Abstract
Purpose
Phosphatidylinositol-3-kinase (PI3K)/AKT pathway activation is an important endocrine resistance mechanism in estrogen receptor positive (ER+) breast cancer. After promising preclinical modeling of MK-2206, an allosteric pan-AKT inhibitor, with either estrogen-deprivation or fulvestrant, we conducted a Phase 1 trial in patients with metastatic ER+HER2− breast cancer to determine the recommended phase II treatment dose (RPTD) of MK-2206 when combined with either anastrozole, fulvestrant, or anastrozole/fulvestrant.
Methods
ER+ breast cancer cell lines were exposed in vitro to MK-2206 plus estrogen-deprivation with or without fulvestrant and monitored for apoptosis. A standard 3+3 design was employed to first determine the maximum tolerated dose (MTD) of MK-2206 plus anastrozole based on cycle 1 toxicity. Each cycle was 28 days. The RPTD was determined based on toxicities observed at MTD level during the first 3 cycles. Subsequent patients received MK-2206, at the RPTD determined above, and fulvestrant or anastrozole/fulvestrant to define RPTD for these additional regimens.
Results
MK-2206 induced apoptosis in parental ER+ but not in long term estrogen deprived cell lines, for which fulvestrant was required for apoptosis induction. Thirty one patients enrolled. The RPTD was defined as MK-2206 150 mg PO weekly with prednisone prophylaxis for each combination. Grade 3 rash was dose limiting. 42% (95% CI: 23%–63%) patients derived clinical benefit without progression within 6 months. Response was not associated with tumor PIK3CA mutation.
Conclusion
MK-2206 plus endocrine treatments were tolerable. MK-2206 in combination with anastrozole is being further evaluated in a phase II neoadjuvant trial for newly diagnosed ER+HER2− breast cancer.
Keywords: Estrogen receptor positive breast cancer, endocrine resistance, AKT, MK-2206, PIK3CA
Introduction
Estrogen receptor positive (ER+) breast cancer accounts for the majority of the breast cancer mortality. The lack of an apoptotic response to standard treatment with chemotherapy (1) and endocrine agents (2) is thought to be an important mechanism of treatment resistance. Accumulating evidence indicates that the phosphatidylinositol-3-kinase (PI3K)/Akt signaling, which is crucial to many aspects of cell growth and survival (3), plays an important role in maintaining cell survival and resistance to endocrine therapy in ER+ breast cancer (4), likely as a result of frequent somatic mutations and epigenetic alterations in the pathway components. For example, mutations in PIK3CA, which encodes the alpha catalytic subunit of PI3K, is the most frequent somatic mutation in ER+ breast cancer (30 to 50%) (4, 5). In addition, lower frequency mutations in phosphatase and tensin homolog (PTEN) (2% to 4%), AKT1 (2% to 3%) and phosphatidylinositol 3-kinase regulatory subunit alpha (PIK3R1: 1% to 2%), have also been observed (4, 5). Furthermore, adaptation of ER+ breast cancer under long-term estrogen deprivation (LTED) is accompanied by up-regulation of PI3K pathway signaling, suppression of which inhibits cell proliferation (6). We previously demonstrated that inhibition of PI3K, either by targeted knockdown of the PIK3CA or pharmacologically using inhibitors of PI3K pathway, induced apoptosis of ER+ breast cancer in a manner that required simultaneous suppression of ER function. These data suggest a synthetic lethal interaction between ER and PI3K pathway targeting (7, 8), supporting the development of clinical strategies to simultaneously inhibitor ER and PI3K/Akt.
AKT, a serine/threonine kinase with 3 isoforms (AKT1, AKT2, and AKT3), is an important downstream target of the PI3K pathway that plays a key role in regulating cell survival, proliferation, growth, and glycogen metabolism (9–11). Increased AKT kinase activity, which signals activated PI3K, is detected in 20–55% of breast cancer specimens (12) and associated with relapse and death in ER+ breast cancer (13). MK-2206 is an allosteric inhibitor of AKT in clinical development for cancer therapeutics. It is highly potent against AKT1 (IC50, 5 nmol/L), AKT2 (IC50, 12 nmol/L), and AKT3 (IC50, 65 nmol/L) and has greater than 100-fold selectivity for AKT versus 256 other kinases (14). In vitro, MK-2206 inhibited auto-phosphorylation of AKT and prevents AKT-mediated phosphorylation of downstream targets (15). MK-2206 demonstrated anti-proliferative activity as a single agent and in combination with other agents in multiple human cancer cell lines including breast cancer (16). In previous Phase I trials MK-2206 was well tolerated in human and demonstrated evidence of AKT inhibition (17).
The goal of this study was to evaluate whether the addition of MK-2206 to endocrine therapy in combination with estrogen deprivation or fulvestrant, an ER down regulator, can induce apoptosis in ER+ breast cancer and to determine the recommended phase II treatment dose (RPTD) of MK-2206 in combination with common endocrine therapy regimens including anastrozole and fulvestrant (NCT01344031). As promising activity was observed for the combination of anastrozole and fulvestrant when compared to anastrozole alone in the first line endocrine therapy setting for patients with advanced ER+ breast cancer (18), we included anastrozole plus fulvestrant as a third endocrine regimen to be combined with MK-2206. To ensure treatment tolerability in future studies and in clinical practice, the RPTD was defined based on toxicities observed in the first 3 cycles rather than 1 cycle of treatments in this trial. Exploratory objectives of this trial included preliminary efficacy assessment and analysis of archival tumor specimens for predictors of response.
Material and Methods
Materials and cell lines for preclinical studies
MK-2206 was obtained through material transfer agreement with Merck & Co., Inc. (Kenilworth, NJ, USA). Fulvestrant (Sigma-Aldrich, St. Louis, MO, USA) and 17β-estradiol (Sigma-Aldrich) were from commercial sources. 17β-Estradiol was dissolved in ethanol. MK-2206 was dissolved in dimethylsulfoxide.
The HCC712 cell line was kindly provided by Dr. Adi Gazdar (19). Other cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA). All cell lines were used within 6 months upon receiving. No further authentication was performed in our laboratory. LTED MCF7, T47D, MDA-MB-415, and HCC712 cell lines were generated by culturing the parental lines for > 6 months in phenol red-free RPMI 1640 containing 5% charcoal stripped FBS (charcoal stripped serum (CSS); Invitrogen, Carlsbad, CA, USA), antibiotics and supplements containing 50 µg/mL gentamycin, pyruvate, 10 mM Hepes and glucose to 4.5 g/L (CSS medium).
In vitro Cell Culture, Western Blot, Apoptosis Assay, and Statistical analysis
Experiments with parental cell lines were performed with low passage number cells used within 2–3 months following revival from the supplier. Cell lines were propagated in RPM1 1640 containing 10% FBS with antibiotic and supplements (50 µg/mL gentamycin, pyruvate, 10 mM Hepes and glucose to 4.5 g/L) in a humidified 37°C incubator containing 5% CO2. Short term estrogen-deprivation was achieved by maintaining in CSS medium for 1–3 weeks prior to treatment with MK-2206. The method for protein extraction, immunoblotting, apoptosis assay, and statistical analysis for in vitro studies were described in our previous publication (8).
Patients
Eligible patients were postmenopausal females aged ≥ 18 years, with stage IV, measurable or evaluable ER+ breast cancer, ECOG performance status ≤ 1, adequate organ and marrow function, total bilirubin not above the institutional upper limit of normal (ULN), AST(SGOT)/ALT(SGPT) <2.5 × institutional ULN, and normal creatinine), fasting glucose ≤ 120 mg/dL and HbA1c ≤ 8%. Patients were allowed any number of prior regimens for the treatment of breast cancer. Patients with known brain metastases were eligible if stable ≥3 months after local therapy and were off steroid. Measurable or evaluable disease by RECIST was allowed. Patients with prior AKT inhibitor, disease progression on anastrozole (MK-2206 plus anastrozole cohort), fulvestrant (MK-2206 plus fulvestrant cohort), or both anastrozole and fulvestrant (MK-2207 plus anastrozole and fulvestrant cohort) or baseline QTcF >470 msec, uncontrolled intercurrent illness, medications or substances that are strong inhibitors or inducers of CYP 450 3A4, or chemotherapy or radiotherapy within 4 weeks were excluded. Medications that may cause QTc interval prolongation were avoided. The protocol was approved by Washington University Institutional Review Board. Informed consent was obtained from each patient. All race and ethnic groups were included. This trial was registered at ClinicalTrials.gov (NCT01344031).
Clinical trial study design and treatment
The phase I study started with the combination of MK-2206 PO weekly and anastrozole 1mg PO daily to determine the recommended phase II treatment dose (RPTD) of this combination. Three dose levels of MK-2206 (100, 150, 200 mg) were tested. Each cycle was 28 days. RPTD was defined as the highest dose level at which no more than 1 of 6 evaluable patients developed a dose limiting toxicity (DLT) within the first 3 cycles. To expedite study progress, a standard 3+3 phase I design was employed to first determine the maximum tolerated dose (MTD) based on cycle 1 toxicity. The MTD was defined as the highest dose level at which no more than 1 of 6 experienced a DLT during cycle 1. RPTD was reached if no more than 1 of the 6 patients treated at MTD experienced a DLT within the first 3 cycles. Otherwise dose de-escalation for MK-2206 would occur. The RPTD of MK-2206 defined in combination with anastrozole was then used as the starting dose of MK-2206 to treat 6 patients each with MK-2206 in combination with either fulvestrant or fulvestrant plus anastrozole to determine the RPTD for these regimens. For RPTD assessments, only patients who completed at least 3 cycles of therapy or those who developed a DLT within the first 3 cycles are considered evaluable. Patients who discontinued study therapy due to reasons other than toxicities (such as disease progression) prior to completing cycle 3 were replaced.
In this trial, DLT was defined as any grade 4 hematological event; uncontrollable hyperglycemia; any grade 3 or 4 non-hematological toxicity; grade 3 or 4 nausea, vomiting, or diarrhea; any intolerable grade 2 non-hematological or grade 3 hematological toxicity requiring a dose reduction; or a delay of MK-2206 or anastrozole for > 1 week during cycle 1 or > 3 total weeks during the first 3 cycles of therapy in RPTD determination.
Patients were evaluated for adverse events (AEs) weekly during the 1st cycle of therapy, then on day 1 of each subsequent cycle. Restaging per RECIST 1.1 was completed every 12 weeks or 3 cycles. Patients were continued on treatment until disease progression, unacceptable toxicity, or withdrawal of consent occurred.
Anastrozole was administered for at least 14 days prior to the first dose of MK-2206 on Day 1 cycle 1 to ensure full estrogen deprivation. Fulvestrant 500 mg was administered monthly following a loading dose of 500 mg on days 1 and 15 during cycle 1. Fulvestrant was administered for at least 28 days prior to the start of cycle 1 day 1 MK-2206. Prophylactic prednisone was administered to the 9th and subsequent patients enrolled in the study following a protocol amendment for rash prophylaxis. Prednisone was administered at a starting dose of 20mg PO daily for three days on the day before, the day of, and the day after each MK-2206. Prednisone dose was tapered as tolerated to the next lower dose level (10mg, 5mg, 0mg) every 4 or more weeks, or up escalated to 40mg if grade 2 or above rash was observed despite 20mg dose of prednisone.
Toxicity Management and Dose Modification
For grade 1 hyperglycemia, MK-2206 was to continue while initiating or to increase oral diabetic agents. For grade 2 hyperglycemia, MK-2206 was held while adjusting diabetic medications. MK-2206 was restarted at the same dose level when glucose level recovered to grade 1 or lower. For grade 3/4 hyperglycemia, consultation with an endocrinologist or other subspecialist was recommended to adjust diabetic medications. MK-2206 was held until hyperglycemia recovered to grade 1. If recovery was more than 7 days, MK-2206 was resumed at 1 dose level reduction.
For rash, if grade 1, patients continued the same dose of MK-2206 and prophylactic prednisone. If grade 2, patients continued MK-2206 with an increase in the dose of prophylactic prednisone to a higher dose level. If grade 3, MK-2206 was held and prednisone was administered as needed. MK-2206 was reduced by one dose level when rash resolved to grade 1 or lower unless MK-2206 was already at the lowest dose level. In addition, prophylactic prednisone dose was increased to the next higher dose level. Patient went off study if grade 3 rash developed despite maximum prophylactic prednisone (40mg). If the rash was grade 4, MK-2206 was discontinued permanently and a dermatologist was consulted to assist management.
For other unspecified AEs, if grade 2, MK-2206 was continued or held at physician’s discretion depending on the nature of the AE. If held, MK-2206 could be restarted at the initial dose or by one dose level reduction when AEs recovered to grade 1 or less. If grade 3, MK-2206 was held until symptoms resolved to grade 1 or lower. MK-2206 was reduced by one dose level when treatment resumed. If grade 4, MK-2206 was discontinued. Resumption of MK-2206 at one dose level reduction could be considered if a patient was deriving benefit from therapy and the grade 4 AE was transient and recovered to no more than grade 1 and unlikely to recur with retreatment.
Archival tumor DNA sequencing
Tumor DNA, extracted from archival formalin fixed paraffin embedded (FFPE) tumor specimens using QIAamp DNA FFPE Tissue Kit (Qiagen, cat# 56404), and matched leukocyte germ-line DNA were subjected to targeted Illumina next generation sequencing by 2×100 paired end reads of an 83-gene panel as described (20) at McDonnell Genome Institute at Washington University. Sequencing analysis was performed as previously described (21–27).
Results
Preclinical data supporting synergistic apoptotic effect of MK-2206 in combination with hormonal therapy
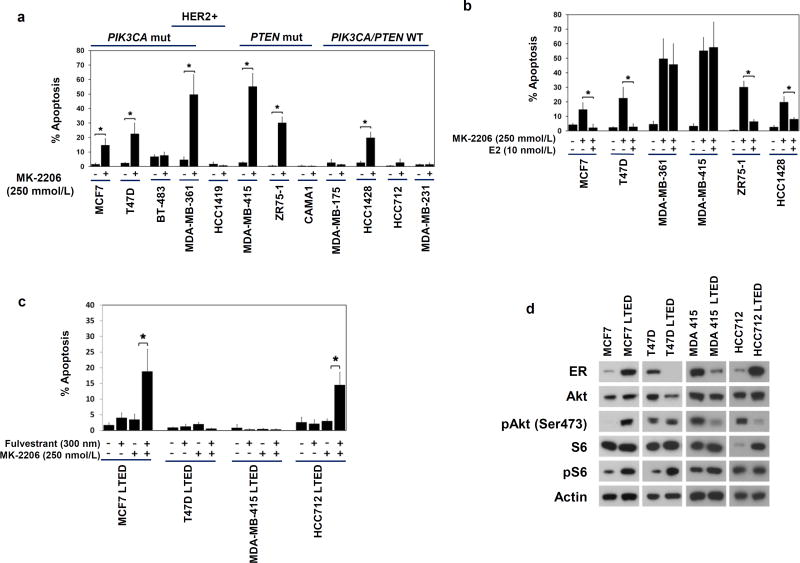
To investigate the apoptotic effect of MK-2206 on ER+ breast cancer, we performed in vitro studies using a panel of ER+ breast cancer cell lines with diverse genetic background in the absence or presence of estradiol (Fig. 1). Although HCC1428, which has normal PIK3CA and PTEN, exhibited sensitivity to MK-2206, majority of the sensitive cell lines carried mutations in PIK3CA or PTEN or harbored HER2 amplification (Fig. 1a). Estrogen deprivation was required for apoptotic induction by MK-2206 in majority of the cell lines tested (Fig. 1b), prompting a clinical investigation of MK-2206 in combination with aromatase inhibitors (AI) in patients with ER+ breast cancers.
Fig. 1. MK-2206 induced apoptosis in ER+ breast cancer cells in vitro.
a, MK-2206 induced apoptosis in ER+ breast cancer cell lines. Mutations in PIK3CA and PTEN, and HER2 amplification were indicated. Cells cultured under estrogen-deprived conditions for 1–3 weeks were treated with MK-2206 250 nmol/L, followed by apoptosis assay by TUNEL. The ER- cell line MDA-MB-231 served as a negative control. b, Estrogen suppressed apoptotic induction by MK-2206 in some ER+ breast cancer cell lines. Cells cultured without or with 10 nmol/L estradiol (E2) were treated with MK-2206 250nmol/L for 4 days followed by apoptosis assay. c, LTED ER+ cell lines were resistant to MK-2206 but sensitive to fulvestrant plus MK-2206 in 2 of the 4 lines tested. ER+ LTED cells were pre-treated with fulvestrant for 3 days prior to the addition of MK-2206 for 4 days followed by apoptosis assessment. Results were from at least 3 replicates for each treatment condition. Significant activation of apoptosis was indicated (*, p<0.05). d, Western Blot of ER and PI3K pathway markers for LTED and parental ER+ cell lines cultured under estrogen deprivation.
To investigate the effect of MK-2206 on AI resistant ER+ breast cancer, long term estrogen deprived (LTED) lines derived from MCF7, T47D, MDA-MB415, and HCC712, were treated with MK-2206 in the presence or absence of fulvestrant (Fig. 1c). Neither agent alone was effective in inducing apoptosis, however combination treatment induced significant apoptosis in 2 of the 4 lines, including MCF7 LTED and HCC712 LTED. In MDA-MB415 LTED and T47D LTED, which were not responsive to the combination, ER levels were low and PI3K activity was not enhanced in the LTED compared to the corresponding parental cell lines (Fig. 1d). These data indicated that MK-2206 in combination with fulvestrant could overcome endocrine resistance in some AI resistant ER+ breast cancer justifying a clinical trial in the second or later line endocrine therapy setting.
Patients Characteristics, Dose Limiting Toxicities, and Recommended Phase II Treatment Dose
Thirty one patients were enrolled in the study at three dose levels of MK-2206 (Table 1). One patient in the MK-2206/Fulvestrant cohort withdrew consent following 1 dose of MK-2206, and therefore was excluded from analysis. Table 2 shows the clinical characteristics of the remaining 30 patients. All patients had ER+HER2− disease. The phase I trial started with MK-2206 150mg PO weekly (dose level 1) in combination with anastrozole, which resulted in a DLT (grade 3 rash) in 2 of 5 patients, leading to dose de-escalation to dose level −1 (MK-2206 100mg), at which 1 of 3 patients experienced a DLT (grade 3 rash). The rashes were typically pruritic and erythematous maculopapular in appearance that occurred post the 2nd dose of MK-2206 and commonly resolved within 1 week in response to prednisone (Suppl. Fig. 1a). Antihistamines were not as successful in treating the rash. Biopsy of the rash in one patient revealed perivascular lymphocytic infiltrate with eosinophils, consistent with hypersensitivity dermatitis (Suppl. Fig. 1b). Because of the exquisite sensitivity of the MK-2206 induced rash to prednisone, the protocol was subsequently amended to include prophylactic prednisone 20mg PO for 3 days, administered on the day before, the day of, and the day after each MK-2206 dose (starting with the 9th patient enrolled to the study). With prophylactic prednisone, 0 of 6 patients experienced a DLT with MK-2206 150mg weekly in combination with anastrozole, however, both patients treated at the next higher dose level of MK-2206 (200mg) experienced a DLT (grade 3 rash) despite prophylactic prednisone. Therefore dose level 1, MK-2206 150mg weekly with prophylactic prednisone, was defined as the MTD. All 6 patients at the MTD dose of MK-2206 in combination with anastrozole were evaluable for RPTD assessment, and none experienced any DLT within the first 3 cycles of treatment. Therefore RPTD was defined as MK-2206 150mg PO weekly with prophylactic prednisone (20mg PO daily on the day before, the day of, and the day after each MK-2206 dose) when combined with anastrozole. Eight patients were then enrolled to received MK-2206 at this dose level with prophylactic prednisone and fulvestrant, among whom 6 were evaluable for RPTD assessment (1 patient withdrew consent after 1 dose of MK-2206, 1 patient discontinued study drug therapy before completing 3 cycles of therapy due to disease progression). Additionally, seven patients received MK-2206 at this dose level with prophylactic prednisone, in combination with anastrozole plus fulvestrant, among whom 6 were evaluable for RPTD assessment (1 patient discontinued study drug therapy before completing 3 cycles of therapy due to disease progression). DLT (grade 3 rash) was observed in 1 of the 6 evaluable patients in each of these two subsequent cohorts. Therefore MK-2206 150mg PO weekly with prophylactic prednisone (20mg PO daily on the day before, the day of, and the day after each MK-2206 dose) was also defined as the RPTD when combined with fulvestrant, or the combination of anastrozole and fulvestrant.
Table 1.
Patient Enrollment and Dose Limiting Toxicities (DLT)
| Cohort | Dose Level | N | MK-2206 (mg) | Hormonal therapy | N (DLT)** |
|---|---|---|---|---|---|
| MK-2206 and anastrozole | 1* | 5 | 150 | Anastrozole | 2 |
| −1 | 3 | 100 | Anastrozole | 1 | |
| 1# | 6 | 150 | Anastrozole | 0 | |
| 2# | 2 | 200 | Anastrozole | 2 | |
| MK-2206 and fulvestrant | 1# | 8a, b | 150 | Fulvestrant | 1 |
| MK-2206 and anastrozole plus fulvestrant | 1# | 7b | 150 | Anastrozole plus Fulvestrant | 1 |
Starting dose
All DLTs were Grade 3 rash
1 patient withdrew consent after 1 dose of MK-2206
1 patient discontinued study drug therapy within 3 cycles due to disease progression.
Prednisone 20mg PO daily for 3 days: the day before, the day of, and the day after each MK-2206.
Table 2.
Patient Characteristics
| Patients (N = 30) | ||
|---|---|---|
|
|
||
| Characteristic | No. | % |
| Age, years | ||
| Median | 55 | |
| Range | 32–79 | |
| Duration on therapy (cycles) | ||
| Median | 5.8 | |
| Range | <1 – 46 | |
| Prior endocrine therapy in adjuvant setting | ||
| Yes | 15 | 50% |
| No | 15 | 50% |
| Endocrine therapy in metastatic setting | ||
| Median | 1 | |
| Range | 0 – 5 | |
| No. prior chemotherapy regimens in metastatic setting | ||
| Median | 0.5 | |
| Range | 0 – 3 | |
| Visceral involvement | ||
| Yes | 19 | 63% |
| No | 11 | 37% |
| Reason off study | ||
| Progressive disease | 20 | 67% |
| Adverse event | 5 | 17% |
| Physician decision (non-compliance) | 1 | 3% |
| Treatment Ongoing | 4 | 13% |
Adverse Events
All 30 patients were evaluable for AE. Treatment was well tolerated (Table 3). The most common all-cycle treatment related grade 2 and above AEs included rash (33.3%), hyperglycemia (20%), hypophosphatemia (16.7%), and fatigue (10%). Despite prophylactic prednisone, the incidence of grade 2 and above hyperglycemia was low: 13.3% (6% grade 3) during cycle 1 and 20% (7% grade 3) in all cycles. There was no grade 4 hyperglycemia. Only 1 grade 4 AE (asymptomatic hypophosphatemia). No grade 5 toxicities were observed in this trial. The 3-cycle AE profile was similar to cycle 1 AE, with slight increased incidence of hyperglycemia, hypophosphatemia, fatigue, diarrhea and reduced lymphocyte counts. Five patients (4 patients in cycle 1 and 1 patient in cycle 3) discontinued study drug therapy due to rash.
Table 3.
Grade 2 and above AE (> 5% incidence) at least possible related to study drug
| Cycle 1 | ||||
| AE (Adverse event) | Grade 2 | Grade 3 | Grade 4 | N (%) |
| Rash | 2 | 6 | 0 | 8 (26.7%) |
| Hyperglycemia | 2 | 2 | 0 | 4 (13.3%) |
| All Cycles | ||||
| AE (Adverse event) | Grade 2 | Grade 3 | Grade 4 | |
| Rash | 3 | 7 | 0 | 10 (33.3%) |
| Hyperglycemia | 4 | 2 | 0 | 6 (20.0%) |
| Hypophosphatemia | 2 | 2 | 1 | 5 (16.7%) |
| Fatigue | 3 | 0 | 0 | 3 (10.0%) |
| Diarrhea | 2 | 0 | 0 | 2 (6.7%) |
| Lymphocyte reduced | 1 | 1 | 0 | 2 (6.7%) |
Anti-tumor Activity
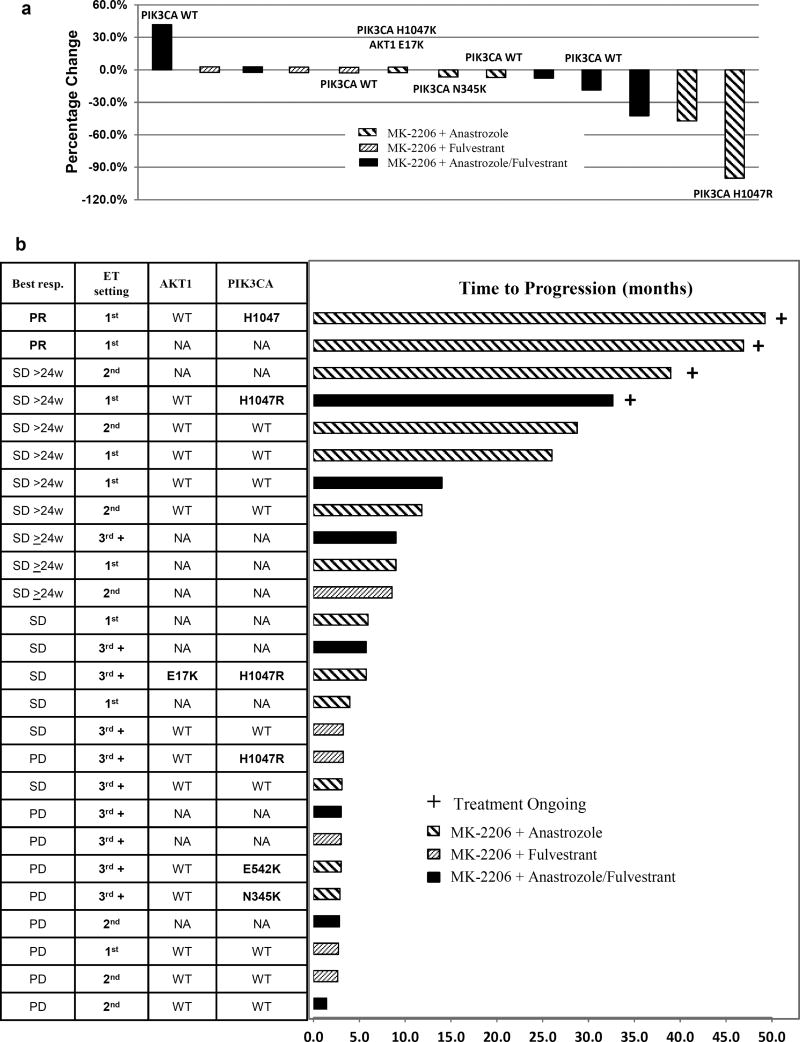
Four patients discontinued study drug therapy within the first cycle of therapy due to adverse events (grade 3 rash), therefore were not evaluable for response (Table 4). Among the 30 intent to treat patient population, the clinical benefit rate (CBR) was 36.7% (95% CI: 20% – 56%), including 2 partial response (PR) and 9 stable disease (SD) ≥ 6 months. Among the 26 patients evaluable for clinical benefit, the median time to progression (TTP) was 5.8 months (inter-quantile range (IQR): 3 – 14 months) and the CBR was 42% (95% CI: 23% – 63%). Among the 13 patients with measurable disease, the overall response rate was 15.4%. The Waterfall plot depicting individual patient response by the percentage change in the sum of the longest diameter of target lesions at best response compared to the baseline is shown in Figure 2a.
Table 4.
Summary of Antitumor Response
| Best Response | N | % |
| Partial response | 2 | 7.7% |
| Stable disease | 57.7% | |
| ≥ 6 months | 9 | |
| < 6 months | 6 | |
| Progressive disease | 9 | 34.6% |
| Not evaluable | 4 | |
| Total | 30 | 100% |
| Clinical Benefit Rate: 11 of 26 evaluable patients (42 %, 95% CI: 23% – 63%) | ||
Fig. 2. Anti-tumor activity.
a, Waterfall plot of tumor diameter change at best response
Percentage change in the sum of the longest diameter of target lesions at best response compared to that at baseline was plotted for patients with measurable disease. Treatment cohort was denoted by the color of the bar graph. PIK3CA mutation status and AKT1 mutation were annotated if available.
b, Individual patient response
This figure shows individual patient response, TTP and tumor AKT1 and PIK3CA mutation status for the 26 evaluable patients. TTP was defined as the duration between the start of treatment to time of progression or off study. TTP was censored to May 10, 2015 in patients continued to receive therapy as of that day.
TTP was further analyzed according to other clinical variables including prior therapy, presence or absence of visceral metastasis, or treatment induced hyperglycemia, none of which led to statistically significant differences in TTP (data not shown). There was a trend toward longer TTP (14 months, IQR: 5.9 months – not reached, vs 3.3 months, IQR: 3 – 9 months, p=0.076) in patients who received study drug as first line endocrine therapy (n=9), defined as no prior ET in the metastatic setting or receiving study drug at recurrence following at least 12 months after completion of adjuvant endocrine therapy. The CBR of the study treatments was 67% (6 of 9), 57% (4 of 7), 10% (1 of 10), respectively when administered in the first, 2nd and the third line or above setting (Suppl. Table S1).
Mutation analysis of archival tumors
Archival tumor specimens were available for next generation sequencing analysis of an 83-gene panel from 16 patients who were evaluable for response (primary site, n=6; metastatic site, n=9). Notable PI3K pathway genes identified with mutations included PIK3CA (n=6, 37.5%) and AKT1 E17K (n=1, 6.3%) (Figure 2b). In this small sample set, PIK3CA mutation status did not obviously associate with TTP (Figure 2b). The AKT1 E17K mutation occurred concomitantly with PIK3CA H1047R in a patient who had a best response of stable disease and went off study due to disease progression at 6 months. Other mutations were rare, which included HER2 S310F mutation identified in the tumor from a patient who did not respond to study drug therapy (Figure 2b).
Discussion
We demonstrated that MK-2206 induced apoptosis in a panel of ER+ breast cancer cell lines, majority of which required estrogen deprivation to elicit this response. Although the LTED cell lines were resistant to apoptotic induction by MK-2206 or fulvestrant alone, the combination of MK-2206 and fulvestrant resulted in significantly increased apoptosis. These results were in line with our prior observations with PI3K inhibitors in ER+ breast cancer (7, 8) and provided the preclinical rationale for the clinical evaluation of MK-2206 in ER+ breast cancer.
We successfully determined the RPTD of MK-2206 in combination with anastrozole, or fulvestrant, or anastrozole plus fulvestrant being 150mg PO weekly, with prednisone prophylaxis. The combination regimens were well tolerated, with common grade 2 and above AEs being rash and hyperglycemia, which were observed in previous studies of MK-2206 (17, 28). Grade 3 rash appeared at lower dose levels of MK-2206 in our trial compared to the single agent MK-2206 phase I study (28). It is unclear whether this was related to the patient population or the concomitant administration of endocrine therapy agents. However, there is no theoretical pharmacokinetic interaction between MK-2206 and anastrozole or fulvestrant. We demonstrated that prednisone prophylaxis could reduce the incidence of rash, allowing chronic drug administration. Although the mechanism of MK-2206 induced rash is not fully understood, pathology of skin biopsy was consistent with hypersensitivity reaction.
In this phase I study in patients with metastatic ER+HER2− breast cancer and heterogeneous treatment histories, MK-2206 in combination with standard endocrine therapy agent(s) induced a clinical benefit rate of 36.7% and 42% in the intent to treat and evaluable patient populations, respectively, including 2 with PR and 9 with SD lasting for ≥ 6 months. There was a trend toward improved response when the study drugs were administered at earlier treatment settings (CBR: 67%, 57%, 10% ; TTP: 14, 8.6, 3.3 months, in the 1st, 2nd and the 3rd line and above endocrine treatment setting, respectively). Majority of the patients (7 out of 9) treated in the first line setting in this trial received MK-2206 and anastrozole combination. As single agent anastrozole resulted in a CBR in the range of 60% and TTP of 11–13 months in the first line endocrine treatment setting for metastatic ER+ breast cancer in previous randomized trials (29, 30), the efficacy data observed in this trial (CBR: 67%, TTP: 14 months) was at least comparable. The 2nd line activity (CBR: 57%, TTP: 8.6 months) observed in this trial was numerically similar to that of everolimus in combination with exemestane (progression free survival: 6.9 – 10.6 months) rather than exemestane alone (PFS: 2.8 – 4.1 months) in BOLERO 2 trial (31), suggesting potential benefit with the addition of MK-2206 in the second line setting. Although limited by the small sample size, the overall impression from the study is that the activity of MK-2206 was modest with the dose and the patient population studied.
The lack of striking tumor regression or disease control beyond what might be expected from standard endocrine therapy could be explained by several factors. The first is that we may not have reached a high enough dose of MK-2206 because of the dose-limiting hypersensitivity rash. The MTD of MK-2206 defined in this trial was lower than that for single agent MK-2206. A second possibility is that patients enrolled in the trial harbored tumors with resistance mechanisms not addressed by MK-2206 inhibition. We were able to retrospectively identify one case in our study with an E17K AKT1 mutation, but this subject did not experience an extreme response of the type described for patients with mTOR gene mutations treated with everolimus (32). To investigate the biomarker effect and anti-tumor activity of MK-2206 in combination with anastrozole treatment naïve tumors, a phase II study in the neoadjuvant setting is being conducted in patients with clinical stage II or III ER+HER2− breast cancer (NCT01776008). Results of this trial are expected to further our understanding regarding the mechanisms of action of this combination therapy. A third possibility is that our preclinical models based on established breast cancer cell lines were misleading. While response to PI3 Kinase pathway inhibition in preclinical models did appear to associate with PIK3CA and other PI3 kinase pathway mutations, in the clinical setting these relationships have not been consistently observed (33–37). Clearly the in vivo growth requirement of ER+ tumors, many of which do not generate cell lines, may be quite different from the in vitro cell culture drug sensitivity studies.
A number of new agents targeting the PI3K pathway are in clinical development, many of which directly target the PI3 kinase isoforms themselves. Although the pan class IA PI3K inhibitor pictilisib did not improve the progression free survival (PFS) when combined with fulvestrant for the treatment of aromatase inhibitor resistant metastatic ER+ HER2− breast cancer in the FERGI phase II trial (37), the dose of pictilisib might not have been optimal due to frequent dose reductions as a result of skin and gastrointestinal toxicities. Buparlisib, another pan PI3K inhibitor, plus fulvestrant, has been examined in a phase III trial in postmenopausal women with ER+HER2− locally advanced or metastatic breast cancer progressed on/after an aromatase inhibitor (BELLE-2: NCT01610284). Recently reported at the 2015 San Antonio Breast Cancer Symposium, BELLE-2 met its primary endpoint for PFS improvement in the full population [6.9 (95% CI 6.8–7.8) months in the buparlisib plus fulvestrant arm (n=576) versus 5.0 (95% CI 4.0–5.2) months in the placebo plus fulvestrant arm (n=571); HR 0.78 (95% CI 0.67–0.89), one-sided p value <0.001)] (35). Although the PFS improvement was not statistically significant in the PI3K activated group defined by PIK3CA mutation and/or loss of PTEN expression in archival tumor tissues, pre-planned evaluation of PIK3CA mutation in circulating tumor DNA (ctDNA) in a subgroup of 587 patients demonstrated a significant improvement in PFS [7.0 versus 3.2 months; HR 0.56 (95% CI 0.39–0.80), one-sided p value <0.001] and overall response rate (18.4% vs 3.5%) with the addition of buparlisib in the ctDNA PIK3CA mutant population but not in the non-mutant population. This data suggests that targeting of the PIK3CA mutant kinase might indeed be most effective. It was worth noting that AEs, including transaminitis, hyperglycemia, rash, and mood disorders, were common, leading to buparlisib dose reduction and interruptions in 46% and 56% of the patients, respectively, which may have limited its efficacy in the BELLE-2 trial. Several alpha specific PI3K inhibitors are in clinical development with the hope for more potent inhibition of PIK3CA and tolerable toxicity profiles (38).
Supplementary Material
Statement of Translational Relevance.
In this study we demonstrated that MK-2206, an allosteric pan-AKT inhibitor in clinical development, induced apoptosis of estrogen receptor positive (ER+) breast cancer cells under estrogen deprived condition or when combined with the ER down-regulator fulvestrant in the preclinical setting. We therefore conducted a Phase 1 trial of MK-2206 in combination with either anastrozole, or fulvestrant, or anastrozole plus fulvestrant, in patients with metastatic ER+ HER2− breast cancer and determined the recommended phase II treatment dose (RPTD). In this heterogeneous patient population, forty two percent (11 of 26) evaluable participants derived clinical benefit without disease progression for at least 6 months. Results of these studies led to an ongoing phase II trial of MK-2206 in combination with anastrozole for ER+ HER2− breast cancer in the neoadjuvant setting to further define its therapeutic potential in a treatment naïve population.
Acknowledgments
This study was funded in part by the Siteman Cancer Center, Fashion Footwear Association of New York, the Mayo PIIC (N01-CM-2011-00099) and Cancer Clinical Investigator Team Leadership Award awarded by the National Cancer Institute though a supplement to P30CA091842 (to C. Ma). We wish to thank the patients and their families for participation in this study. We would like to acknowledge the McDonnell Genome Institute for DNA sequencing and analysis, Ms. Shana Thomas for editorial assistance of the manuscript, and Ms. Jing Han for tumor specimens collection, and clinical research and regulatory coordinators.
Footnotes
Conflict of Interest Statement: Authors disclose no conflict of interest
Presented in part at the 2014 Annual meeting for the American Society of Clinical Oncology
References
- 1.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–54. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin. Cancer Res. 2006;12:1024s–30s. doi: 10.1158/1078-0432.CCR-05-2127. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Ma CX, Crowder RJ, Ellis MJ. Importance of PI3-kinase pathway in response/resistance to aromatase inhibitors. Steroids. 2011;76:750–2. doi: 10.1016/j.steroids.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer research. 2009;69:3955–62. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez CG, Ma CX, Crowder RJ, Guintoli T, Phommaly C, Gao F, et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011;13:R21. doi: 10.1186/bcr2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 10.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 12.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 13.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–46. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 14.Yan L. Abstract #DDT01-1: MK-2206: A potent oral allosteric AKT inhibitor. AACR Meeting Abstracts. 2009 Abstract: DDT01-1. [Google Scholar]
- 15.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin. Cancer Res. 2012;18:5816–28. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 17.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin. Oncol. 2011;29:4688–95. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. New Engl. J. Med. 2012;367:435–44. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wistuba II, Behrens C, Milchgrub S, Syed S, Ahmadian M, Virmani AK, et al. Comparison of features of human breast cancer cell lines and their corresponding tumors. Clin. Cancer Res. 1998;4:2931–8. [PubMed] [Google Scholar]
- 20.Griffith OL, Griffith M, Luo J, Hundall J, Miller CA, Larson DE, et al. Prognostic effects of gene mutation in estrogen receptor positive breast cancer. San Antonio Breast Cancer Symposium; 2014. Abstract S1-02. [Google Scholar]
- 21.Griffith M, Griffith OL, Smith SM, Ramu A, Callaway MB, Brummett AM, et al. Genome Modeling System: A Knowledge Management Platform for Genomics. PLoS Comput Biol. 2015;11:e1004274. doi: 10.1371/journal.pcbi.1004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson DE, Harris CC, Chen K, Koboldt DC, Abbott TE, Dooling DJ, et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics. 2012;28:311–7. doi: 10.1093/bioinformatics/btr665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–76. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28:1811–7. doi: 10.1093/bioinformatics/bts271. [DOI] [PubMed] [Google Scholar]
- 27.Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25:2730–1. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yap TA, Yan L, Patnaik A, Tunariu N, Biondo A, Fearen I, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin. Cancer Res. 2014;20:5672–85. doi: 10.1158/1078-0432.CCR-14-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin. Oncol. 2000;18:3758–67. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 30.Robertson JF, Lindemann JP, Llombart-Cussac A, Rolski J, Feltl D, Dewar J, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized 'FIRST' study. Breast Cancer Res Treat. 2012;136:503–11. doi: 10.1007/s10549-012-2192-4. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. New Engl. J. Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. Genome Sequencing Identifies a Basis for Everolimus Sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hortobagyi GN, Piccart-Gebhart MJ, Rugo HS, Burris HA, Campone M, Noguchi S, et al. Correlation of molecular alterations with efficacy of everolimus in hormone receptor–positive, HER2-negative advanced breast cancer: Results from BOLERO-2. Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. 2013. Abstract: LBA509. [Google Scholar]
- 34.Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. Stand up to cancer phase ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2014;32:1202–9. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baselga J, Im S-A, Iwata H, Clemons M, Ito Y, Awada A, et al. PIK3CA status in circulating tumor DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resistant HR+/HER2– advanced breast cancer (BC): First results from the randomized, phase III BELLE-2 trial. 2015 San Antonio Breast Cancer Symposium; San Antonio, Texas. 2015. Abstract: S6-01. [Google Scholar]
- 36.Ma CX, Luo J, Naughton M, Ademuyiwa FO, Suresh R, Griffith M, et al. A phase 1 trial of BKM120 (Buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor positive metastatic breast cancer. Clin. Cancer Res. 2015 Nov 12; doi: 10.1158/1078-0432.CCR-15-1745. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krop I, Johnston S, Mayer IA, Dickler M, Ganju V, Forero-Torres A, et al. The FERGI phase II study of the PI3K inhibitor pictilisib (GDC-0941) plus fulvestrant vs fulvestrant plus placebo in patients with ER+, aromatase inhibitor (AI)-resistant advanced or metastatic breast cancer – Part I results. 2014 San Antonio Breast Cancer Symposium; San Antonio, Texas. 2014. Abstract: S2-02. [Google Scholar]
- 38.Ma CX. The PI3K Pathway as a Therapeutic Target in Breast Cancer. American Journal of Hematology / Oncology. 2015 Mar; http://www.gotoper.com/publications/ajho/2015/mar/the-pi3k-pathway-as-a-therapeutic-target-in-breast-cancer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.