Abstract
Depression, stress, and diet can all alter inflammation. This double-blind, randomized crossover study addressed the impact of daily stressors and a history of major depressive disorder (MDD) on inflammatory responses to high-fat meals. During two separate 9.5 hour admissions, 58 healthy women (38 breast cancer survivors and 20 demographically-similar controls), mean age 53.1 years, received either a high saturated fat meal or a high oleic sunflower oil meal. The Daily Inventory of Stressful Events assessed prior day stressors and the Structured Clinical Interview for DSM-IV evaluated MDD. As expected, for a woman with no prior day stressors, C-reactive protein (CRP), serum amyloid A (SAA), intercellular adhesion molecule-1 (sICAM-1), and vascular cell adhesion molecule-1 (sVCAM-1) were higher following the saturated fat meal than the high oleic sunflower oil meal after controlling for pre-meal measures, age, trunk fat, and physical activity. But if a woman had prior day stressors, these meal-related differences disappeared – because the stressors heightened CRP, SAA, sICAM-1, and sVCAM-1 responses to the sunflower oil meal, making it look more like the responses to the saturated fat meal. In addition, women with an MDD history had higher post-meal blood pressure responses than those without a similar history. These data show how recent stressors and an MDD history can reverberate through metabolic alterations, promoting inflammatory and atherogenic responses.
Introduction
Adherence to a Mediterranean-type diet can reduce the risk for depression, cardiovascular disease, type 2 diabetes, and total mortality (1–4). Reduced inflammation may be the cornerstone for the Mediterranean diet’s benefits (5–8).
The central fat source in the Mediterranean diet, olive oil, appears to be a key anti-inflammatory dietary component (8, 9). Indeed, a meta-analysis concluded that olive oil interventions lowered CRP and interleukin 6 (IL)-6 compared to control conditions (9). The major dietary fatty acids in the North American diet are palmitic acid, a saturated fatty acid found in meat and dairy products, and the monounsaturated oleic acid, the main fatty acid in olive oil, and many vegetable oils (10).
Saturated fatty acids trigger proinflammatory signaling pathways (11). For example, palmitic acid activated toll-like receptor 4 (TLR4), leading to nuclear transcription factor κB (NF-κB) signaling and increased gene expression of IL-6, IL-8, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) in contrast, the monounsaturated palmioleate did not have inflammatory sequelae (11). A meal made with butter, a rich source of saturated fatty acids, activated NF-κB in peripheral blood mononuclear cells and also increased sICAM-1 concentrations, but a meal with olive oil, a rich source of oleic acid, did not (12). Palmitate-stimulated monocytes also fuel ICAM-1 expression in endothelial cells via an IL-1 signaling pathway (13).
High saturated fat meals raise adhesion molecule concentrations while monounsaturated fat meals lower concentrations (14). For example, high palmitate milkshakes boosted sICAM-1 levels while olive oil milkshakes decreased sICAM-1 (14). Similarly, a high-palmitic fatty acid oil breakfast heightened post-meal sICAM-1 and sVCAM-1, but a refined olive oil breakfast lowered their concentrations (15). Postprandial sICAM-1 levels were amplified by a 12-week high saturated fat diet and reduced by a monounsaturated fat diet (16).
These meal-related differences are important because adhesion molecules play a central role in the development of atherosclerosis and diabetes (17–23). Plasma sICAM-1 reflects a more generalized inflammatory process, while sVCAM-1 appears to be a better indicator of plaque burden (19). In healthy people sICAM-1 serves as a more reliable prognostic indicator of cardiovascular disease than sVCAM-1, but sVCAM-1 better predicts cardiovascular events among patients with atherosclerotic disease than sICAM-1 (17–19). Furthermore, adhesion molecules and inflammation are associated with an increased risk of hypertension (24, 25), and monounsaturated fat interventions have reduced blood pressure (26, 27).
Elevated sICAM-1 levels also predict type II diabetes (20–23). For example, data from the Nurses’ Health Study showed that sICAM-1 independently predicted incident diabetes (21). In a large population-based prospective, sICAM-1 levels were higher among those participants who developed diabetes than in those who did not (20).
In addition to these dietary fat influences, depression and stressors can also boost NF-κB signaling, thus augmenting inflammation and increasing adhesion molecule concentrations (1, 28–30). For example, sICAM-1 levels were higher among all 18 physicians following an academic oral presentation compared to their own values two hours earlier, as well as their data from parallel assessments obtained on a control day (31). Comparisons of 22 patients who developed posttraumatic stress disorder (PTSD) following a myocardial infarction (MI) and 22 who did not develop post-MI PTSD showed that those with PTSD had higher concentrations of sICAM-1 and sVCAM-1 at rest, as well as following a trauma-specific interview than their PTSD-free counterparts (32). Among patients who had experienced a recent acute coronary syndrome, sICAM-1 levels were higher among those with major depression than those who did not meet criteria (33). Young adults with major depression had higher levels of sICAM-1 than nondepressed controls (34), and heightened sICAM-1 concentrations have also been found in healthy adults with elevated depressive symptoms (35–38).
People with a history of depression experience more major and minor stressors than those without a similar history, and past depression can also boost emotional reactivity to stressors (39, 40). Thus a mood disorder history could act synergistically with stress through multiple pathways.
Indeed, other data from this same sample of healthy breast cancer survivors and controls demonstrated that women with a history of depression who had also experienced more prior day stressors had a higher peak postprandial triglyceride response than other participants (41). Similarly, in our subsequent study with married couples that included the same high-fat meals, men and women who had a mood disorder history and who also displayed more hostile behaviors during the laboratory marital conflict discussions had lower post-meal resting energy expenditure, higher insulin, and higher peak triglyceride responses than other participants (42).
Accordingly, this secondary analysis assessed the impact of prior day stressors and past depression on metabolic responses to two different high-fat meals (41). We expected that our high saturated fat meal would increase inflammatory markers and adhesion molecules, but not our high oleic sunflower oil meal (14–16). We also hypothesized that both prior day stressors and an MDD history would be associated with higher post-meal concentrations of inflammatory markers and adhesion molecules.
METHODS AND MATERIALS
Design and Overview
This double-blind, randomized crossover study assessed metabolic responses following high-fat meals. Women received one high saturated fat meal and one high oleic sunflower oil meal during two separate 9.5 hour Clinical Research Center (CRC) visits spaced 1–4 weeks apart. The Ohio State institutional review board approved this study, and each participant provided informed consent.
After fasting for 12 hours, a catheter was inserted in the arm on admission. Women had 20 minutes to eat the meal. Blood samples were obtained before and 2, 4, and 7 hours after the meal. Body composition was assessed by dual x-ray absorptiometry (DXA) (43).
Participants
The parent study was designed to assess whether a high-fat diet in combination with a depression history increased fatigue in post-treatment cancer survivors and benign controls, and thus the 58 healthy women included 38 breast cancer survivors and 20 benign controls (women who had an initial abnormal mammogram) were recruited from a large observational study (41). Exclusions included a history of any other prior cancer, chronic obstructive pulmonary disease, symptomatic ischemic heart disease, alcohol/drug abuse, and immune-related conditions such as diabetes, autoimmune disease, and major inflammatory diseases. Medication exclusions included blood lipid medications, angiotensin type I receptor blockers, and medications with major immunological or endocrinological consequences, e.g., steroids. We invited 91 women from the large observational study who met these criteria to participate; of those, 3 had exclusionary health problems not previously identified, 18 declined to participate, and 14 did not respond to our recruitment letter. Demographic and laboratory data did not differ between groups with the exception of higher sICAM-1 in survivors compared to controls (Table 1). Survivors averaged 27.03 months (SD=17.45) since diagnosis and 19.87 months (SD=6.47) since treatment completion. Only one participant failed to return for her second visit.
Table 1.
Subject Characteristics
| Control Subjects (n=20) | Breast Cancer Survivors (n=38) | P-value | |
|---|---|---|---|
| Age, years | 55.0 (10.2) | 52.1 (7.3) | 0.23 |
| Body Mass Index, kg/m2 | 26.7 (4.1) | 28.8 (5.3) | 0.13 |
| Waist, cm | 91.2 (10.3) | 96.6 (12.8) | 0.11 |
| Trunk fat, g (DXA) | 13994.2 (5218.7) | 16983.8 (5758.6) | 0.06 |
| Lean body mass, g (DXA) | 40559.1 (3839.9) | 42541.1 (5239.7) | 0.14 |
| Caloric expenditure per week, moderate intensity exercise | 1331 (1285) | 1066 (1196) | 0.44 |
| Systolic blood pressure, mmHg | 124.0 (18.5) | 124.9 (20.1) | 0.89 |
| Diastolic blood pressure, mmHg | 72.6 (7.9) | 75.5 (8.6) | 0.21 |
| Pulse pressure, mmHg | 51.4 (15.0) | 49.5 (14.7) | 0.60 |
| Fasting sICAM-1 | 290.7 (86.9) | 326.7 (78.4) | 0.04 |
| Fasting sVCAM-1 | 423.5 (137.0) | 416.4 (114.2) | 0.95 |
| Fasting CRP | 2.9 (5.3) | 2.6 (2.9) | 0.72 |
| Fasting SAA | 6892.4 (10338.1) | 6018.3 (5208.3) | 0.74 |
| Post-menopausal | 13 (65%) | 32 (84%) | 0.10 |
| CES-D score | 8.23 (5.70) | 11.01 (7.54) | 0.15 |
| Number of prior day stressors | 1.2 (1.1) | 1.1 (1.2) | 0.56 |
| History of major depression | 3 (15%) | 14 (37%) | 0.08 |
Data shown are mean (SD) or N (%). Body Mass Index, blood pressure, CES-D, and number of stressors were measured at both study visits; data shown are aggregated across the two measurements per subject.
CES-D, Center for Epidemiological Studies Depression Scale; DXA, dual x-ray absorptiometry
Standardized Pre-Study Meals
Participants were instructed to avoid alcohol use the day before study visits, and any strenuous physical activity two days previously (44). They were also asked not to take aspirin, vitamins, antioxidants, or other dietary supplements for the 7 days before each admission.
On the day before each of the two study visits participants received three standardized meals from the CRC’s metabolic kitchen to reduce the variability associated with recent intake. Equations from the Dietary Reference Intakes calculated the total kcal requirements for each participant based on age, height, weight, and physical activity (45). Macronutrient targets (as percent of total energy) for these meals were 54.9 ± 2.68% carbohydrate, 27.6 ±2.13% fat, and 17.6 ± 0.95% protein. The fat content was 9.10 ± 1.20 % saturated fats, 9.43 ± 1.55% monounsaturated fats, and 7.26 ± 1.25% polyunsaturated fats. Participants ate their last meal no later than 7:30 pm the night before admission; the dinner was light and low in fat (44). Compliance was good: women consumed 91.83 ± 8.41% of these meals.
Research Meals
Both research meals included eggs, turkey sausage, biscuits, and gravy for a total of 930 kcals, with 60 grams fat, 59 grams carbohydrate, and 36 grams protein (percent of total kcals = 60, 25, 15, respectively). However, the saturated:unsaturated fatty acid ratio varied between the meals; the high saturated fat meal contained 16.84 g palmitic and 13.5 g oleic (ratio=1.93), compared to 8.64 g palmitic and 31.21 g oleic for the high oleic sunflower oil meal (ratio=0.67).
Assays
Serum CRP, serum amyloid A (SAA), sICAM-1 and sVCAM-1 were measured in duplicate with the Vascular Injury Panel 2 Multispot Kit on an MSD Imager 2400 (both Meso Scale Discovery, Rockville, MD), following kit instructions. Both samples for each subject were analyzed within the same plate, and all plates were from the same kit/lot. The intra-assay coefficient of variation (CV) and inter-assay CV for each analyte were: CRP 6.28% and 7.36%, SAA 4.90% and 3.09%, sICAM-1 4.19% and 6.92%, and sVCAM-1 2.14% and 5.16%.
Blood Pressure
Blood pressure was assessed using the Critikon Dinamap 1846 SX (GE, Tampa, FL) every 2 minutes for 20 minutes at baseline, and then every 2 minutes for 10 minutes immediately following the meal. Subsequently, 2 readings were taken 2 minutes apart every hour for the remainder of the visit.
Interview Data
The mood disorder modules of the Structured Clinical Interview for DSM-IV (SCID) provided data on lifetime prevalence of major depressive disorder (MDD) (46). SCID data showed that 29% (n=17) had met MDD criteria. The average time since diagnosis was 8.32 years (SD=10.94).
The Daily Inventory of Stressful Events (DISE) assessed the number of daily stressors within the last 24 hours (47). The transcribed interview responses were rated in a consensus meeting using the DISE’s extensive electronic “dictionary” which specifies codable events (47). In our sample 31 women reported at least one recent stressor at one visit, 21 at both visits, and 6 women reported no stressors.
Questionnaires
Following CRC admission participants completed several questionnaires. The Center for Epidemiological Studies Depression Scale (CES-D) assessed depressive symptomatology in the last week (48). The Community Healthy Activities Model Program for Seniors questionnaire (CHAMPS) assessed the weekly frequency and duration of various physical activities (49, 50). These measures have well-established reliability and validity (48–50).
Statistical methods
The primary analyses used linear mixed models, since these models allow explicit modeling of the within-subject correlations due to repeated study visits and repeated measurements during each visit.
Demographics and other pre-meal characteristics were compared across groups (cancer, control) using two-sample t-tests and chi-square tests for data collected at only one visit (e.g., age) and linear mixed models with a random subject effect for data collected at both visits (e.g., body mass index). Four primary outcome measures (CRP, SAA, sICAM-1, and sVCAM-1) were right-skewed and were natural-log transformed to better approximate normality of residuals. Linear mixed effects models were used to model post-meal outcome values, with the premeal (fasting) outcome measurement included as a covariate. All models included the three-way interaction of group (cancer, control) by meal type by time point and all lower-order terms to properly account for the design of the parent study. However, there were no significant effects of group (p > 0.2 for all tests) and thus reported results are averaged over group.
Of primary interest was the impact of prior day stressors and MDD history on post-meal responses. Initial models contained interactions of these predictors with time post-meal (categorical) and with meal type; nonsignificant interactions were removed for final models. All models further controlled on additional covariates to guard against confounding, including age, trunk fat, physical activity (caloric expenditure per week), and study visit (first vs. second). Additional models also controlled for menopausal status, but results were unchanged, thus final models did not include this factor. To capture the within-subject correlations within and across visits, all models included a pair of correlated subject-specific random meal effects, which allowed the magnitude of within-subject correlation for the two meal types to differ. The Kenward-Roger adjustment to the degrees of freedom was used to control type I error (51). All analyses were conducted in SAS version 9.3 (Cary, North Carolina).
Results
There were no associations between fasting levels of CRP, SAA, sICAM-1, or sVCAM-1 and either prior day stressors (p > 0.2 for all tests) or MDD history (p > 0.15 for all tests), after controlling for visit, age, trunk fat, and physical activity. Trunk fat was significantly positively associated with fasting levels of CRP (p<0.0001), SAA (p = 0.004), and sVCAM-1 (p = 0.05), and marginally associated with fasting sICAM-1 (p = 0.09). Each 1 kg increase in trunk fat was associated with a 12.7% increase in the geometric mean of CRP, a 5.0% increase in the geometric mean of SAA, a 1.0% increase in the geometric mean of sICAM-1, and a 1.2% increase in the geometric mean of sVCAM-1. Physical activity was marginally negatively associated with fasting SAA (p = 0.09), with a 100 kcal increase in caloric expenditure associated with a 1.3% decrease in the geometric mean of SAA. Both fasting sICAM-1 and fasting CRP were elevated at the first study visit compared to the second, with a 9.5% higher geometric mean (p<0.0001) for sICAM-1 and a 21% higher geometric mean for CRP (p = 0.06). Age and cancer status were not significantly associated with fasting levels of any outcome (p > 0.1 for all tests).
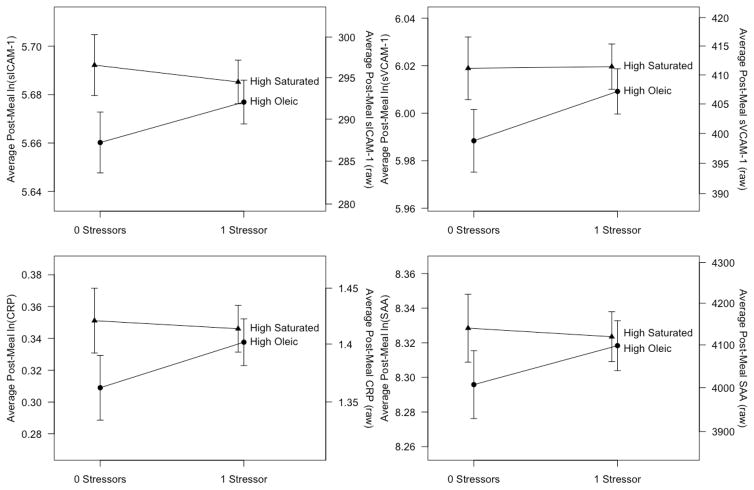
When modeling the postprandial responses, controlling for pre-meal levels, there were significant two-way interactions of prior day stressors by meal type in the models for CRP (p = 0.04), sICAM-1 (p = 0.02), and sVCAM-1 (p = 0.05), and a borderline interaction for SAA (p = 0.09). Thus the effect of stressors on post-meal inflammation depended on the meal type, and differences between the meals depended on a woman’s number of prior day stressors. A larger number of stressors was associated with higher levels of postprandial CRP, SAA, sICAM-1, and sVCAM-1 after the high oleic sunflower oil meal but not after the high saturated fat meal (Figure 1, Table 2). After the high oleic sunflower oil meal, each additional prior day stressor was associated with a 2.9% increase in the geometric mean of CRP, a 2.3% increase in the geometric mean of SAA, a 1.7% increase in the geometric mean of sICAM-1, and a 2.1% increase in the geometric mean of sVCAM-1. In contrast, after the high saturated fat meal there was no significant effect of stressors (Table 2). As shown in Figure 1, for a woman with no prior day stressors, post-meal inflammation was higher following the high saturated fat meal than the high oleic sunflower oil meal. But having prior day stressors shifted the response to the high oleic sunflower oil meal such that it looked like the response to the high saturated fat meal. There were no significant effects of MDD history on these outcomes (p > 0.07 for all tests).
Figure 1.
Mean post-meal sICAM-1, sVCAM-1, SAA, and CRP as a function of the number of prior day stressors and meal type. Results are estimates from linear mixed effects models controlling for pre-meal values, cancer status, visit, age, trunk fat, physical activity, and depression history. Bars are +/− 1 standard error.
Table 2.
Estimated changes in post-meal sICAM-1, sVCAM-1, CRP, and SAA for each additional prior day stressor, for each meal type. Estimates from linear mixed models controlling for cancer status, visit, age, trunk fat, physical activity, and depression history.
| High oleic sunflower oil | High saturated fat oil | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | 95% CI | P-value | Estimate | 95% CI | P-value | |
| sICAM-1 | 1.7% | (0.2%,3.1%) | 0.02 | −0.7% | (−2.1%,0.8%) | 0.34 |
| sVCAM-1 | 2.1% | (0.6%,3.6%) | 0.007 | 0.1% | (−1.5%,1.6%) | 0.93 |
| CRP | 2.9% | (0.6%,5.3%) | 0.02 | −0.5% | (−2.9%,2.0%) | 0.68 |
| SAA | 2.3% | (0.1%,4.5%) | 0.04 | −0.5% | (−3.0%,2.0%) | 0.69 |
There were no associations between pre-meal levels of SBP or DBP, and prior day stressors (p > 0.15 for all tests) or MDD history (p > 0.15 for all tests), after controlling for visit, age, trunk fat, and physical activity. Subjects with MDD history had marginally higher SBP (p = 0.06) and DBP (p = 0.10).
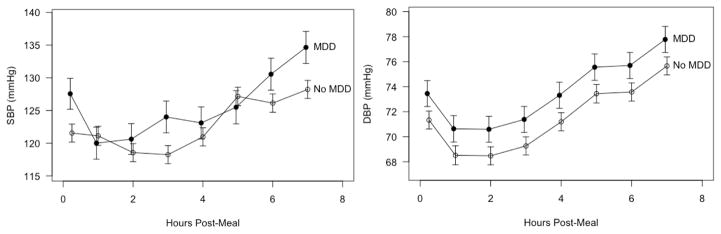
There were no significant effects of prior day stressors or meal type on the blood pressure outcomes (p > 0.25 for all tests). There was a significant main effect of MDD history on postprandial DBP (p = 0.05), even after controlling for pre-meal levels. In the post-meal period, DBP was higher for subjects with a history of MDD compared to those without (Figure 2). There was also a significant interaction between MDD history and time on postprandial SBP (p = 0.02) despite controlling for pre-meal levels. Subjects with a history of MDD had elevated SBP at most time points, particularly at around 3 hours and 6–7 hours post-meal (Figure 2).
Figure 2.
Post-meal trajectories of mean SBP and DBP as a function of major depression history. Results are estimates from linear mixed effects models controlling for pre-meal values, cancer status, visit, age, trunk fat, physical activity, and prior day stressors. Bars are +/− 1 standard error.
Discussion
This study is the first to demonstrate that stressors can heighten inflammatory responses to high oleic sunflower oil meals. Importantly, the stress- and depression-related metabolic responses to the two meals revealed adverse effects that were not evident when fasting. As expected, CRP, sICAM-1, and sVCAM-1 were higher following the saturated fat meal than the high oleic sunflower oil meal. However, a higher number of prior day stressors was associated with higher postprandial CRP, SAA, sICAM-1, and sVCAM-1 after the high oleic sunflower oil meal, but not after the high saturated fat meal. Putting it another way, for a woman with no prior day stressors, outcomes were higher after the saturated fat meal than after the sunflower oil meal. But if a woman had prior day stressors, the differences disappeared – because the stressors heightened responses to the sunflower oil meal, making it look more like the responses to the saturated fat meal.
The endothelial activation that was reflected by the heightened post-meal sICAM-1 and sVCAM-1 concentrations may also mirror a more chronic inflammatory responsiveness associated with atherosclerosis of brain arteries, a process that could itself promote depression (33, 52). Among adults with moderate to severe traumatic brain injury, higher levels of sICAM-1, sVCAM-1, and soluble Fas in cerebrospinal fluid had a 3.92-fold increase in the odds for developing posttraumatic depression at 6 months (53); indeed, having any one of these markers above the 75th percentile had a predictive value of 85.7% for posttraumatic depression at six months (53). Similarly, among patients treated with interferon-α for melanoma, sICAM-1 levels were correlated with the development of depressive symptoms during treatment (54). Postmortem studies of depressed older adults revealed elevated ICAM-1 expression in the dorsolateral prefrontal cortex compared to nondepressed controls (55, 56). ICAM-1 is thought to play a key role in blood-brain barrier (BBB) permeability, and enhanced levels of depressive symptoms may reflect greater BBB porousness (54). The fact that a Mediterranean diet offers some protection against the development of both depressive symptoms and depressive disorders (4, 57, 58) may be related to its anti-inflammatory actions (8, 9).
Women with an MDD history had higher post-meal blood pressure responses than those without a similar history, with no differences evident in the fasted state; MDD history was not related to the inflammatory responses. A meta-analysis of prospective cohort studies concluded that depression increases the risk of hypertension, an important risk factor for cardiovascular disease (59). Other research from this sample has demonstrated that women with an MDD history who had also experienced more prior day stressors had a higher peak postprandial triglyceride response than other participants (41). Larger post-meal triglyceride responses are reliably associated with enhanced cardiovascular risk and the progression of atherosclerosis (60–62). Depression has well-established effects on cardiovascular morbidity and mortality, and these two different meal-related changes highlight previously unrecognized depression-sensitive mechanistic pathways (63, 64).
Strengths of our study included control of both the composition and energy content of each woman’s diet on the day prior to admission as well as during admission. The only difference between the two challenge meals was the fat source. Both meals included 930 kcal and 60 g fat, values that are quite comparable to common fast food choices. For example, a Burger King Double Whopper with cheese has 990 kcal and 64 g fat, while a Big Mac cheeseburger and medium French fries contain 930 kcals and 58 g fat.
It is unclear why, with the addition of a stressor to the high saturated fat meal, there were no further increase in levels of CRP, sICAM-1 and sVCAM-1, while stress-related changes were observed in response to the high oleic sunflower oil meal. Speculatively, inflammatory markers may have already reached their ceiling level in response to the high saturated fat condition, a reasonable possibility because our participants did not have overt signs of cardiac disease or other diseases associated with chronically elevated inflammation.
Our two meals, regardless of their fat source, were high in energy and fat, and they included refined flour and little fiber. We do not know whether a relatively healthier meal (e.g., energy balanced, lower in total fat, higher in polyunsaturated oils and containing more protein and fiber with fewer simple carbohydrates) would attenuate the adverse postprandial inflammatory and metabolic changes, one limitation. Nonetheless, our study meals reflected typical everyday dietary choices because they were designed to mimic common fast food meals.
The inclusion of healthy breast cancer survivors could have affected our results, another limitation. However, we found no reliable post-meal differences between cancer survivors and controls in these analyses, or in other metabolic data from this study (41). We selected our healthy survivors using the same inclusion/exclusion criteria as our controls; had we used a larger and more representative sample of cancer survivors we might have seen group differences related to cancer treatment. Additionally, depressive symptoms did not differ between cancer survivors and controls, and the relatively low levels of depressive symptoms limited our ability to see depression-related effects.
These data are important because heightened inflammation characterizes a number of disorders and systemic diseases including cardiovascular disease, diabetes, metabolic syndrome, rheumatoid arthritis, asthma, multiple sclerosis, chronic pain, and psoriasis; each of these also features an elevated risk for depression (65, 66). Our data show how recent stressors and a depression history can reverberate through these metabolic alterations to fuel inflammation, which can, in turn, promote depression.
Acknowledgments
The study was supported in part by NIH grants CA154054, CA172296, UL1TRR025755, and CA016058. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We are grateful to Michael Di Gregorio, M.A., for his role as a key organizer and experimenter, and to Bryon Laskowski for laboratory analyses.
Footnotes
Conflicts of Interest
NIH has funded work by Drs. Kiecolt-Glaser, Malarkey, Fagundes, and Belury. Dr. Andridge and Ms. Peng declare no conflict of interest.
References
- 1.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: Depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075–91. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salas-Salvado J, Garcia-Arellano A, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, et al. Components of the mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008;62(5):651–9. doi: 10.1038/sj.ejcn.1602762. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Gonzalez MA, Dominguez LJ, Delgado-Rodriguez M. Olive oil consumption and risk of CHD and/or stroke: A meta-analysis of case-control, cohort and intervention studies. Br J Nutr. 2014;112(2):248–59. doi: 10.1017/S0007114514000713. [DOI] [PubMed] [Google Scholar]
- 4.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann Neurol. 2013;74(4):580–91. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 5.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292(12):1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 6.Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, et al. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation. 2008;117(2):169–75. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milaneschi Y, Bandinelli S, Penninx BW, Vogelzangs N, Corsi AM, Lauretani F, et al. Depressive symptoms and inflammation increase in a prospective study of older adults: A protective effect of a healthy (Mediterranean-style) diet. Mol Psychiatry. 2011;16(6):589–90. doi: 10.1038/mp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutrition Metabolism and Cardiovascular Diseases. 2014;24(9):929–39. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function-a systematic review and meta-analysis. Nutrients. 2015;7(9):7651–75. doi: 10.3390/nu7095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsheim E, Kien CL, Pearl WM. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metabolism. 2006;55(9):1215–21. doi: 10.1016/j.metabol.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillon NJ, Azizi PM, Li YE, Liu J, Wang C, Chan KL, et al. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am J Physiol Endocrinol Metab. 2015;309(1):E35–44. doi: 10.1152/ajpendo.00611.2014. [DOI] [PubMed] [Google Scholar]
- 12.Bellido C, Lopez-Miranda J, Blanco-Colio LM, Perez-Martinez P, Muriana FJ, Martin-Ventura JL, et al. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappab in peripheral blood mononuclear cells from healthy men. Am J Clin Nutr. 2004;80(6):1487–91. doi: 10.1093/ajcn/80.6.1487. [DOI] [PubMed] [Google Scholar]
- 13.Shikama Y, Aki N, Hata A, Nishimura M, Oyadomari S, Funaki M. Palmitate-stimulated monocytes induce adhesion molecule expression in endothelial cells via IL-1 signaling pathway. J Cell Physiol. 2015;230(3):732–42. doi: 10.1002/jcp.24797. [DOI] [PubMed] [Google Scholar]
- 14.Peairs AD, Rankin JW, Lee YW. Effects of acute ingestion of different fats on oxidative stress and inflammation in overweight and obese adults. Nutr J. 2011;10:122. doi: 10.1186/1475-2891-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacheco YM, Lopez S, Bermudez B, Abia R, Villar J, Muriana FJ. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J Nutr Biochem. 2008;19(3):200–5. doi: 10.1016/j.jnutbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Martinez P, Moreno-Conde M, Cruz-Teno C, Ruano J, Fuentes F, Delgado-Lista J, et al. Dietary fat differentially influences regulatory endothelial function during the postprandial state in patients with metabolic syndrome: From the LIPGENE study. Atherosclerosis. 2010;209(2):533–8. doi: 10.1016/j.atherosclerosis.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Silvestro A, Brevetti G, Schiano V, Scopacasa F, Chiariello M. Adhesion molecules and cardiovascular risk in peripheral arterial disease - soluble vascular cell adhesion molecule-i improves risk stratification. Thromb Haemost. 2005;93(3):559–63. doi: 10.1160/TH04-07-0440. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 19.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 20.Hoogeveen RC, Ballantyne CM, Bang H, Heiss G, Duncan BB, Folsom AR, et al. Circulating oxidised low-density lipoprotein and intercellular adhesion molecule-1 and risk of type 2 diabetes mellitus: The Atherosclerosis Risk in Communities study. Diabetologia. 2007;50(1):36–42. doi: 10.1007/s00125-006-0533-8. [DOI] [PubMed] [Google Scholar]
- 21.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–86. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Manson JE, Tinker L, Rifai N, Cook NR, Hu FB, et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007;56(7):1898–904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorand B, Baumert J, Chambless L, Meisinger C, Kolb H, Doring A, et al. Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle-aged men and women from the general population. Arterioscler Thromb Vasc Biol. 2006;26(2):398–405. doi: 10.1161/01.ATV.0000198392.05307.aa. [DOI] [PubMed] [Google Scholar]
- 24.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38(3):399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 25.Rohde LE, Hennekens CH, Ridker PM. Cross-sectional study of soluble intercellular adhesion molecule-1 and cardiovascular risk factors in apparently healthy men. Arterioscler Thromb Vasc Biol. 1999;19(7):1595–9. doi: 10.1161/01.atv.19.7.1595. [DOI] [PubMed] [Google Scholar]
- 26.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients. 2012;4(12):1989–2007. doi: 10.3390/nu4121989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hohmann CD, Cramer H, Michalsen A, Kessler C, Steckhan N, Choi K, et al. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine. 2015;22(6):631–40. doi: 10.1016/j.phymed.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72:365–9. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–2. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 30.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism for converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100(4):1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinz A, Hermann D, Smolka M, Rieks M, Gräf K-J, Pöhlau D, et al. Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: Implications for coronary heart disease. Psychopharmacology (Berl) 2003;165(2):111–7. doi: 10.1007/s00213-002-1244-6. [DOI] [PubMed] [Google Scholar]
- 32.von Kanel R, Abbas CC, Begre S, Saner H, Gander ML, Schmid JP. Posttraumatic stress disorder and soluble cellular adhesion molecules at rest and in response to a trauma-specific interview in patients after myocardial infarction. Psychiatry Res. 2010;179(3):312–7. doi: 10.1016/j.psychres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–7. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88(2):196–8. A7. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- 35.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men - the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2005;111(18):2299–305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 36.Tchalla AE, Wellenius GA, Sorond FA, Travison TG, Dantoine T, Lipsitz LA. Elevated circulating vascular cell adhesion molecule-1 (sVCAM-1) is associated with concurrent depressive symptoms and cerebral white matter hyperintensities in older adults. BMC Geriatrics. 2015:15. doi: 10.1186/s12877-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimopoulos N, Piperi C, Salonicioti A, Mitsonis C, Liappas L, Lea RW, et al. Elevation of plasma concentration of adhesion molecules in late-life depression. Int J Geriatr Psychiatry. 2006;21(10):965–71. doi: 10.1002/gps.1592. [DOI] [PubMed] [Google Scholar]
- 38.van Sloten TT, Schram MT, Adriaanse MC, Dekker JM, Nijpels G, Teerlink T, et al. Endothelial dysfunction is associated with a greater depressive symptom score in a general elderly population: The Hoorn study. Psychol Med. 2014;44(7):1403–16. doi: 10.1017/S0033291713002043. [DOI] [PubMed] [Google Scholar]
- 39.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555–61. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 40.Husky M, Mazure C, Maciejewski P, Swendsen J. Past depression and gender interact to influence emotional reactivity to daily life stress. Cognit Ther Res. 2009;33(3):264–71. [Google Scholar]
- 41.Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, et al. Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biol Psychiatry. 2015;77(7):653–60. doi: 10.1016/j.biopsych.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, et al. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology. 2015;52:239–50. doi: 10.1016/j.psyneuen.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy AP, Shea JL, Sun G. Comparison of the classification of obesity by BMI vs. Dual-energy x-ray absorptiometry in the Newfoundland population. Obesity. 2009;17(11):2094–9. doi: 10.1038/oby.2009.101. [DOI] [PubMed] [Google Scholar]
- 44.Lairon D, Lopez-Miranda J, Williams C. Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61(10):1145–61. doi: 10.1038/sj.ejcn.1602749. [DOI] [PubMed] [Google Scholar]
- 45.Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington, DC: National Academy Press; 2002. [DOI] [PubMed] [Google Scholar]
- 46.First M, Gibbon M, Spitzer R, Williams J. User’s guide for the Structured Clinical Interview for DSM-IV Axis I disorders—research version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 47.Almeida DM, Kessler RC. Everyday stressors and gender differences in daily distress. J Pers Soc Psychol. 1998;75:670–80. doi: 10.1037//0022-3514.75.3.670. [DOI] [PubMed] [Google Scholar]
- 48.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 49.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. Champs physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Harada ND, Chiu V, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–70. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–97. [PubMed] [Google Scholar]
- 52.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109–18. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute inflammatory biomarker profiles predict depression risk following moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2015;30(3):207–18. doi: 10.1097/HTR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer M, Horn M, Schmidt F, Schmid-Wendtner MH, Volkenandt M, Ackenheil M, et al. Correlation between sICAM-1 and depressive symptoms during adjuvant treatment of melanoma with interferon-alpha. Brain Behav Immun. 2004;18(6):555–62. doi: 10.1016/j.bbi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Thomas AJ, Ferrier IN, Kalaria RN, Davis S, O’Brien JT. Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulite cortex in major depression in the elderly. Br J Psychiatry. 2002;181:129–34. doi: 10.1017/s0007125000161847. [DOI] [PubMed] [Google Scholar]
- 56.Thomas AJ, Ferrier IN, Kalaria RN, Woodward SA, Ballard C. Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am J Psychiatry. 2000;157(10):1682–4. doi: 10.1176/appi.ajp.157.10.1682. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Majem LS, et al. Association of the Mediterranean dietary pattern with the incidence of depression: The Seguimiento Universidad de Navarra/University of Navarra Follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090–8. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- 58.Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: Results from a large community-based prospective study. Eur J Clin Nutr. 2013;67(1):75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- 59.Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. J Hypertens. 2012;30(5):842–51. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- 60.Pollin TI, Damcott CM, Shen HQ, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human apoc3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322(5908):1702–5. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boquist S, Ruotolo G, Tang R, Bjorkegren J, Bond MG, de Faire U, et al. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation. 1999;100(7):723–8. doi: 10.1161/01.cir.100.7.723. [DOI] [PubMed] [Google Scholar]
- 62.Teno S, Uto Y, Nagashima H, Endoh Y, Iwamoto Y, Omori Y, et al. Association of postprandial hypertriglyceridemia and carotid intima/media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23(9):1401–6. doi: 10.2337/diacare.23.9.1401. [DOI] [PubMed] [Google Scholar]
- 63.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 Suppl 2):S20–7. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak-the link between depression and cardiovascular disease. Nature Reviews Cardiology. 2012;9(9):526–39. doi: 10.1038/nrcardio.2012.91. [DOI] [PubMed] [Google Scholar]
- 65.Shelton RC, Miller AH. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91(4):275–99. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]