Abstract
This letter describes the continued optimization of M5 NAM ML375 (VU0483253). While a valuable in vivo tool compound, ML375 has an excessively long elimination half-life in rat (t1/2 = 80 hours), which can be problematic in certain rodent addiction paradigms (e.g., reinstatement). Thus, we required an M5 NAM of comparable potency to ML375, but with a rat t1/2 of less than 4 hours. Steep SAR plagued this chemotype, and here we detail aniline replacements that offered some improvements over ML375, but failed to advance. Ultimately, incorporation of a single methyl group to the 9b-phenyl ring acted as a metabolic shunt, providing (S)-11 (VU6008667), an equipotent M5 NAM, with high CNS penetration, excellent selectivity versus M1–4 and the desired short half-life (t1/2 = 2.3 hours) in rat.
Keywords: M5, Muscarinic acetylcholine receptor, pharmacokinetics, CNS penetration, Structure-Activity Relationship (SAR)
Graphical Abstract
To create your abstract, type over the instructions in the template box below. Fonts or abstract dimensions should not be changed or altered.9.0_/0
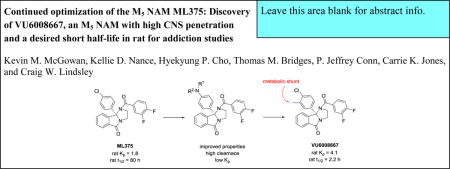
Recently, we reported on the discovery of highly selective muscarinic acetylcholine receptor subtype 5 (M5) inhibitors (both negative allosteric modulators (NAMs), represented by ML375 (VU0483253), 1, and VU6000181, 2)1,2 and an orthosteric antagonist, ML381 (VU0488130), 33 (Figure 1). Based on these chemotypes, we also performed a ligand-based virtual screening campaign, but no highly subtype selective M5 inhibitors were identified.4 Pharmacological recapitulation of the strong genetic data (from M5 knock-out mice) linking this receptor to a role in addiction5–7 with a selective small molecule inhibitor is of great interest. Excitingly, we have now achieved that validation in models of cocaine addiction with ML375.8 However, the excessively long elimination half-life of ML375 in rat (t1/2 = 80 hours)1 is problematic in the context of addiction studies involving reinstatement paradigms and washout periods. Both 2 and 3 possessed PK profiles not suitable as in vivo tool compounds.2,3 Ideally, we desired an M5 NAM with high CNS penetration and reasonable potency but with a short to moderate half-life in rat (t1/2 < 4 hours) to further assess this novel mechanism for the treatment of addiction. Yet, these series typify the worst caveats of allosteric modulator SAR – tremendously steep SAR.1–3,9–11 In this Letter, we detail the continued optimization of ML375 that ultimately afforded an M5 NAM with high CNS penetration and the desired short half-life in rat useful for evaluation in rat models of drug addiction.
Figure 1.
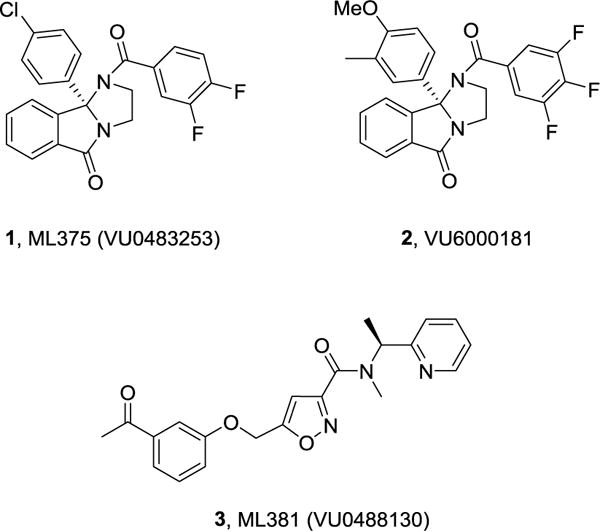
Structures of M5 NAMs ML375 (1, VU0483253), VU6000181 (2) and the highly selective orthosteric M5 antagonist ML381 (3, VU0488130).
As SAR to almost all portions of the ML375 scaffold resulted in largely inactive analogs (Figure 2), we directed our focus to further modifications to the 9b-phenyl moiety and explored installing aniline moieties to replace the chlorine atom. This was attractive for three reasons: 1) the ability to form a salt (and hopefully improve physiochemical and DMPK properties), 2) the ease of installation onto chiral 1 (which we had in bulk) and 3) the diversity of amines for the requisite Buchwald coupling. In the event (Scheme 1), analogs 4 were prepared in a single step, employing a microwave-assisted Buchwald coupling in moderate to good yields (45–92%). In short order, over 100 analogs 4 were synthesized, purified, and screened in our hM5 functional assay (intracellular calcium mobilization); however, and true to the steep SAR in observed with this chemotype,1,2 only about 10% of the analogs displayed M5 NAM activity (Table 1). While none of the analogs 4 proved to more potent than 1, several (4d, 4f and 4m) were comparable in terms of hM5 NAM activity (hM5 IC50s in the 1.3 to 1.8 μM range and rat M5 potency with 2-fold), but superior in terms of aqueous solubility as the respective HCl salts (cf 10 mg/mL versus <0.01 mg/mL for 1). However, only lipophilic amine moieties provided M5 NAMs in this subseries 4, which resulted in high clogPs (>4), high predicted hepatic clearance (rCLhep >65 mL/min/kg, hCLhep >18 mL/min/kg) based on microsomal CLint data, and low fraction unbound (rat and human fu,plasma <0.01). The series also displayed a robust in vitro/in vivo correlation (IVIVC) for clearance in rat, displaying CLps of >70 mL/min/kg and very short elimination half-lives (t1/2 <30 min). Within analogs 4, CNS distribution was variable in rat, affording brain:plasma partition coefficients (Kps) either greater than 1, or below the limit of quantitation (no detectable brain levels). Thus, while we were excited to identify tractable SAR for this M5 NAM chemotype and improvements in aqueous solubility, the uniformly poor disposition in rat precluded analogs 4 from further consideration as in vivo tools for addiction studies. Although, the improved solubility of congeners 4 should provide better tools for in vitro electrophysiology studies.
Figure 2.
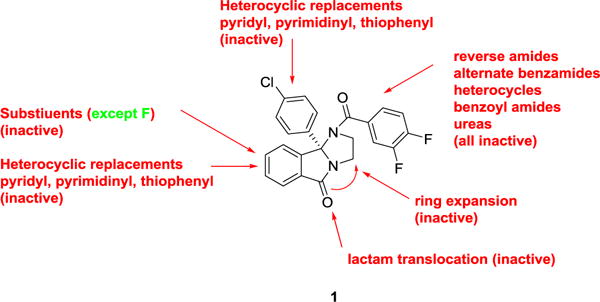
Summary of unproductive SAR with the M5 NAM ML375 (1, VU0483253). Only fluoro substituents retained activity, but led to higher clogPs and very poor physiochemical properties.
Scheme 1.
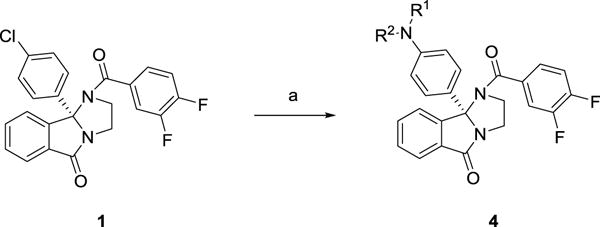
Synthesis of M5 NAM analogs 4.a
aReagents and conditions: (a) HNR1R2 (3.0 equiv.), 5 mol% Pd(OAc)2, 15 mol% SPhos, toluene (0.25 M), microwave, 20 min, 45–92%.
Table 1.
Structures and activities for hM5 NAM 1 and analogs 4.
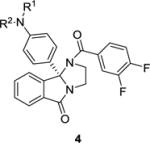
| |||
|---|---|---|---|
| Cpd | NR1R2 | hM5 IC50 (μM)a [% ACh Min ± SEM] |
hM5 pIC50 (±SEM)a |
| 1 ML375 | “Cl” | 1.11 [2.3 ± 0.3] |
5.98±0.04 |
| 4a |
|
6.04 [10.5±2.8] |
5.22±0.04 |
| 4b |
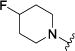
|
4.28 [13.6 ± 1.0] |
5.37 ± 0.04 |
| 4c |
|
6.36 [6.9 ± 1.7] |
5.20 ± 0.03 |
| 4d |
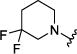
|
1.46 [3.1 ± 0.2] |
5.84 ± 0.04 |
| 4e |
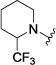
|
5.70 [2.6 ± 0.1] |
5.84 ± 0.04 |
| 4f |
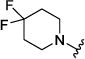
|
1.82 [3.1 ± 0.2] |
5.74 ± 0.04 |
| 4g |
|
3.42 [3.2 ± 0.2] |
5.47 ± 0.03 |
| 4h |
|
4.01 [3.6 ± 0.2] |
5.40 ± 0.4 |
| 4i |
|
7.29 [26.3 ± 6.2] |
5.14 ± 0.05 |
| 4k |
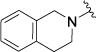
|
8.21 [20.1 ± 7.7] |
5.11 ± 0.10 |
| 4l |
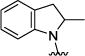
|
4.55 [27.0 ± 7.7] |
5.35 ± 0.05 |
| 4m |
|
1.32 [2.6 ± 0.9] |
5.89 ± 0.08 |
| 4n |
|
6.92 [25.5 ± 9.3] |
5.17 ± 0.08 |
| 4o |
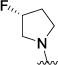
|
3.09 [3.3 ± 0.3] |
5.52 ± 0.05 |
| 4p |
|
>10 [26.2 ± 3.5] |
>5 |
| 4q |
|
3.50 [6.0 ± 0.3] |
5.46 ± 0.03 |
| 4r |
|
9.49 [7.5 ± 2.4] |
5.06 ± 0.14 |
Mean of three independent determinations performed in triplicate via an intracellular calcium mobilization assay with recombinant hM5 cells in the presence of an ACh EC80.
At this point, and with over 600 analogs synthesized and assayed in the ML375 series, it was not immediately clear where to go in the optimization effort to provide a short half-life analog of 1. The M5 NAM 2, harboring a 9b-(4-methoxy-3-methylphenyl) moiety, was of comparable potency to 1, but demonstrated poor rat PK (t1/2 <30 min, CLp >70 mL/min/kg), as well as limited CNS penetration.1,2 Thus, we postulated that a hybrid of NAMs 1 and 2, with incorporation of a 3-methyl group into the 9b-phenyl ring of 1, might engender a metabolic shunt, and thus provide a balance between M5 NAM activity, physiochemical properties and disposition (i.e., reduced rat t1/2).
The synthesis of the 9b-(4-chloro-3-methylphenyl)-containing M5 NAM was straightforward (Scheme 2).1,12 Lithium-halogen exchange on 5, and addition of anhydride 6 affords benzoic acid 7 in 37% yield. Condensation with ethylenediamine, under microwave irradiation, provides the 1,2,3,9b-tetrahydro-5H-imidazo[2,1-a]isoindol-5-one core 8 in 70%. Finally, acylation with 3,4-difluorobenzoyl chloride delivers racemic 9. Chiral resolution by super criterial fluid chromatography gave (R)-10 and (S)-11.1,12 Racemic 9 was indeed an M5 NAM (IC50 = 1.8 μM, pIC50 = 5.75±0.03, 2.9±0.29 % ACh min). As has held true for this series, the (R)-enantiomer 10 was devoid of M5 NAM activity (IC50 >10 μM), while the (S)-enantiomer 11 (human M5 IC50 = 1.2 μM, pIC50 = 5.93±0.02, 2.3±0.03 % ACh min and rat M5 IC50 = 1.6 μM, pIC50 = 5.78±0.02, 2.6±0.03 % ACh min) was the active enantiomer (Figure 3A), and equipotent to 1. Moreover, 11 (VU6008667) was selective for M5 over M1–4 (Figure 3B). Next, we sought to assess if incorporation of a putative metabolic shunt into the framework of 1, with 11, would lower the 80 hour half-life in rat.
Scheme 2.
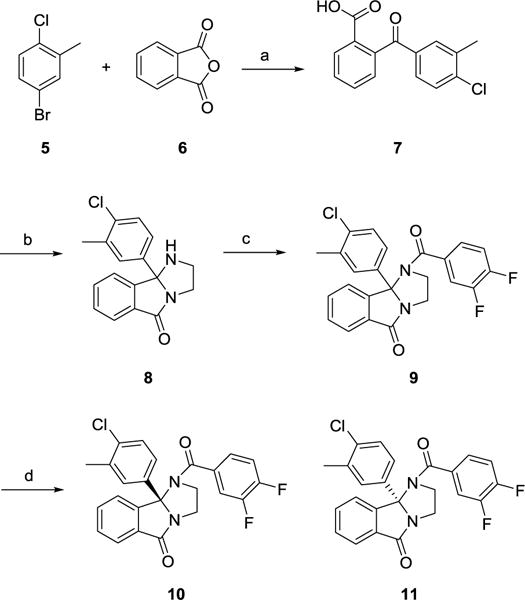
Synthesis of M5 NAM analogs 9–11.a
aReagents and conditions: (a) nBuLi, THF, −78 °C, 2h, 50%; (b) ethylenediamine, pTsOH, 4Å sieves, toluene, 150°C μW 25 min, 70%; (c) 3,4-difluorobenzoyl chloride, DIPEA, DCM, 80%; (d) SFC, (CHIRALPAK IE, 20 mm × 250 mm column at 40 °C, back-pressure regulated at 100 bar, IPA cosolvent, 30% isocratic prep over 7 min at 80 mL/min).
Figure 3. Molecular pharmacology profile of M5 NAMs 9–11.
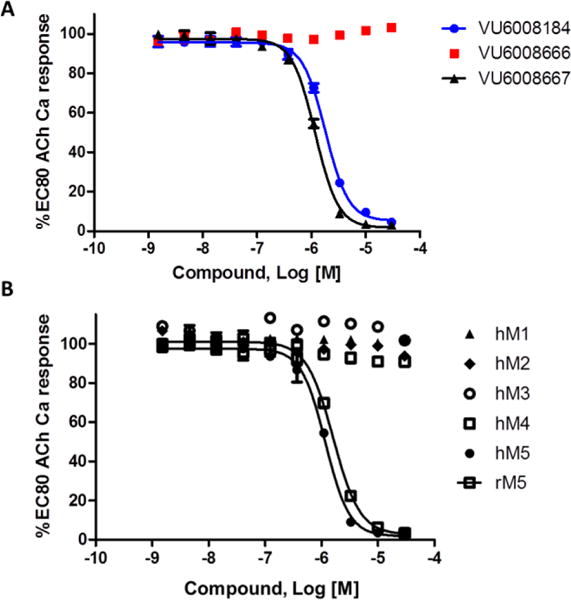
A) hM5 concentration response curves (CRCs) for 9, 10 and 11; B) mAChR CRCs for hM1–5, highlighting the exquisite selectivity for (S)-11 as an M5 NAM, as well as rat M5, showing no significant species differences. Data represent means from at least three independent determinations performed in triplicate via intracellular calcium mobilization assays in recombinant Chinese Hamster Ovary (CHO) cells stably transfected with the individual mAChRs.
The in vitro DMPK profile of 11 was similar to 1 in terms of plasma protein binding (fu,plasma rat = 0.014,human = 0.006) and rat brain homogenate binding (fu,brain = 0.003), but the predicted hepatic clearance, as we had hoped, was an order of magnitude higher (rat CLhep = 67 mL/min/kg and human CLhep = 15 mL/min/kg). Moreover, 11 was highly brain penetrant (Kp = 4.1, Kp,uu = 0.88). In a rat IV (1 mg/kg)/PO (3 mg/kg, solution dose) PK study, 11 displayed the desired diminished elimination half-life (t1/2 = 2.3 hr) driven by a smaller (yet still large) volume (Vss = 7.4 L/kg) and higher clearance (CLp = 82 mL/min/kg), with moderate oral bioavailability (17% F). These results reveal a dramatic impact of the addition of a single methyl group to 1 as a metabolic shunt, decreasing rat t1/2 by ~35-fold while maintaining M5 NAM potency and favorable CNS penetration. Again, an M5 NAM with this PK profile was key for addiction studies in rat examining reinstatement paradigms and washout, where 1 could be problematic.
In summary, the continued optimization of the long half-life M5 NAM ML375 resulted in the discovery of (S)-11 (VU6008667), an equipotent human and rat M5 NAM with a desired short half-life in rat (t1/2 = 2.3 hr). For many of the addiction studies underway on our labs, a short half-life M5 NAM was required. New studies are underway to directly compare ML375 to (S)-11 in a variety of paradigms and with multiple drugs of abuse, and results will be reported in due course.
Acknowledgments
We thank the NIH for funding via the National Institute of Drug Abuse (1R01DA037207). We also thank William K. Warren, Jr. and the William K. Warren Foundation who funded the William K. Warren, Jr. Chair in Medicine (to C.W.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gentry PR, Kokubo M, Bridges TM, Byun N, Cho HP, Smith E, Hodder PS, Niswender CM, Daniels JS, Conn PJ, Lindsley CW, Wood MR. J Med Chem. 2014;57:7804–7810. doi: 10.1021/jm500995y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurata H, Gentry PR, Kokubo M, Cho HP, Bridges TM, Niswender CM, Byers FW, Wood MR, Daniels JS, Conn PJ, Lindsley CW. Bioorg Med Chem Lett. 2015;25:690–694. doi: 10.1016/j.bmcl.2014.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentry PR, Kokubo M, Bridges TM, Cho HP, Smith E, Chase P, Hodder PS, Utley TJ, Rajapakse A, Byers F, Niswender CM, Morrison RD, Daniels JS, Wood MR, Conn PJ, Lindsley CW. ChemMedChem. 2014;9:1677–1682. doi: 10.1002/cmdc.201402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geanes AR, Cho HP, Nance KD, McGowan KM, Conn PJ, Jones CK, Meiler J, Lindsley CW. Bioorg Med Chem Lett. 2016;26:4487–4491. doi: 10.1016/j.bmcl.2016.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile AS, Fedorova I, Zapata A, Liu X, Shippenberg T, Duttaroy A, Yamada M, Wess J. Proc Natl Acad Sci USA. 2002;99:11452–11457. doi: 10.1073/pnas.162371899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fink-Jensen A, Fedorova I, Wörtwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile AJ. Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 7.Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manuscript in preparation.
- 9.Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, Conn PJ, Lindsley CW. J Med Chem. 2012;55:1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conn PJ, Lindsley CW, Meiler J, Niswender CM. Nat Rev Drug Discov. 2014;13:692–708. doi: 10.1038/nrd4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsley CW, Emmitte KA, Hopkins CR, Bridges TM, Gregory KA, Niswender CM, Conn PJ. Chem Rev. 2016;116:6707–6741. doi: 10.1021/acs.chemrev.5b00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preparation of 12 (VU6008667): To a stirring solution of 4-bromo-1-chloro-2-methyl-benzene 5 (4.92 mL, 37.1 mmol) in THF (75 mL) cooled to −78 °C was added n-butyllithium (14.9 mL, 37.1 mmol) dropwise. The solution was stirred at −78 °C for 30 min and then was added to a stirring solution of phthalic anhydride 6 (5.00 g, 33.8 mmol) in THF (90 mL) at −78 °C. The reaction was stirred at −78 °C for 2 hrs before being quenched with 1N HCl (100 mL). The solution was allowed to warm to rt, the layers were separated and the aqueous layer was extracted with EtOAc (3 × 100 mL). The combined organic layers were passed through a phase separator and concentrated. The crude material was purified using Teledyne ISCO Combi-Flash system (solid loading, 40G column, 40–80% EtOAc, 20 min run) to give 2-(4-chloro-3-methyl-benzoyl)benzoic acid 7 (4.67 g, 50.4% yield) as a white solid. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.10 (d, J = 7.8 Hz, 1H), 7.68 (dt, J = 7.6, 1.2 Hz, 1H), 7.64–7.62 (m, 1H), 7.59 (dt, J = 7.9, 1.2 Hz, 1H), 7.44 (dd, J = 8.2, 2.2 Hz, 1H), 7.39–7.35 (m, 2H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ (ppm): 170.1, 136.8, 135.7, 133.6, 131.8, 131.7, 131.2, 131.1, 129.9, 129.6, 128.5, 127.9, 127.8, 20.3. To a solution of 2-(4-chloro-3-methyl-benzoyl)benzoic acid 7 (175.0 mg, 0.640 mmol) in toluene (1.59 mL) in a microwave vial was added ethylenediamine (0.09 mL, 1.27 mmol) followed by p-toluenesulfonic acid (8.48 mg, 0.040 mmol). The reaction subjected to microwave irradiation at 150 °C for 30 min. The reaction was diluted with EtOAc (10 mL) and quenched with saturated aqueous NaHCO3 (5 mL). The layers were separated and the organic layer was washed with water (2 × 5 mL). The organic layer was dried (MgSO4) before concentration to dryness. The crude material was purified using Teledyne ISCO Combi-Flash system (solid loading, 24G column, 80–100% EtOAc, 18 min run) to give 9b-(4-chloro-3-methyl-phenyl)-2,3-dihydro-1H-imidazo[2,1-a]isoindol-5-one 8 (135 mg, 70.9% yield) as a clear oil. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.82–7.77 (m, 1H), 7.55–7.53 (m, 1H), 7.50–7.45 (m, 3H), 7.34–7.28 (m, 2H), 3.86–3.78 (m, 1H), 3.68–3.64 (m, 1H), 3.29–3.15 (m, 2H), 2.37 (s, 3H); 13C NMR (100 MHz, CDCl3) δ (ppm): 172.8, 147.8, 137.8, 136.7, 134.7, 133.0, 131.9, 130.1, 129.6, 129.0, 125.3, 124.7, 123.5, 89.3, 50.9, 41.9, 20.4. To a stirring solution of 9b-(4-chloro-3-methyl-phenyl)-2,3-dihydro-1H-imidazo[2,1-a]isoindol-5-one 8 (1.40 g, 4.69 mmol) in DCM (46.8 mL) was added 3,4-difluorobenzoyl chloride (0.884 mL, 7.03 mmol) followed by diisopropylethylamine (2.05 mL, 11.72 mmol). The reaction was stirred for 1.5 hrs. The reaction was quenched with saturated aqueous NH4Cl (50 mL) and the layers were separated. The aqueous layer was extracted with DCM (2 × 50 mL) and the combined organic layers were passed through a phase separator and concentrated to dryness. The crude material was dissolved in DMSO and purified using the Gilson LC system (30 × 100 mm column, 55–95%, 0.1% TFA water and ACN, 10 min run, 6 runs). The desired fractions were neutralized with saturated aqueous NaHCO3 and concentrated to give racemic 9b-(4-chloro-3-methyl-phenyl)-1-(3,4-difluorobenzoyl)-2,3-dihydroimidazo[2,1-a]isoindol-5-one 9 (1.66 g, 80.7% yield). The second eluting pure enantiomer 11 was separated via CO2 supercritical fluid chromatography (CHIRALPAK IE, 20 mm × 250 mm column at 40 °C, back-pressure regulated at 100 bar, IPA cosolvent, 30% isocratic prep over 7 min at 80 mL/min) and was determined to have 98% ee by chiral HPLC analysis CHIRALPAK IE, 4.6 mm × 250 mm column at 40 °C, backpressure regulated at 100 bar, IPA cosolvent, 30% over 7 min at 3.5 mL/min). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.03 (dd, J = 6.4, 1.3 Hz, 1H), 7.91–7.87 (m, 1H), 7.62 (dpentet, J = 7.6, 1.6 Hz, 2H), 7.37–7.31 (m, 2H), 7.25–7.18 (m, 2H), 7.06–7.02 (m, 2H), 4.34 (ddd, J = 12.2, 7.8, 1.8 Hz, 1H), 4.01–3.93 (m, 1H), 3.79 (ddd, J = 9.6, 7.5, 1.8 Hz, 1H), 3.31 (ddd, J = 17.9, 9.9, 7.6 Hz, 1H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ (ppm): 172.2, 167.0, 145.9, 136.9, 136.8, 135.3, 133.6, 132.0, 130.7, 129.6, 129.1, 128.7, 124.9, 124.1, 123.8, 123.3, 118.2, 118.0, 117.0, 116.8, 87.5, 52.4, 39.8, 20.7. Specific Rotation [α]23D = −147.2° (c = 1.00, CHCl3).


