumor stage predicted for inferior laryngectomy-free survival.
TABLE 3.
Cox proportional hazards univariate analysis of local control, laryngectomy-free survival (LFS), distant metastasis, and overall survival in T1-2N0 glottic carcinoma.
| Univariate Analysis | Local Control | LFS | Distant Metastasis | Overall Survival | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| IMRT vs Conventional | 0.96 (0.38-2.47) | 0.938 | 1.39 (0.73-2.65) | 0.318 | 2.47 (0.48-12.65) | 0.278 | 1.85 (0.89-3.86) | .100 |
| T2 vs. T1 stage | 2.38 (1.26-4.49) | 0.008 | 1.81 (1.16-2.83) | 0.009 | 3.57 (0.88-14.40) | 0.076 | 1.37 (0.83-2.25) | .215 |
| Age | 1.00 (0.97-1.02) | 0.874 | 1.04 (1.01-1.05) | <0.001 | 1.07 (0.99-1.14) | 0.068 | 1.07 (1.04-1.09) | <.001 |
| Smoking (yes vs no) | 0.45 (0.22-0.91) | 0.027 | 1.19 (0.64-2.22) | 0.583 | 0.48 (0.10-2.37) | 0.365 | 1.80 (0.83-3.92) | .138 |
| Gender (Male vs female) | 1.18 (0.46-3.01) | 0.729 | 1.14 (0.65-2.00) | 0.649 | 0.51 (0.10-2.54) | 0.409 | 1.01 (0.55-1.86) | .977 |
| Fractionation (2.25 vs 2 Gy) | 1.03 (0.54-1.96) | 0.925 | 1.077 (0.684-1.696) | 0.748 | 1.32 (0.30-5.77) | 0.710 | 1.31 (0.78-2.22) | .312 |
| Total dose | 1.00 (0.999-1.001) | 0.796 | 1.00 (1.000-1.001) | 0.690 | 1.00 (0.998-1.004) | 0.377 | 1.00 (0.999-1.001) | .836 |
| Year of treatment | 1.00 (0.95-1.06) | 0.973 | 1.01 (0.97-1.05) | 0.720 | 1.08 (0.93-1.26) | 0.291 | 1.01 (0.97-1.06) | .605 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
FIGURE 2.
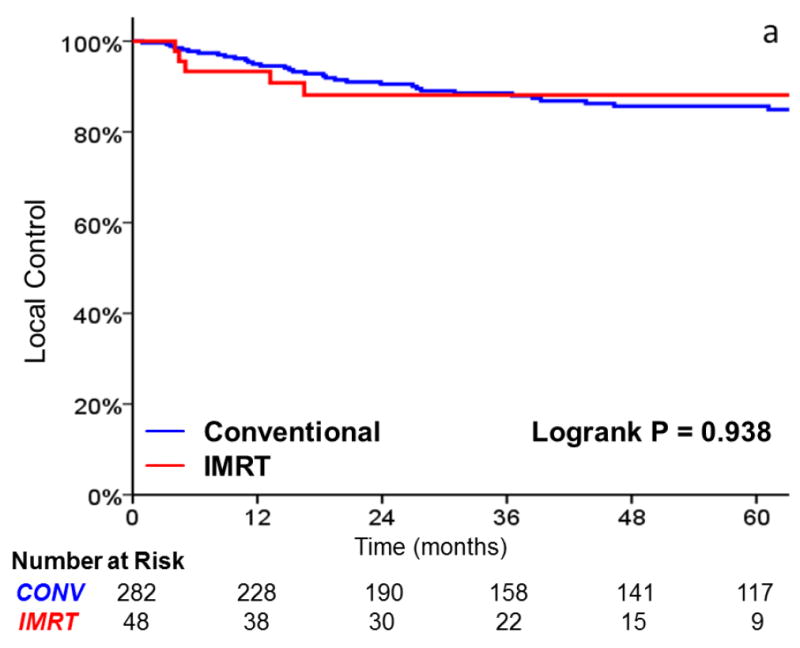
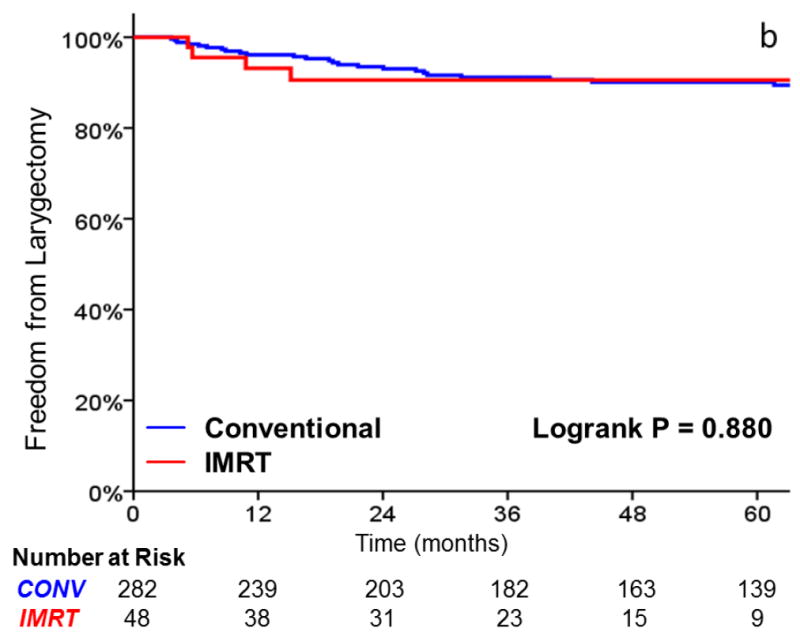
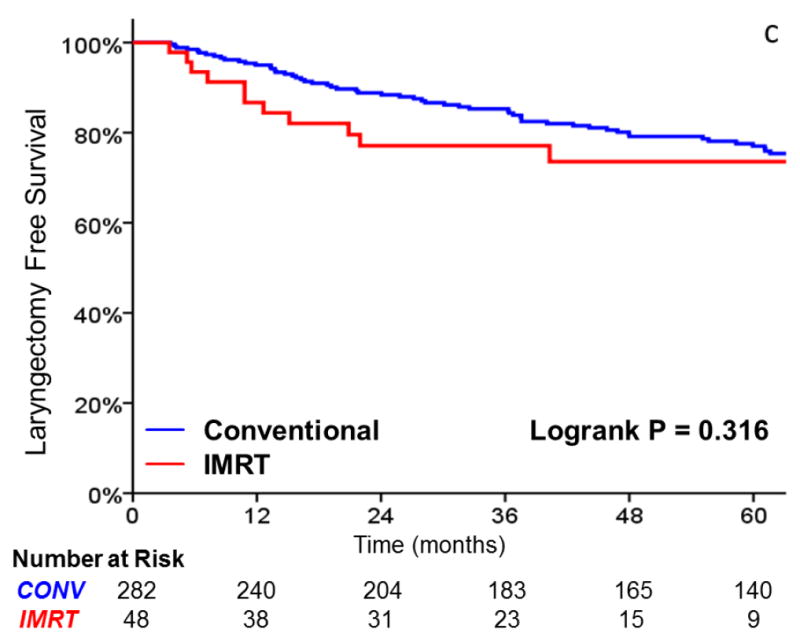
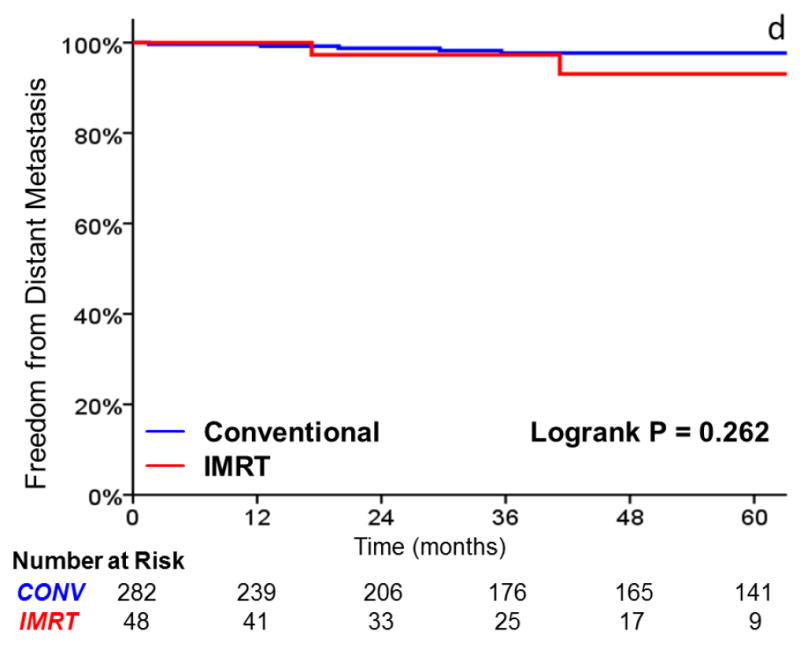
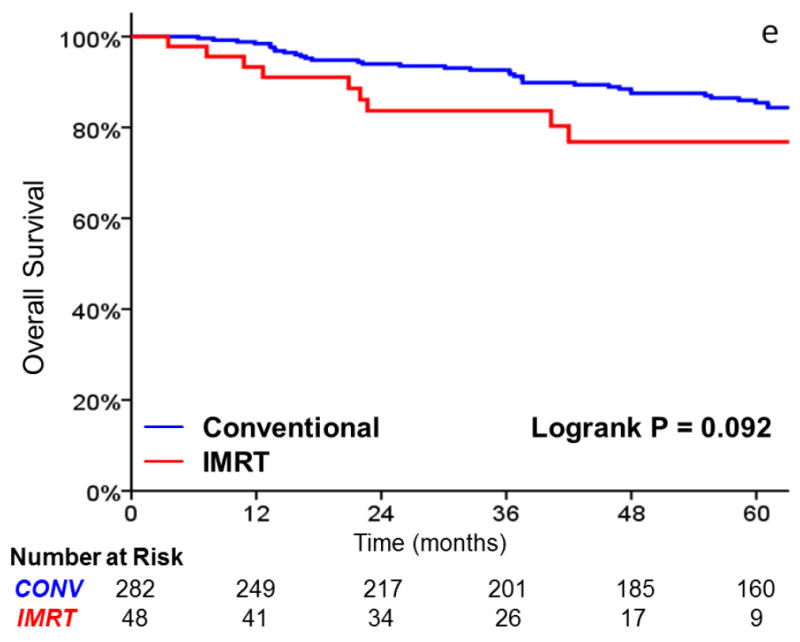
A comparison of A) local control, B) larynx preservation rates, C) laryngectomy-free survival, D) distant metastasis, and E) overall survival in patients undergoing intensity-modulated radiotherapy (IMRT) and conventional radiotherapy
Patterns of locoregional failure are summarized in Table 4. In 41 locoregional recurrences, 36 (88%) involved the ipsilateral true vocal cord, including all 5 in the IMRT group. Among these patients, 18 (44%) had recurrences with subglottic or supraglottic extension and 2 (5%) had simultaneous cervical lymph node recurrence. No patient had contralateral true vocal cord recurrence without ipsilateral true vocal cord recurrence. Of the 5 patients not recurring in the glottic larynx, one recurred in the supraglottic larynx alone, one failed in the hypopharynx and cervical lymph nodes, and three recurred in the cervical lymph nodes alone. Twenty-one patients had salvage total laryngectomy, 13 had organ-preserving salvage surgery, one had laser ablation, one had salvage radiation to a level 6 lymph node recurrence, and 5 did not receive salvage local therapy.
TABLE 4.
Patterns of locoregional failures following definitive radiotherapy for early-stage glottic carcinoma.
| IMRT | Conventional | |
|---|---|---|
| Patients with LRR | 5 | 36 |
| Site of first failure | ||
| True vocal cord (isolated) | 5 (1) | 31 (17) |
| Ipsilateral cord only | 5 | 17 |
| Bilateral cords | 0 | 14 |
| Contralateral cord only | 0 | 0 |
| Supraglottic extension (isolated) | 3 (0) | 10 (1) |
| Subglottic extension (isolated) | 4 (0) | 10 (0) |
| Hypopharynx | 0 | 1 |
| Cervical lymph node (isolated) | 2 (0) | 4 (3) |
Abbreviation: IMRT, intensity-modulated radiotherapy; LRR, locoregional recurrence.
DISCUSSION
We have previously demonstrated that IMRT is feasible and results in excellent locoregional control for locally advanced laryngeal cancer.19 Here we further demonstrate that IMRT for early glottic cancer results in local control comparable to CRT. Overall, we achieved local control in 91% of T1 tumors and 80% of T2 tumors at 3 years, which is comparable to previous reports.1-4 Thus, the similar local control outcomes for IMRT and CRT cannot be attributed to unexpectedly poor control in CRT patients. We found only a relatively mild decrease in carotid artery dose with this technique, likely attributable to the relatively generous 1cm circumferential PTV margin we used to the time period of this study and a conservative CTV that included the entire larynx. In contrast, other groups have proposed limiting the treatment volume only to the involved vocal cord to maximize carotid sparing.20 For example, Janssen and colleagues recently reported excellent local control rates in 39 patients with T1-2N0 glottic carcinoma treated with IMRT, including 21 patients that underwent ipsilateral vocal cord irradiation with bilateral carotid sparing resulting in a mean contralateral carotid dose of 20.2 Gy.21 Since the time period of this study, we have chosen to employ an intermediate approach, using daily image guidance and decreasing our circumferential margin to 0.5cm, with as little as 0.3cm margin allowable near the carotid arteries in order to achieve a more significant reduction in carotid dose. Notably, in this study we found that the benefit of carotid sparing IMRT varied significantly based on a given patient’s anatomy, with mean carotid artery doses ranging from 20.5 Gy to 61.5 Gy. Thus, the benefit of carotid sparing IMRT is likely to depend on patient anatomy.
Although radiation techniques for many anatomic subsites in head and neck cancer have radically evolved over the past 25 years, with IMRT nearly universally replacing older techniques due to its superior dosimetry and decreased long-term morbidity,22-25 radiation therapy for early-stage glottic cancer has remained essentially unchanged at most institutions. This stems from the fact that early-stage cancer of the glottic larynx, uniquely amongst squamous cell cancers in the head and neck, rarely involves the cervical lymph nodes. Thus, unlike other anatomic subsites, where radiation fields can stretch from the skull base to the clavicle, a relatively small field is used, usually consisting of a 5×5 or 6×6 cm box that excludes the major salivary glands, pharyngeal constrictors, and oral cavity. Thus, although this small field does treat normal structures not included in the target volume, the rationale for employing IMRT for early-stage glottic carcinoma may appear less compelling than for other head and neck sites.
Nevertheless, in a disease where nearly all patients will die of causes unrelated to their cancer, it is important to consider the impact of therapy on long-term morbidity. There is a growing body of literature suggesting that radiation for head and neck cancer induces radiation vasculopathy, thereby increasing the risk of carotid artery stenosis and cerebrovascular morbidity.26 These adverse effects of radiation on the vasculature can be seen within 1-2 years of treatment.8,9 Additionally, the effects on carotid artery stenosis appear to continue to progress with longer follow-up after radiotherapy.10
These early effects on carotid artery stenosis may have clinically relevant consequences. For example, a study from the Netherlands found that in 367 patients with head and neck cancer undergoing definitive radiotherapy prior to age 60, including 162 with T1-2N0 laryngeal cancer, the risk of stroke was 5.6 times higher than expected based on age- and sex-matched controls from the general population.11 A similar study from the University of Pennsylvania found a more than twofold increase in stroke among 413 patients undergoing definitive radiation compared with a population-based estimate derived from unirradiated patients from Stockholm.12 In a recent SEER database study exclusively of patients with T1N0 glottic larynx cancer radiation was associated with a 75% relative increase in risk of fatal stroke compared with surgery alone, although the absolute increase in risk was relatively small.13
Given this reported increased risk of cerebrovascular morbidity, multiple studies have convincingly demonstrated that IMRT is capable of significantly reducing radiation dose to the carotid arteries for patients with early stage glottic cancer.14-16 Although these studies suggested that IMRT was capable of reducing the carotid dose by approximately 75%, the magnitude of carotid sparing was significantly less in our study for several reasons. First of all, the University of Florida used a 3mm PTV margin,16 whereas the study from MD Anderson used no PTV margin at all.15 By contrast, we used a conservative 1cm PTV circumferential margin for the patients included in this study given that we lacked experience with this technique when we first implemented it and wanted to minimize the potential for marginal misses. Additionally, we did not use daily image guidance for the patients included in this study. However, now that our results have demonstrated equivalent local control with IMRT and CRT, we have placed a much stronger emphasis on further reducing the carotid artery dose. Currently, we use daily kV-imaging and a 5mm circumferential PTV expansion around our CTV, with as little as 3mm expansion of the CTV near the carotid arteries, allowing much lower carotid doses to be achieved. Further, it should also be noted that we chose to define the carotid artery structure at risk as only segments of the carotid artery on CT slices containing a PTV contour in order to most fairly compare the carotid dose delivered by our IMRT and oppose lateral beam plans. This is in contrast to the University of Florida study, which defined the carotid artery structure as extending 1cm above and below the PTV.16 Because this carotid artery definition will artificially lower the reported mean and median carotid doses, dosimetric results from this should not be directly compared to our reported doses.
It should be pointed out that there is no definitive data relating carotid-artery dose to cerebrovascular morbidity, and the impact of carotid-sparing IMRT on vasculopathy remains unproven. Nevertheless, extrapolating from a breast cancer study demonstrating a linear increase of 7.4% in the rate of major coronary artery events for each additional gray of mean heart radiation dose,7 it seems possible that a clinically significant decrease in carotid-associated adverse sequelae may potentially result from the magnitude of reduction in carotid-artery dose achievable with carotid sparing IMRT, particularly with tighter margins than those employed in our study. However, this is a hypothesis in need of evidence and investigation.
Nevertheless, IMRT for early-stage glottic cancer remains controversial.17,18 In addition to anecdotal reports of increased failure with IMRT, the major concerns are the potential for underdosing the tumor through target delineation errors, complications resulting from hot spots, increased cost, and increased treatment complexity leading to prolonged contouring, planning, and treatment time. Although marginal misses resulting from IMRT have been reported in head and neck cancer previously,27-29 there is no plausible reason to hypothesize that IMRT will result in increased marginal misses when delivering an equivalent radiation dose if a clinical target volume including the entire larynx is properly contoured, a reasonable PTV expansion is employed, and a bolus is used to ensure anterior coverage. In fact, several studies have previously shown that IMRT is capable of producing superior target coverage compared with two-dimensional treatment with a lower maximum dose (i.e., a hot spot),14,15 In our study, in addition to demonstrating comparable local control for patients undergoing IMRT and CRT, all patients with local recurrence following IMRT had involvement of the ipsilateral true vocal cord, suggesting that the failures occurred mostly within the field itself rather than at the field margins. Further, although there is a theoretical concern that intrafraction motion from mobile organs such as the larynx may be more greatly impacted by IMRT than CRT given the potential interaction of the dynamic multileaf collimator and the target motion, we did not note an increased rate of infield failures with IMRT compared to CRT. It was notable that two of the five local failures in the IMRT arm were in patients initially with anterior commissure involvement treated without bolus, and four of five local recurrences had subglottic extension. Because of this, it is our current practice to employ a 5 mm bolus for all early-stage glottic carcinoma IMRT patients and to carefully check the inferior extent of the field to ensure that it is at least 2cm below gross disease.
Although we believe that tumor control should be similar with carotid-sparing IMRT and CRT, there are some potential dosimetric trade-offs to consider. For example, IMRT planning can lead to a higher integral dose, and increased volume of tissue receiving low radiation doses. The effect of these dosimetric changes on long-term toxicity, especially secondary malignancies, is unclear and warrants careful investigation. However, other arguments against the use of IMRT, such as increased physician time for contouring, increased planning complexity, and increased treatment time, do not preclude its use. Unlike IMRT treatments for other sites in the head and neck, the amount of contouring needed for a glottic larynx IMRT plan is minimal, consisting of defining the larynx, bilateral carotid arteries, and spinal cord. Similarly, although there is some added treatment-planning complexity with IMRT vs CRT, at our institution we use a four-beam technique, and meeting dose-volume constraints is generally straightforward. Analogously, simplified IMRT with opposed tangents is used at our institution routinely for breast cancer given its superior dose homogeneity.30 Finally, treatment time is not significantly prolonged compared with conventional treatments, and in fact a study from the MD Anderson Cancer Center found that overall time in the treatment room decreased with IMRT for early-stage laryngeal cancer.15 This may partially be because therapists do not have to enter the treatment room to place wedges with IMRT.
This study has several weaknesses that warrant further discussion. This is a retrospective series, and thus imbalances in unmeasured confounding factors between the IMRT and CRT groups could have affected the results. For example, patients undergoing IMRT, representing a more contemporary cohort, were significantly more likely to receive treatment with 2.25 Gy fraction sizes, a regimen that to improved local control in the randomized trial compared with 2 Gy fractions.31 However, in our data set, fraction size did not predict for local control, and thus this imbalance likely did not impact our results. Furthermore, given the small number of local recurrences, we were not able to use multivariate models to adjust for possible confounding variables. However, given that T-stage was the most significant predictor for local control, we adjusted for T-stage and found essentially identical results. A further important limitation to our study is that, in comparison to the group treated with CRT, patients undergoing IMRT had relatively shorter follow-up. However, the majority of local failures in early-stage glottic carcinoma occur during the first 3 years after treatment, and thus it is unlikely that longer follow-up will reveal late local failures that alter the conclusions of this study. Additionally, our results should not be extrapolated to IMRT strategies that target only a single vocal cord or part of the larynx, such as the approach recently reported by the Princess Margaret Hospital that resulted in relatively high rates of local failure for patients with T2N0 laryngeal cancer.18 Finally, a major weakness of our study is a lack of long-term toxicity data, given the relatively short follow-up of our cohort and incomplete non-oncologic post-radiotherapy medical information in our institution’s medical record.
Despite these caveats, and given that IMRT is capable of decreasing radiation dose to the carotid arteries without compromising local control, we believe that a prospective comparison of CRT to IMRT is warranted. However, even if such a study were undertaken in the future, it would require large numbers of patients and many years of follow-up to adequately understand the impact of radiation technique on long-term cerebrovascular morbidity. Given this, prospective studies using surrogate outcomes, like carotid artery stenosis, would be valuable and provide more timely results. Towards this end, we are currently developing a single-arm IMRT protocol at our institution that will prospectively evaluate toxicity and obtain serial carotid ultrasounds following radiotherapy. Nevertheless, we acknowledge that there are many uncertainties regarding its costs and benefits not addressed by our study. Thus, although carotid-sparing IMRT is a safe, effective treatment in this disease and warrants further study, we do not believe that it should broadly replace conventional radiotherapy at this time.
Highlights.
IMRT produces similar outcomes to conventional radiotherapy in early stage glottic carcinoma.
All failures in the IMRT group involved the ipsilateral vocal cord.
Anterior bolus and careful subglottic coverage may reduce the local failure risk with IMRT.
Maximal carotid sparing will likely require tight margins and image guidance.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frata P, Cellai E, Magrini SM, et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int J Radiat Oncol Biol Phys. 2005;63(5):1387–1394. doi: 10.1016/j.ijrobp.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Cellai E, Frata P, Magrini SM, et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int J Radiat Oncol Biol Phys. 2005;63(5):1378–1386. doi: 10.1016/j.ijrobp.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Chera BS, Amdur RJ, Morris CG, Kirwan JM, Mendenhall WM. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(2):461–466. doi: 10.1016/j.ijrobp.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 4.Khan MK, Koyfman SA, Hunter GK, Reddy CA, Saxton JP. Definitive radiotherapy for early (T1-T2) glottic squamous cell carcinoma: a 20 year Cleveland Clinic experience. Radiat Oncol. 2012;7:193. doi: 10.1186/1748-717X-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol. 2011;29(14):1885–1892. doi: 10.1200/JCO.2010.32.8427. [DOI] [PubMed] [Google Scholar]
- 6.Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97(18):1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 7.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 8.Muzaffar K, Collins SL, Labropoulos N, Baker WH. A prospective study of the effects of irradiation on the carotid artery. Laryngoscope. 2000;110(11):1811–1814. doi: 10.1097/00005537-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chang YJ, Chang TC, Lee TH, Ryu SJ. Predictors of carotid artery stenosis after radiotherapy for head and neck cancers. J Vasc Surg. 2009;50(2):280–285. doi: 10.1016/j.jvs.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SW, Ting AC, Ho P, Wu LL. Accelerated progression of carotid stenosis in patients with previous external neck irradiation. J Vasc Surg. 2004;39(2):409–415. doi: 10.1016/j.jvs.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282–288. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 12.Haynes JC, Machtay M, Weber RS, Weinstein GS, Chalian AA, Rosenthal DI. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112(10):1883–1887. doi: 10.1097/00005537-200210000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Swisher-McClure S, Mitra N, Lin A, et al. Risk of fatal cerebrovascular accidents after external beam radiation therapy for early-stage glottic laryngeal cancer. Head Neck. 2014;36(5):611–6. doi: 10.1002/hed.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez D, Cahlon O, Mechalakos J, Lee N. An investigation of intensity-modulated radiation therapy versus conventional two-dimensional and 3D-conformal radiation therapy for early stage larynx cancer. Radiat Oncol. 2010;5:74. doi: 10.1186/1748-717X-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal DI, Fuller CD, Barker JL, Jr, et al. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1-2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):455–461. doi: 10.1016/j.ijrobp.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chera BS, Amdur RJ, Morris CG, Mendenhall WM. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77(5):1380–1385. doi: 10.1016/j.ijrobp.2009.07.1687. [DOI] [PubMed] [Google Scholar]
- 17.Feigenberg SJ, Lango M, Nicolaou N, Ridge JA. Intensity-modulated radiotherapy for early larynx cancer: is there a role? Int J Radiat Oncol Biol Phys. 2007;68(1):2–3. doi: 10.1016/j.ijrobp.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Tiong A, Huang S, O’Sullivan B, et al. Outcomes for T2N0M0 glottic squamous cell carcinoma treated with IMRT compared with conventional parallel opposed fields. Int J Radiat Oncol Biol Phys. 2011;81:S106–S107. [Google Scholar]
- 19.Lee NY, O’Meara W, Chan K, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2007;69(2):459–468. doi: 10.1016/j.ijrobp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Levendag PC, Teguh DN, Keskin-Cambay F, Al-Mamgani A, van Rooij P, Astreinidou E, Kwa SL, Heijmen B, Monserez DA, Osman SO. Single vocal cord irradiation: a competitive treatment strategy in early glottic cancer. Radiother Oncol. 2011 Dec;101(3):415–9. doi: 10.1016/j.radonc.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Janssen S, Glanzmann C, Huber G, Studer G. Risk-adapted partial larynx and/or carotid artery sparing modulated radiation therapy of glottic cancer. Radiat Oncol. 2014 Jun;139:136. doi: 10.1186/1748-717X-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 25.Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104(3):343–348. doi: 10.1016/j.radonc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42(9):2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 27.Damast S, Wolden S, Lee N. Marginal recurrences after selective targeting with intensity-modulated radiotherapy for oral tongue cancer. Head Neck. 2012 Jun;34(6):900–6. doi: 10.1002/hed.21677. [DOI] [PubMed] [Google Scholar]
- 28.Chen AM, Farwell DG, Luu Q, et al. Marginal misses after postoperative intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2011 Aug 1;80(5):1423–9. doi: 10.1016/j.ijrobp.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Chen AM, Farwell DG, Luu Q, et al. Misses and near-misses after postoperative radiation therapy for head and neck cancer: Comparison of IMRT and non-IMRT techniques in the CT-simulation era. Head Neck. 2010 Nov;32(11):1452–9. doi: 10.1002/hed.21343. [DOI] [PubMed] [Google Scholar]
- 30.Chui CS, Hong L, Hunt M, McCormick B. A simplified intensity modulated radiation therapy technique for the breast. Med Phys. 2002 Apr;29(4):522–9. doi: 10.1118/1.1460875. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]