Abstract
Objectives
To assess whether sparing neck level IB in target delineation of node positive (N+) oropharyngeal carcinoma (OPC) can improve xerostomia outcomes without compromising local-regional control (LRC).
Methods
125 N+ OPC patients with a median age of 57 years underwent chemoradiation between 5/10 and 12/11. 74% of patients had T1-2 disease, 26% T3-4, 16% N1, 8% N2A, 48% N2B, 28% N2C; 53% base of tongue, 41% tonsil, and 6% other. Patients were divided into those who had target delineation sparing of bilateral level IB (the spared cohort) vs. no sparing (the treated cohort). Sparing of contralateral high level II nodes was also performed more consistently in the spared cohort. A prospective xerostomia questionnaire (patient reported) was given at each patient follow-up visit to this cohort of patients to assess late xerostomia. Clinical assessment (observer rated) at each patient follow-up visit was also recorded.
Results
The 2-year LRC for the spared and treated cohorts was 97.5% and 93.8%, respectively (median follow-up, 23.2 months). No local-regional failures occurred outside of treatment fields. The spared cohort experienced significant benefits in patient-reported xerostomia summary scores (P = 0.021) and observer-rated xerostomia scores (P = 0.006). In addition, there were significant reductions in mean doses to the ipsilateral submandibular gland (SMG; 63.9 Gy vs. 70.5 Gy; P < 0.001), contralateral SMG (45.0 Gy vs. 56.2 Gy; P < 0.001), oral cavity (35.9 Gy vs. 45.2 Gy; P < 0.001), and contralateral parotid gland (20.0 Gy vs. 24.4 Gy; P < 0.001).
Conclusions
Target delineation sparing of bilateral level IB nodes in N+ OPC reduced mean doses to salivary organs without compromising LRC. Patients with reduced target volumes had better patient-reported xerostomia outcomes.
Keywords: sparing IB, submandibular, salivary gland, xerostomia, oropharyngeal cancer
INTRODUCTION
The rate of subclinical neck level IB nodal involvement in node positive (N+) oropharyngeal carcinoma (OPC) is low.1–3 The level IB nodal region includes the submandibular glands (SMGs) and lies in proximity to the oral cavity. Therefore, we postulated that the level IB nodes may be spared radiation treatment with the goal of reducing toxicity.
Late xerostomia is the most common adverse effect of intensity-modulated radiation therapy (IMRT) for OPC.4 A Phase III multicenter randomized controlled trial (PARSPORT) showed improved observer-rated xerostomia and improved quality of life when using parotid-sparing with IMRT.5 Sparing additional major and minor salivary glands may further improve xerostomia.6–8 The SMGs produce saliva in the non-stimulated state and the minor salivary glands produce mucin, both of which may be inhibited by current treatment methods.9,10 However, a number of uncertainties remain concerning the actual benefits from sparing these salivary glands.11
The purpose of this study is to evaluate whether sparing bilateral IB nodes in OPC N+ disease can improve xerostomia outcomes, in particular, patient-reported outcomes without compromising local-regional control. A secondary objective is to correlate dosimetric parameters with xerostomia outcomes.
MATERIALS AND METHODS
Patient Selection
This study is focused on patients with N+ OPC who were consecutively treated with definitive IMRT at our center between May 2010 and December 2011. Patients were excluded if they had level 1 nodal involvement (n=11), N3 disease (n=3), prior head and neck irradiation (n=21), distant metastasis on presentation (n=3), histology other than squamous cell carcinoma (n=5), gross total resection (n=24), or neck dissection prior to radiation therapy (n=3). In total, 125 patients were included in this analysis. All patients were staged according to the American Joint Committee on Cancer (AJCC) 7th edition with a complete history, physical exam, focused head and neck evaluation, direct flexible fiber-optic endoscopic examination, complete blood counts, liver function tests, chest X-ray, and dental evaluation. Additionally, computed tomography (CT) and/or MRI of the head and neck region as well as positron-emission tomography (PET)/CT were typically obtained.
Sparing of Low-Risk Nodes
Treatment plans were reviewed for coverage of bilateral level IB nodes according to the Radiation Therapy Oncology Group (RTOG) consensus guidelines (Fig. 1).12 Target delineation sparing of neck level IB was performed by limiting elective treatment of the ipsilateral IB to the posterior half or the perisubmandibular gland portion.1 The clinical target volume and planning target volume (PTV) may extend into the posterior aspect of level IB to provide sufficient coverage when a node is abutting level IB (Fig. 1). Sparing ipsilateral neck level IB started at our institution in May 2010. However, the practice of sparing had not been implemented by all treating radiation oncologists within our institution, which allows us to perform this analysis. Level IB was never spared with disease extension into the oral cavity or level IB nodes. After retrospective review of treatment plans, patients were divided into two cohorts: sparing of bilateral level IB (the spared cohort) vs. no sparing of bilateral level IB (the treated cohort). Dose constraints were also applied to the oral cavity more consistently in the spared cohort. Target delineation sparing of bilateral neck level IB nodes was important in allowing treatment planners to meet those dose constraints. Additional target delineation sparing of the contralateral high level II nodes were performed more consistently in the spared cohort.
FIGURE 1.
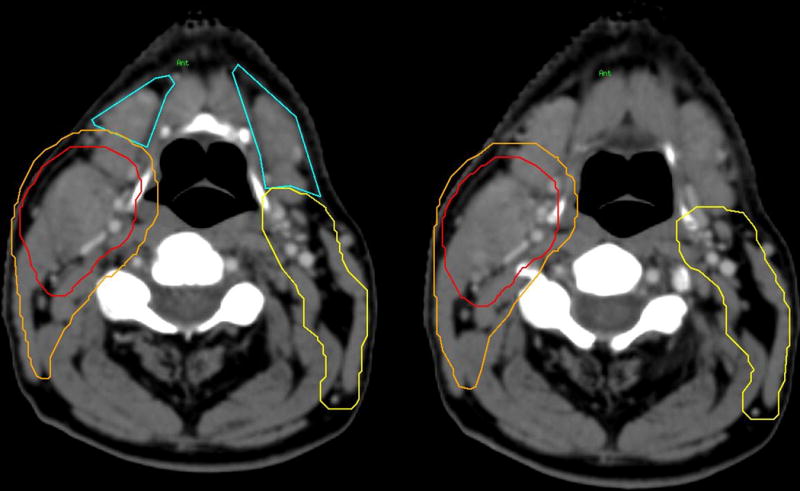
Bilateral level IB (blue) with PTV70 (red) and PTV59.4 (orange). Left: Contouring (blue) of bilateral level IB lymph nodes. Right: Sparing of bilateral level IB lymph nodes. The clinical target volume (CTV) and planning target volume (PTV) may extend into the posterior aspect of level IB to provide sufficient coverage when a node is abutting level IB.
Chemotherapy
Chemotherapy was administered concurrently in all but one patient (99%) due to pretreatment thrombocytopenia. Patients received high-dose cisplatin alone (n=82, 65.6%), consisting of 100 mg/m2 for a planned 2 or 3 cycles; cetuximab alone (n=9, 7.2%), administered as an initial loading dose of 400 mg/m2 followed by 7 weekly cycles administered at 250 mg/m2; cisplatin and bevacizumab (n=11, 8.8%), administered at 100 mg/m2 and 15 mg/kg, respectively, in 2 to 3 cycles; and other chemotherapy regimens (n=20, 16.0%), which consisted of paclitaxel/carboplatin (n=6), paclitaxel/cetuximab (n=5), carboplatin/5-fluorouracil (n=4), paclitaxel/cisplatin (n=2), docetaxel/cisplatin (n=1), and carboplatin alone (n=2).
Intensity-Modulated Radiation Therapy
The guidelines for treatment planning have been described in detail.13,14 Briefly, all patients were treated with IMRT with a median dose of 70 Gy to the PTV (PTV70), 59.4 Gy to the high-risk subclinical disease (PTV59.4), and 54 Gy to the lower-risk subclinical disease (PTV54). The median dose per fraction was 2.12 Gy to the PTV70, 1.8 Gy to the PTV59.4, and 1.64 Gy to the PTV54.
Self-Reported Assessments
A prospective, previously validated, self-reported xerostomia questionnaire (XQ) that was approved by our Institution Review Board was collected during patient visits.15 The XQ consisted of 9 questions (Table 1) in which patients rate each item on a scale of 0 to 10; the higher the score, the worse the xerostomia. These scores were then averaged and converted to a summary score scaled from 0 to 100. The XQ was collected at patient pretreatment and follow-up visits starting in June 2011. The first completed questionnaire between 9 months and 24 months after day 1 of radiation treatment was used to represent self-reported late xerostomia.
TABLE 1.
Xerostomia Questionnaire
| Dryness Questionnaire | |
|---|---|
| Xerostomia (0 to 10, 10 denoting the greatest difficulty) | |
| 1 | Difficulty in talking due to dryness |
| 2 | Difficulty in chewing due to dryness |
| 3 | Difficulty in swallowing solid food due to dryness |
| 4 | Frequency of your sleeping problems due to dryness |
| 5 | Mouth dryness while not eating food |
| 6 | Throat dryness while not eating food |
| 7 | Mouth and throat dryness when eating food |
| 8 | Frequency of sipping liquids to aid swallowing food |
| 9 | Frequency of sipping liquids for oral comfort when not eating |
Observer-Rated Assessments
Late toxicities were assessed by the treating radiation and medical oncology physicians using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 in post-treatment follow-up visits. The maximum late xerostomia grade was used for analysis.
Follow-up and Response Assessment
Follow-up visits were planned for 4, 8, and 12 weeks after completion of treatment, then 3 months for 2 years, followed by every 6 months thereafter. At follow-up visits, physical examination and direct flexible fiber-optic endoscopic examinations were performed. PET/CT scans were performed at 3–4 months after radiation treatment. Further imaging with CT, PET/CT, and MRI was performed on suspicion of recurrence. Biopsies were performed to verify recurrences.
Dosimetric Analysis
Dosimetric analysis of the salivary glands was performed. Mean doses of the parotid glands, SMGs, and oral cavity were evaluated. The oral cavity was defined as including the base of the tongue, the floor of the mouth, and the hard palate and did not exclude gross tumor volume.16
Statistical Analysis
Differences in proportions of treatment characteristics were determined using chi-square test. The comparison of mean values of questionnaire scores and doses was assessed using the two-tailed t-test. Correlation between doses and questionnaire scores were determined using Pearson’s correlation. Local-regional control, freedom from distant metastasis, and overall survival were determined using the Kaplan-Meier method. Time to events was calculated from the first day of radiation treatment.
RESULTS
Patient characteristics are listed in Table 2. Among the 125 patients, 40 patients were in the spared cohort and 85 patients were in and the treated cohort. No significant differences in patient or disease characteristics were found between the two cohorts.
TABLE 2.
Patient and disease characteristics
| IB Spared (n=40) | IB Treated (n=85) |
P Value | |
|---|---|---|---|
| Age | 0.34 | ||
| Mean | 60 | 57 | |
| Range | 43–84 | 38–88 | |
|
| |||
| n (%) | n (%) | ||
|
| |||
| Gender | 0.92 | ||
| Male | 36 (90%) | 77 (91%) | |
| Female | 4 (10%) | 8 (9%) | |
| Site | 0.73 | ||
| Tonsil | 15 (38%) | 36 (42%) | |
| Base of tongue | 23 (58%) | 43 (51%) | |
| Other | 2 (5%) | 6 (7%) | |
| T stage | 0.56 | ||
| T1 | 12 (30%) | 16 (19%) | |
| T2 | 21 (53%) | 44 (52%) | |
| T3 | 6 (15%) | 18 (21%) | |
| T4 | 1 (3%) | 7 (8%) | |
| N stage | 0.57 | ||
| N1 | 6 (15%) | 14 (16%) | |
| N2A | 4 (10%) | 6 (7%) | |
| N2B | 22 (55%) | 38 (45%) | |
| N2C | 8 (20%) | 27 (32%) | |
| HPV Status | 0.86 | ||
| Positive | 21 (53%) | 47 (55%) | |
| Negative | 3 (8%) | 5 (6%) | |
| Smoking | 0.15 | ||
| Current | 4 (10%) | 13 (15%) | |
| Former | 13 (33%) | 39 (46%) | |
| Never | 23 (58%) | 33 (39%) | |
HPV indicates human papillomavirus.
Local-Regional Control
With a median follow-up of 23.2 months, the 2-year local-regional control for the spared cohort spared and the treated cohort was 97.5% and 93.8%, respectively (Fig. 2). All local-regional failures in the spared cohort occurred within treated volumes with a median time to failure of 2.5 months. No patient experienced a local-regional recurrence outside of the treatment fields. The spared cohort had only one local-regional failure, which occurred in an ipsilateral level II node that had been treated to 70 Gy.
FIGURE 2.
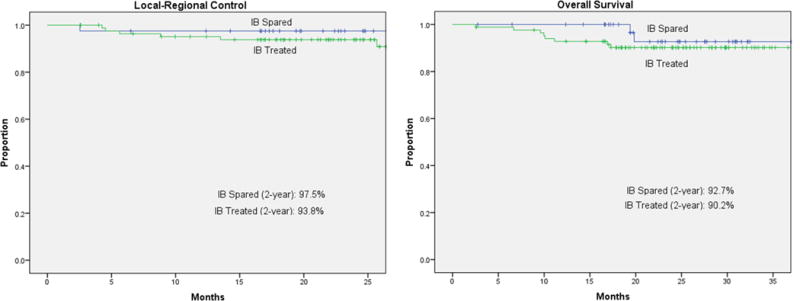
Local-regional control and overall survival.
Xerostomia Questionnaire and Dosimetric Analysis
In total, there were 87 (70%) patients with evaluable questionnaires completed between 9 and 24 months after the start of radiation treatment. The completion rates for the spared and treated cohorts were 68% and 71%, respectively. The mean time to questionnaire completion for the spared and treated cohorts was 11.3 months and 13.0 months, respectively.
Patients in the spared cohort reported less xerostomia toxicity compared with patients in the treated cohort based on XQ summary scores (39.5 vs. 52.4; P = 0.021); where lower scores indicate better salivary function (Table 3). The spared cohort received lower mean doses to the ipsilateral SMG (63.9 Gy vs. 70.5 Gy; P < 0.001), contralateral SMG (45.0 Gy vs. 56.2 Gy; P = 0.001), and the oral cavity (36.1 Gy vs. 45.2 Gy; P < 0.001) (Table 3).
Table 3.
Xerostomia and dosimetric outcomes
| IB Spared | IB Treated | P Value | |
|---|---|---|---|
| Xerostomia | |||
| Self-reported (summary scores) | 39.5 | 52.4 | 0.021 |
| Observer-rated (CTCAE) | 1.4 | 1.7 | 0.006 |
| Dosimetric analysis (mean doses in Gy) (SD) | |||
| Parotid (ipsilateral) | 31.0 (6.9) | 33.8 (9.7) | 0.127 |
| Parotid (contralateral) | 20.0 (5.1) | 24.4 (4.6) | <0.001 |
| SMG (ipsilateral) | 63.9 (7.2) | 70.5 (2.8) | <0.001 |
| SMG (contralateral) | 45.0 (13.8) | 56.2 (13.6) | <0.001 |
| Oral cavity | 36.1 (8.1) | 45.2 (11.0) | <0.001 |
CTCAE indicates Common Terminology Criteria for Adverse Events; SD, standard deviation, SMG, submandibular glands.
Mean doses to the contralateral SMG (P = 0.012) and contralateral parotid (P = 0.047) correlated with XQ summary scores. Mean doses to the oral cavity (P = 0.0138) and the ipsilateral parotid (P = 0.161) showed a trend towards correlation with XQ summary scores. Mean doses to the ipsilateral SMG (P = 0.688) did not correlate with XQ summary scores.
Observer-Rated Late Xerostomia
Patients in the spared cohort experienced a lower grade of observer-rated late xerostomia (1.4 vs. 1.7; P = 0.006) (Table 3 and Fig. 3). Observer-rated xerostomia correlated with XQ summary scores (P = 0.039) as well as doses to the contralateral parotid gland (P = 0.023).
FIGURE 3.
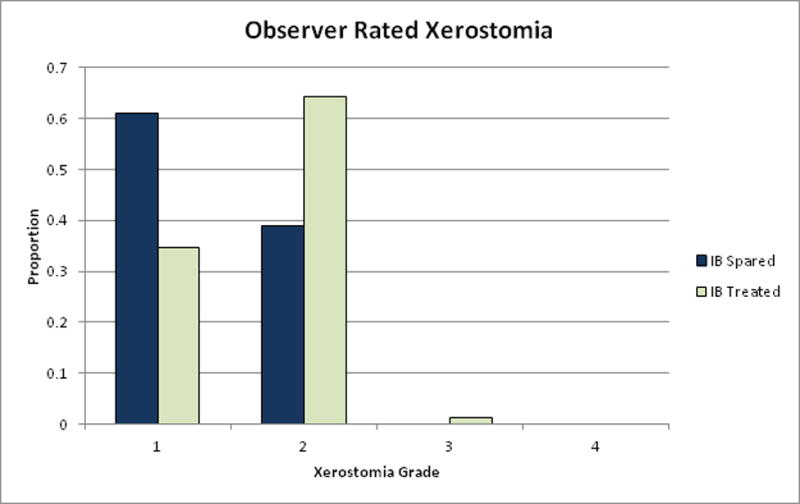
Observer-rated late xerostomia.
Distant Metastasis and Overall Survival
The 2-year freedom from distant metastasis in the spared cohort vs the treated cohort was 97.3% vs. 93.9%, respectively. The 2-year overall survival in the spared cohort vs the treated cohort was 92.7% vs. 90.2% (Fig. 2).
CONCLUSIONS
The rate of subclinical disease in bilateral level IB N+ OPC has been shown to be low; however, it remains unclear whether sparing these low-risk nodes can reduce toxicity without resulting in unwarranted failures. In this study, we report significant benefits in patient-reported quality of life outcomes when omitting prophylactic radiotherapy to level IB lymph nodes. Target delineation sparing of level IB nodes reduced mean doses to the SMGs and the oral cavity without compromising 2-year local-regional control. Additionally, no local-regional failures occurred in untreated fields.
We were able to achieve a mean dose of <50 Gy to the contralateral SMG and <40 Gy to the oral cavity through the sparing of level IB. Our data showed that lower mean doses to the contralateral SMG correlated with xerostomia outcomes, which has reported by several studies.6,7,17,18 Mean doses to the oral cavity also showed a trend in predicting xerostomia outcomes and is consistent with literature.15,18 Although no clear threshold exists, these mean dose levels have been associated with improved xerostomia outcomes.18
Our data did not show a correlation between xerostomia outcomes and mean doses to the ipsilateral SMG. Thus, a reduction in mean dose from 71 Gy to 64 Gy may not be clinically significant. The normal tissue complication probability of submandibular glands needs to be further investigated, as current predictive models of SMG dose and xerostomia are imprecise.11
Additional factors may have contributed to the lower doses to the salivary organs including the use of dose constraints in the oral cavity and the target delineation sparing of the contralateral high level II nodes. Both of these factors were more consistently performed in the spared cohort. Our data did show a correlation between lower mean dose to the parotid gland and xerostomia outcomes, which is also consistent with the literature.11
Sparing of the ipsilateral IB nodal level has remained controversial, with a previously published series showing a moderate rate of subclinical level IB involvement in an era before routine cross-axial and PET imaging.19 Our study found that sparing the uninvolved level IB nodes did not result in worse local-regional control, which supports the notion of a very low risk of subclinical disease in level IB as reported by Sanguineti et al.1 Even though the Johns Hopkins Hospital series included only early T-stage disease, our study included 15% T3 and 3% T4 disease in the spared cohort with promising results. However, this is a limited sample size of T3-T4 disease and therefore more data is required. It is important to note that we excluded patients with direct tumor involvement in IB and/or those with disease extension into the oral cavity, which drains into the IB nodes.
A significant majority of our patients with known human papillomavirus (HPV) status were HPV-positive, which has important effects on outcomes.20 Patients with HPV-positive tumors are living much longer and, therefore, late treatment toxicities will have a greater impact on quality of life outcomes. Thus, we need to consider treatment de-escalation as the demographics of our population changes and oropharyngeal carcinoma continues to shift from an etiology based on tobacco to an etiology based on HPV.
Limitations to this study include non-randomization and a lack of pretherapy xerostomia scores. An analysis of xerostomia outcomes relative to baseline scores is currently ongoing. Furthermore, dose reductions to several salivary organs limit our ability to isolate the effect of the mean dose to the ipsilateral SMG on xerostomia outcomes.
In conclusion, our study found that target delineation sparing of bilateral level IB nodes in OPC reduces mean dose to the submandibular glands and the oral cavity without compromising local-regional control. Patients with reduced target delineation volumes experienced better xerostomia outcomes.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Sanguineti G, Califano J, Stafford E, et al. Defining the risk of involvement for each neck nodal level in patients with early T-stage node-positive oropharyngeal carcinoma. International journal of radiation oncology, biology, physics. 2009;74:1356–64. doi: 10.1016/j.ijrobp.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. International journal of radiation oncology, biology, physics. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. American journal of surgery. 1990;160:405–9. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 4.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. International journal of radiation oncology, biology, physics. 2012;82:291–8. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. The lancet oncology. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strigari L, Benassi M, Arcangeli G, Bruzzaniti V, Giovinazzo G, Marucci L. A novel dose constraint to reduce xerostomia in head-and-neck cancer patients treated with intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2010;77:269–76. doi: 10.1016/j.ijrobp.2009.07.1734. [DOI] [PubMed] [Google Scholar]
- 7.Saarilahti K, Kouri M, Collan J, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2006;78:270–5. doi: 10.1016/j.radonc.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Murdoch-Kinch CA, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol. 2008;72:373–82. doi: 10.1016/j.ijrobp.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milne RW, Dawes C. The relative contributions of different salivary glands to the blood group activity of whole saliva in humans. Vox sanguinis. 1973;25:298–307. doi: 10.1111/j.1423-0410.1973.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 10.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annual review of physiology. 1995;57:547–64. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 11.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. International journal of radiation oncology, biology, physics. 2010;76:S58–63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2003;69:227–36. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 13.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. International journal of radiation oncology, biology, physics. 2006;64:363–73. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Lee NY. Intensity-modulated radiation therapy in the treatment of head and neck cancer involving the base of the skull. International journal of radiation oncology, biology, physics. 2007;69:S43–5. doi: 10.1016/j.ijrobp.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 15.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. International journal of radiation oncology, biology, physics. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoebers F, Yu E, Eisbruch A, et al. A Pragmatic Contouring Guideline for Salivary Gland Structures in Head and Neck Radiation Oncology: The MOIST Target. American journal of clinical oncology. 2013;36:70–6. doi: 10.1097/COC.0b013e31823a538e. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZH, Yan C, Zhang ZY, et al. Impact of salivary gland dosimetry on post-IMRT recovery of saliva output and xerostomia grade for head-and-neck cancer patients treated with or without contralateral submandibular gland sparing: a longitudinal study. International journal of radiation oncology, biology, physics. 2011;81:1479–87. doi: 10.1016/j.ijrobp.2010.07.1990. [DOI] [PubMed] [Google Scholar]
- 18.Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. International journal of radiation oncology, biology, physics. 2012;83:1007–14. doi: 10.1016/j.ijrobp.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head & neck. 1990;12:197–203. doi: 10.1002/hed.2880120302. [DOI] [PubMed] [Google Scholar]
- 20.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]


