Abstract
Severe knee trauma, such as an ACL disruption, produces aggrecan degradation as evidenced by elevated synovial fluid (SF) N-terminal (393) Alanine–Arginine–Glycine–Serine (ARGS) neoepitope (or ARGS-aggrecan) and is associated with inflammatory activity soon after injury. However, it is not known if this process persists for a substantial time interval following the initial trauma. The purpose of this study was to evaluate relationships between SF ARGS concentrations and an array of cytokines, matrix metalloproteases (MMPs), and tissue inhibitor of metalloproteases (TIMPs) during the initial 6 months following ACL rupture. SF samples from 67 ACL-injured subjects (29 women) were analyzed within 6 months of injury (18–155 days), immediately prior to surgical ACL reconstruction. Relationships between ARGS and individual analyte concentrations, as well as MMP/TIMP ratios were evaluated. Statistically significant relationships were found between ARGS and basic fibroblast growth factor (FGF2) (p = 0.03) and TIMP-3 (p = 0.01). Our findings suggest that FGF2, considered to be primarily catabolic in articular cartilage, is not downregulated as ARGS concentration declines over time since injury. In addition, these results support the hypothesis that an upregulation of TIMP-3, the primary aggrecanase inhibitor, is elicited in response to increased aggrecan degradation, which may inhibit further cleavage.
Keywords: ARGS, ACL, knee trauma, cytokine, MMP
There is an increased risk of Post-Traumatic Osteoarthritis (PTOA) following significant knee trauma, such as rupture of the Anterior Cruciate Ligament (ACL), regardless of treatment.1–11 Mechanical stresses incurred at the time of the index injury may result in structural and biochemical changes within the tibiofemoral joint,1,4,12,13 causing an acute inflammatory response and altered cartilage biology.14,15 The resulting imbalance in articular cartilage homeostasis and physiologically driven chondrodestructive events may be associated with the onset of PTOA through numerous mechanisms.14–22 Unfortunately, by the time patients suffering from these injuries become symptomatic, irreversible damage to the articular cartilage and surrounding tissues has likely occurred. Consequently, much attention has been devoted to the characterization of the early localized physiological events that occur following joint trauma, in hopes of identifying promising prognostic markers of PTOA onset and early disease progression23–26 that may serve as therapeutic targets for early intervention to prevent future articular cartilage degradation.13,24–28
The onset of OA includes deleterious physiologic changes within the articular cartilage, and it is believed that one of the initial events involves degradation of matrix proteoglycans.26,29–34 One mechanism by which this may occur following knee trauma is through the enzymatic cleavage of aggrecan at the (392)Glu–(393)Ala bond in its interglobular domain, which results in the release of N-terminal (393)Alanine–Arginine–Glycine–Serine (ARGS) neoepitopes into the surrounding synovial fluid (SF).29,35,36
Recent investigations have provided evidence that a biochemical response occurs very soon after acute knee trauma, and aggrecan degradation (evidenced by elevated SF levels of ARGS) is associated with inflammatory activity during the initial few weeks following acute ACL rupture,15,19,36,37 as well as at long-term follow-up after menisectomy30,37 compared to healthy, matched controls. It is not known, however, if this process persists for a substantial time interval following acute trauma, or if it is exclusive to the initial acute inflammatory phase following injury. In addition, the association between SF ARGS concentrations and inflammatory and non-inflammatory cytokines, as well as relationships between ARGS concentrations and the levels of matrix metalloproteases (MMPs) or tissue inhibitors of metalloproteases (TIMPs) found in the synovial fluid following ACL disruption, have not been reported. Consequently, the purpose of this study was to evaluate relationships between SF ARGS levels and an array of cytokines, MMPs, and TIMPs within the 6 months following acute ACL rupture.
MATERIALS AND METHODS
Research Participants
This was a cross-sectional study of 67 subjects (29 women) with a mean age of 30 years (SD: 11.4) and a mean BMI of 25.6 (SD: 4.7). These subjects were enrolled in two different prospective cohort studies at the time of sample collection, and as such, a full description of specific inclusion/exclusion criteria has been previously reported. (Ref’s removed for blinding) Both studies were approved by our institutional review board prior to data accrual, and all study participants provided written informed consent prior to enrollment. Briefly, entry criteria for both studies were similar and included: Tegner activity score greater than or equal to 5, no history of substantial trauma to any joint defined as that requiring medical evaluation and/or more than 3 days modified activity, no relevant knee pathologies other than those sustained during the index ACL injury event; no abnormal capsular laxity or appreciable injury to the collateral ligaments or posterior cruciate ligament; no radiographic evidence of fracture or pre-existing OA; no visual malalignment of the tibiofemoral joint as determined with the International Knee Documentation Committee (IKDC) Knee Examination Criteria; less than 1/3 meniscectomy performed at the time of surgical reconstruction (only one subject had just over 1/3 meniscectomy and was consequently graded “2/3 menisectomy” as a conservative measure per grading criteria guidelines); and articular cartilage lesions of grade 3A or less in the tibiofemoral and patellofemoral joints (based on International Cartilage Repair Society grading criteria38). At the time of surgical reconstruction, 42 subjects had intact menisci,39 including 26 knees with no meniscal pathology and 16 that received a meniscal repair or had a stable tear that was left in-situ. Twenty-five subjects were treated with a partial meniscectomy, which also included very minor debridement.
Synovial Fluid Collection
Synovial fluid samples were obtained from the injured knee of subjects via non-lavage arthrocentesis under sterile conditions immediately prior to surgical ACL-reconstruction. Following collection, samples were centrifuged (4,000g) at 4°C for 20 min, and supernatants were stored at −80°C until assay processing. All surgical reconstructions occurred within 6 months of the index injury (mean = 67.0 days, SD = 28.7, range = 13–155 days). Samples were analyzed to evaluate concentrations of ARGS, 42 cytokines, 8 MMPs, and 4 TIMPs.
ARGS Evaluation
SF aggrecan ARGS neoepitope, from aggrecanase cleavage at TEGE392↓393ARGS, was quantified using electrochemiluminescence (ECLC) immunoassay as described previously.15,30 Briefly, SF was deglycosylated with chondroitinase ABC, keratanase, and keratanase II. High-bind MA600 96-well microtiter plates (#L11XB-1 Meso Scale Discovery [MSD] Gaithersburg, MD) were coated with an antibody against the G1 and G2 globular domains of human aggrecan (#AHP0022, Invitrogen Life Technologies, Grand Island, NY). ARGS-aggrecan was detected by a biotinylated monoclonal anti-ARGS antibody (MAb OA-1)9,31 and incubated with Sulfo-tagged streptavidin (MSD) before analysis in a Sector Imager 6000 (MSD). Reported concentrations are in pmol/ml synovial fluid.
Cytokine, MMP, and TIMP Evaluation
Concentrations of analytes were evaluated in synovial fluid using commercially available bead-based multiplex immunoassays (MilliPlex 42-Plex Kit, Millipore Corporation, Billerica, MA), which were evaluated on a Bio-Plex 200 testing system equipped with high-throughput fluidics (Bio-Rad Laboratories, Hercules, CA) using neat SF. Evaluated analytes for this assay included epidermal growth factor (EGF), eotaxin, basic fibroblast growth factor (FGF2), Fms-related tyrosine kinase 3 ligand (Flt-3L), fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM–CSF), growth related oncogene (GRO), interferon alpha 2 (IFN-α2), interferon gamma (IFN-γ), monocyte chemotactic protein-1 (MCP-1), monocyte chemotactic protein-3 (MCP-3), macrophage-derived chemokine (MDC), macrophage inflammatory protein-1 alpha (MIP-1α), macrophage inflammatory protein-1 beta (MIP-1β), platelet-derived growth factor-AA (PDGF-AA), platelet-derived growth factor-AB (PDGF-AB), regulated on activation-normal T-cell expressed and secreted (RANTES, aka CCL5), transforming growth factor alpha (TGFα), tumor necrosis factor-α (TNF-α), tumor necrosis factor-β (TNF-β), vascular endothelial growth factor (VEGF), soluble CD40 ligand (sCD40L), interferon gamma-induced protein 10 (IP-10), and the following interleukins (IL) and receptors: IL-1ra, IL-1α, IL-1β, IL-2, soluble interleukin-2 receptor alpha (sIL-2r-α), IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, and IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, and IL-17. In addition, MMP 1, 2, 3, 7, 9, 10, 12, and 13 (Milliplex MMP panels 1 and 2), and TIMP 1, 2, 3, and 4 (Milliplex TIMP panel 1) were evaluated using similar methods.
Per manufacturer guidelines, RANTES, PDGF-AA, and PDGF-AB/BB are generally not evaluated concurrently with other analytes in this multiplex assay due to the need for a 1:100 dilution to adequately quantify concentrations of these cytokines in plasma and serum. However, preliminary optimization and dilution experiments performed with synovial fluid samples from injured knees and those of non-injured subjects demonstrated that all three analytes were within the detectable range of the assay in neat synovial fluids samples.
All assays were performed in duplicate according to manufacturer’s instructions. Briefly, 25 μl of undiluted synovial fluid, standard, quality control, or assay buffer (background) was added to each well of a pre-wet 96-well vacuum filter plate. Twenty-five microliters of assay buffer plus 25 μl of conjugated beads were added to each well and the plates were covered, shaken vigorously for 1 min on an IKA (Wilmington, NC) MTS 2/4 digital microtiter plate shaker and then moderately shaken overnight at 4°C. After washing using a Bio-Rad Bio-Plex Pro II wash station, 25 μl of biotinylated detection antibodies were added to the appropriate wells for 2 h followed by addition of 25 μl of streptavidin-PE to all wells for 30 min. The wells were washed and the beads were resuspended in 125 μl sheath fluid. Data were acquired using the Bio-Rad Bio-Plex suspension array system and Bio-Plex Manager 6.0 software. Fluorescence intensity of the background was subtracted from the values for each sample, standard, or control for each specific bead. Standard curves were generated from standards provided in the Milliplex kits, which were analyzed using 5-place logistic regression from standards within the limits of detection of the assay (70–130% of expected values). Reported concentrations are in pg/ml synovial fluid.
Statistical Analysis
The strengths of the relationships between ARGS concentrations and individual analyte concentrations, as well as MMP/TIMP ratios were evaluated statistically with the Partial Spearman Correlation test, due to the non-normality of the concentration data, while adjusting for age, sex, and the time interval between injury and SF sample acquisition. This non-parametric method uses the ranks of the data for analysis, and was chosen because it is valid regardless of non-normality of the data, and is not strongly influenced by outliers. As a conservative measure, correlations between ARGS and other analytes were evaluated only if 80% of sample concentrations fell within the detectable range of the assay. The purpose of this study was to explore relationships between ARGS and other SF analytes while maintaining an adequate level of power to detect correlations of 0.25 (absolute) or greater as statistically significant. Analysis was done without and then with adjustment for multiple comparisons using the Benjamini–Hochberg procedure for controlling False Discovery Rate. The alpha level for determining statistical significance was set a-priori at 0.05 for all analyses, which were conducted using SAS 9.2 software (SAS, Inc., Cary, NC).
RESULTS
All of the synovial fluid samples that were analyzed in this study had ARGS concentrations that fell within the limits of detectability (Table 1). Twenty three of the 42 cytokines, and 5 of the 12 MMP//TIMP had at 80% or greater of the synovial sample concentrations that were within the limits of detectability (Tables 1 and 2). Because of concerns about incomplete data sets, we chose not to perform statistical analysis on the data sets that had less that 80% of the synovial fluid samples with the limits of detectability (see Tables 1 and 2 for details).
Table 1.
Cytokine Concentrations and Correlations With ARGS
| Concentrations Within Limits of Detectability
|
||||||
|---|---|---|---|---|---|---|
| SF Analytes | Mean Concentration (SD) pg/ml (Unless Otherwise Noted) | Partial Spearman Correlation Coefficient versus ARGS | p-Value | N | min, max | % (Of total N), Concentrations Within Detectability Limits |
| ARGS | 27.75† (21.80) | 67 | 4.53, 90.66 | 100.00 | ||
| Eotaxin | 18.75 (11.55) | −0.146 | 0.25 | 67 | 4.87, 71.77 | 100.0 |
| FGF2 | 14.68 (16.61) | −0.266 | 0.03* | 64 | 1.14, 83.84 | 95.5 |
| Flt-3L | 32.37 (25.38) | −0.207 | 0.10 | 64 | 2.17, 111.89 | 95.5 |
| Fractaline | 36.1 (60.95) | −0.038 | 0.76 | 65 | 0.21, 480.02 | 97.0 |
| G-CSF | 4.64 (5.43) | −0.035 | 0.78 | 65 | 0.02, 38.58 | 97.0 |
| GM-CSF | 19.11 (68.12) | 0.044 | 0.73 | 65 | 0.09, 541.62 | 97.0 |
| IFN-α2 | 4.59 (4.71) | 0.068 | 0.59 | 60 | 0.04, 27.6 | 89.6 |
| IFN-γ | 6.49 (11.45) | −0.015 | 0.90 | 55 | 0.01, 73.6 | 82.1 |
| IL-10 | 7.2 (35.48) | 0.119 | 0.35 | 55 | 0.05, 289.11 | 82.1 |
| IL-12p4 | 16.17 (28.1) | −0.077 | 0.54 | 55 | 0.14, 203.61 | 82.1 |
| L-15 | 5.94 (3.5) | −0.156 | 0.22 | 67 | 0.19, 19.61 | 100.0 |
| IL-1β | 0.78 (1.08) | 0.167 | 0.19 | 62 | 0.01, 6.27 | 92.5 |
| IL-2 | 0.48 (0.62) | 0.004 | 0.98 | 66 | 0.03, 2.84 | 98.5 |
| sIL-2r-α | 86.02 (91.72) | 0.062 | 0.63 | 67 | 0.73, 607.01 | 100.0 |
| IL-6 | 38.22 (115.91) | 0.121 | 0.34 | 54 | 0.08, 654.56 | 80.6 |
| IL-8 | 11.02 (37.24) | −0.041 | 0.75 | 65 | 0.11, 303.77 | 97.0 |
| IP-10 | 1004.65 (1084.97) | 0.234 | 0.06 | 67 | 82, 8375.07 | 100.0 |
| MCP-1 | 655.84 (310.69) | −0.062 | 0.62 | 67 | 247.18, 1983.49 | 100.0 |
| MDC | 1663.48 (1109.18) | 0.101 | 0.43 | 67 | 478.81, 8107.06 | 100.0 |
| MIP-1α | 9.52 (7.42) | −0.071 | 0.58 | 62 | 1.06, 44.15 | 92.5 |
| PDGF-AA | 155.77 (551.9) | 0.015 | 0.91 | 66 | 0.03, 4413.84 | 98.5 |
| sCD40L | 201.75 (660.84) | 0.004 | 0.97 | 67 | 1.63, 5173.91 | 100.0 |
| TNF-α | 0.79 (1.1) | −0.141 | 0.26 | 63 | 0.04, 8.28 | 94.0 |
|
| ||||||
| EGF | 4.97 (6.86) | — | — | 32 | 2.36, 31.02 | 47.8 |
| GRO | 0 (0) | — | — | 0 | 0, 0 | 0.0 |
| IL-12p7 | 1.92 (5.83) | — | — | 39 | 0.05, 39.36 | 58.2 |
| IL-13 | 0.7 (2.73) | — | — | 14 | 0.03, 19.71 | 20.9 |
| IL-17 | 1.43 (5.27) | — | — | 11 | 0.07, 34.16 | 16.4 |
| IL-1ra | 7.05 (26.5) | — | — | 36 | 0.09, 98.83 | 53.7 |
| IL-1α | 2.22 (5.53) | — | — | 33 | 0.04, 39.56 | 49.3 |
| IL-3 | 1.72 (2.86) | — | — | 42 | 0.02, 12.33 | 62.7 |
| IL-4 | 1.42 (4.28) | — | — | 30 | 0.2, 25.26 | 44.8 |
| IL-5 | 0.07 (0.3) | — | — | 12 | 0.01, 1.95 | 17.9 |
| IL-7 | 0.75 (1.84) | — | — | 25 | 0.05, 10.49 | 37.3 |
| IL-9 | 0.98 (1.28) | — | — | 44 | 0.01, 5.67 | 65.7 |
| MCP-3 | 1.26 (1.82) | — | — | 33 | 0.47, 8.02 | 49.3 |
| MIP-1β | 7.78 (7.92) | — | — | 50 | 0.71, 35.5 | 74.6 |
| PDGF-AB | 267.53 (1402.36) | — | — | 3 | 2698.81, 10000 | 4.5 |
| RANTES | 998.01 (1939.82) | — | — | 42 | 1.43, 10007 | 62.7 |
| TGF-α | 0.12 (0.42) | — | — | 13 | 0.01, 2.92 | 19.4 |
| TNF-β | 1.68 (4.87) | — | — | 30 | 0.04, 31.39 | 44.8 |
| VEGF | 44.15 (67.63) | — | — | 40 | 7.67, 381.79 | 59.7 |
Bold p-value indicates borderline significance. Shaded rows have less than 80% of sample concentrations within detectable limits, correlation analyses were not performed.
p <0.05.
ARGS concentration presented in pmol/ml.
Table 2.
MMP and TIMP Concentrations and Correlations With ARGS
| Concentrations Within Limits of Detectability
|
||||||
|---|---|---|---|---|---|---|
| SF Analytes | Concentration Mean (SD) | Partial Spearman Correlation Coefficient versus ARGS | p-Value | N | min, max | % (Of Total N), Concentrations Within Detectability Limits |
| MMP-2 | 17944.49 (3735.48) | 0.150 | 0.24 | 66 | 8998.71, 25735.7 | 100.0 |
| MMP-7 | 290.29 (269.6) | 0.084 | 0.51 | 62 | 103.82, 1918.24 | 93.9 |
| TIMP-2 | 20310.46 (8868.52) | −0.042 | 0.75 | 60 | 712.77, 45546.29 | 96.8 |
| TIMP-3 | 9160.18 (5946.23) | 0.330 | 0.01* | 55 | 598, 25108.05 | 87.3 |
| TIMP-4 | 648.76 (276.07) | 0.103 | 0.44 | 62 | 120.02, 1781.7 | 98.4 |
|
| ||||||
| MMP-1 | 4928.08 (6112.83) | — | — | 36 | 131.47, 19475.78 | 54.5 |
| MMP-3 | 17175.52 (20786.34) | — | — | 27 | 18342.04, 48429.03 | 42.2 |
| MMP-9 | 0 (0) | — | — | 0 | 0, 0 | 0.0 |
| MMP-10 | 20.68 (31.07) | — | — | 29 | 19.39, 142.38 | 43.9 |
| MMP-12 | 18.64 (59.8) | — | — | 6 | 134.66, 276.71 | 9.4 |
| MMP-13 | 24.02 (70.12) | — | — | 22 | 9.02, 449.94 | 34.4 |
| TIMP-1 | 1841461.36 (9405495.4) | — | — | 3 | 13455.87, 18346.06 | 5.4 |
Bold p-value text indicates borderline significance. Shaded rows have less than 80% of sample concentrations within detectable limits, and therefore, correlation analyses were not performed.
p <0.05.
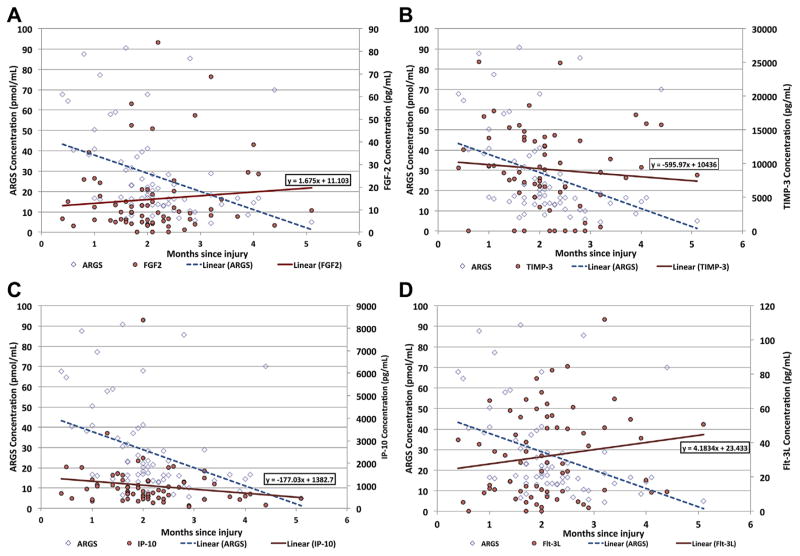
The ARGS concentrations decreased over time following ACL injury (Fig. 1A). Results of the Spearman Partial Correlation tests revealed statistically significant relationships between ARGS concentrations and FGF2 (rs = −0.27, p = 0.03; Fig. 1A; Table 1). Similarly there was a statistically significant relationship between ARGS concentrations and TIMP-3 (rs = 0.33, p = 0.01; Fig. 1B; Table 2). Although not statistically significant, IP-10 (Fig. 1C), and Flt-3L (Fig. 1D), had correlations greater than 0.20 (absolute values) (Table 2). All other analytes tested received partial correlation values less than 0.2 and were not statistically significant. Concentrations for these analytes as well as their correlation with ARGS are presented in Tables 1 and 2.
Figure 1.
A: Concentrations of ARGS (pmol/mL) and FGF2 (pg/ml) in synovial fluid obtained from the ACL injured knee plotted against the time interval (months) between the index ACL injury and acquisition of the sample from the knee joint via non-lavage arthrocentesis. B: Concentrations of ARGS (pmol/ml) and TIMP-3(pg/ml) in synovial fluid obtained from the ACL injured knee plotted against the time interval (months) between the ACL injury and acquisition of the sample from the knee joint with the used of non-lavage arthrocentesis. C: Concentrations of ARGS (pmol/ml) and IP-10 (pg/ml) in synovial fluid obtained from the ACL injured knee plotted against the time interval (months) between ACL trauma and acquisition of the sample from the knee joint with non-lavage arthrocentesis. D: Concentrations of ARGS (pmol/ml) and Flt-3L (pg/ml) in synovial fluid obtained from the ACL injured knee plotted against the time interval (months) between ACL trauma and acquisition of the sample from the knee joint with non-lavage arthrocentesis.
Findings When Using Adjustment for Multiple Comparisons With the Benjamini–Hochberg Procedure for Controlling False Discovery Rate
Results of the Spearman Partial Correlation tests showed a statistically significant relationship between ARGS concentration and TIMP-3 (p = 0.05 after Hochberg correction for False Discovery Rate). Although not statistically significant after Hochberg correction, FGF2, IP-10, and Flt-3L had correlations greater than 0.20 (absolute values). The unadjusted p-value for FGF2 was significant (p = 0.03). These results seem to be indicators of meaningful relationships between ARGS and these analytes, and worthy of further study.
DISCUSSION
In this study, we found that SF-ARGS concentrations decreased over time since injury, which supports previously reported findings.29 To our knowledge, this is the first investigation to examine a large array of cytokines, MMPs, and TIMPs as they relate to SF ARGS concentration during the first 6 months following acute ACL trauma and builds on information reported previously.15,29 This work represents an important first step in our understanding of the initial response of the knee to severe ligament trauma.
During the initial period following ACL injury when ARGS concentrations are high, FGF2 concentrations are low, and as ARGS concentrations decrease over time, FGF2 concentrations gradually increase. The specific role of FGF2 as a catabolic or anabolic mediator of articular cartilage homeostasis remains controversial40 depending upon which of the four FGF receptor subtypes (FGFR1, 2, 3, or 4) expressed on the surface of the articular chondrocyte is selectively activated. Activation of FGFR1 by members of the FGF protein family (FGF2, -8, and -18) is associated with a catabolic response, whereas activation of FGFR2is associated with an anabolic/chondroprotective response.3,11,41 Since FGFR1 is expressed in substantially higher quantities on the chondrocyte surface compared toFGFR2, elevated concentrations of FGF2 are believed to result in primarily catabolic responses.11 Human articular cartilage explant studies3,42 have demonstrated the rapid release of FGF2 soon after mechanically induced trauma. In those investigations, increased FGF2 concentration resulted in the up-regulation of MMP-13 through mitogen activated protein kinase (MAPK) pathways, as well asup-regulation of ADAMTS-4 and -5 (A disintegrin and a metalloproteinase domain with thrombospondin motifs, proteins 4 and 5). These proteins, also commonly referred to as aggrecanase 1 and 2, respectively, are considered to be the primary enzymes responsible for the degradation of aggrecan through cleavage at the Glu392–Ala393 bond in the interglobular domain.43 The FGF2-induced upregulation of aggrecanase-1 and -2 is mediated through activation of Runt-related transcription factor 2 (RUNX2) and activator protein 1 (AP-1) pathways.11 In addition to its participation in the upregulation of aggrecanase enzymes, FGF2 is also implicated in pathways responsible for suppression of genes involved in aggrecan production.11 Due to the purported catabolic effects of increased FGF2 concentration, it seems counter-intuitive that this protein displayed an inverse/negative relationship with ARGS levels as was observed in the current investigation. This finding is especially intriguing in light of previous findings that FGF2 up-regulates aggrecanase (primarily ADAMTS-4 and-5) production.11,41 It may be that in the current study, FGF2 was, in-fact, upregulated during the initial days following the index ACL trauma, resulting in higher levels of aggrecanases (and consequent production of ARGS through aggrecanase cleavage) and returned to lower levels while ARGS remained elevated prior to SF acquisition. An alternative hypothesis may be that, following injury (and after the acute inflammatory phase has subsided), chondrocytes may increase FGFR2 production concurrently with increased levels of FGF2 in an effort to upregulate the anabolic/chondro protective response from chondrocytes. This study was not designed to evaluate relationships between ARGS concentration and other analytes during the initial acute inflammatory response immediately following ACL injury, as these relationships have been previously reported.15 The purpose of this investigation was to build on this prior work,15 and evaluate the temporal response that occurred following the acute inflammatory phase (within the first 2 weeks of injury) through surgical ACL reconstruction (22 weeks post injury).
Our results regarding ARGS concentration following ACL injury concur with those we have reported previously.15 In that investigation, SF samples were obtained from 111 ACL injured knees within the first 23 days following injury to characterize ARGS and pro-inflammatory concentrations during the acute inflammatory phase following injury. The results of this earlier work demonstrated that ARGS concentration levels are significantly elevated during the first few weeks following ACL injury compared to non-injured controls. In addition, the pro-inflammatory cytokines that were evaluated (IL-1β, IL-6, IL-8, and TNF-α) were also significantly elevated in injured subjects compared to controls from the time of injury through 23 days post. In the present study, we found that ARGS levels are generally higher during the first 2 months following injury, and decline as a function of time (Fig. 1). We did not, however, find substantially elevated pro-inflammatory cytokine concentrations of IL-1ra, IL-1β, IL-6, IL-8, or TNF-α in our cross-sectional analysis over 6 months post-injury. The levels of these SF analytes appear to be similar to those previously reported with healthy subject reference values,15,20 albeit using different assay techniques (i.e., Luminexvs MSD), and consequently may not be directly comparable. This finding is in concurrence with those previously reported during this time-frame following ACL injury,22,44 and indicates that (as a group) the inflammatory response following injury likely subsided during this period.
This study was designed to characterize how ACL disruption is associated with the short-term temporal response of ARGS in synovial fluid with a focus on understand the mechanism in which it is controlled following joint trauma but prior to reconstruction and rehabilitation. We took steps to control for known confounding variables that may lead to the identification of spurious results through our statistical analyses by adjusting for subject age, sex, and time since injury, it is possible that unknown variables that were not controlled for could be influencing these results. In addition, whereas the multiplex assays used for our synovial fluid analyses have been validated for use in human serum, the manufacturer has not specifically validated their use for the analysis of other body fluids such as urine, cerebral spinal fluid, or synovial fluid at this time. Nevertheless, Milliplex assays have been used successfully for the measurement of synovial fluid.6,45 Another potential limitation associated with our study is that it did not include an evaluation of matched, non-injured control subjects with normal joints, or measurement of aggrecanases (ADAMTS-4 and -5). We did not expect subjects with normal knees to have variable levels of the analytes that were considered in the current study as prior work has demonstrated that they either do not vary over time or are very low and cannot be detected in subjects with normal knees and no history of prior injury or disease.46,47 This study drew from two separate cohorts that have similar characteristics. Consequently, we performed the Partial Spearman Correlation tests on each cohort separately, and for all comparisons this resulted in the same relationships as presented in the analysis of the combined cohorts.
This study was cross-sectional by design and did not have the capacity to definitively establish cause–and –effect relationships between the change in ARGS matrix component of cartilage and mediator responsible for producing the change. These finding suggests that there are likely additional variables associated with change in ARGS concentration following injury and healing that have not yet been identified, which may include other cellular mediators, damage incurred at the time of injury (articular cartilage, menisci, synovium, etc), or the rate at which SF ARGS fragments are produced and cleared by the synovial membrane.
Strengths of this study include the evaluation of only first-time isolated ACL injuries or ones with relatively minor concomitant injuries to the articular cartilage and menisci, obtained from knees with no radiographic characteristics indicative of pre-existing OA. Our approach was to study the temporal response of ARGS following ACL disruption and prior to reconstruction in an effort to begin to understand the initial response of the knee to ACL trauma. It is important for us to point out, however, that the time interval between ACL injury and acquisition of SF samples was dependent on the recruitment and retention of study participants and the surgical schedule (the day when SF was acquired) with the cross-sectional study design that was used. Consequently we did not have a homogenous distribution of data over time. Instead, the time interval between injury and SF acquisition was used as a covariate in the statistical analysis. Future studies would benefit from the longitudinal assessment of ARGS concentration as it relates to various inflammatory and non-inflammatory cytokines, MMPs and their inhibitors, as well as the evaluation of aggrecanase concentrations, with Post Traumatic Osteoarthritis outcomes evaluated at a multiple-year follow-up and compared to a group of healthy, matched control subjects.
In conclusion, the results of this cross-sectional investigation indicate that ARGS concentrations are negatively correlated with FGF2 concentrations, and positively correlated with TIMP-3 over time following acute ACL injury. Our results support the hypothesis that during the initial period following ACL injury (when SF ARGS concentrations are highest), there is an up-regulation of TIMP-3 in response to increased aggrecan degradation, which may inhibit further cleavage. TIMP-3 is one of the primary inhibitors of ADAMTS-4 and -5.43,48 Although the evaluation of ADAMTS proteins was beyond the scope of this preliminary investigation, we posit that as aggrecanase activity is up-regulated following injury (as evidenced by the increased concentration of ARGS in SF) the corresponding up regulation of TIMP-3 serves as a regulatory mediator of aggrecan degradation. This also lends to the hypothesis that an imbalance of aggrecanase to TIMP-3 ratio may play a role in tibiofemoral joint space width changes (increased or decreased), which present within months following ACL injury in some individuals.12,49,50
Acknowledgments
Funding for this investigation was provided by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01 AR051477-01 (BDB), the Swedish Rheumatism Association (AS), the Kock Foundation (AS), the King Gustaf V 80-year Anniversary Foundation (AS), the Faculty of Medicine Lund University (AS), Österlunds Foundation (AS), and the Crafoord Foundation (AS). The authors have no additional professional or financial disclosures. We would like to thank L. Stefan Lohmander, MD, PhD for his insight with this project. We would also like to thank Robert J. Johnson, MD and James R. Slauterbeck, MD for their assistance in referring study participants and for performing the SF arthrocentesis procedures, Maria Hansson for her work with the SF-ARGS analysis, as well as Shelly Naud, PhD for her preliminary assistance with statistical analyses.
Grant sponsor: National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases grant; Grant number: R01 AR051477-01; Grant sponsor: Swedish Rheumatism Association; Grant sponsor: Kock Foundation; Grant sponsor: King Gustaf V 80-year Anniversary Foundation; Grant sponsor: Faculty of Medicine Lund University; Grant sponsor: Österlunds Foundation; Grant sponsor: Crafoord Foundation.
Footnotes
AUTHORS’ CONTRIBUTIONS
All authors have substantially contributed to the conception and design of the study, acquisition of data, or analysis and interpretation of data. All authors have participated in the writing process and approved the final version of the manuscript. Bruce D. Beynnon (bruce.beynnon@uvm.edu) takes responsibility for the integrity of the work.
References
- 1.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TD, Johnston RC, Saltzman CL, et al. Post-traumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 3.Keays SL, Newcombe PA, Bullock-Saxton JE, et al. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med. 2010;38:455–463. doi: 10.1177/0363546509350914. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, et al. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Louboutin H, Debarge R, Richou J, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–244. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Murray JR, Lindh AM, Hogan NA, et al. Does anterior cruciate ligament reconstruction lead to degenerative disease? Thirteen-year results after bone-patellar tendon-bone autograft. Am J Sports Med. 2012;40:404–413. doi: 10.1177/0363546511428580. [DOI] [PubMed] [Google Scholar]
- 7.Muthuri SG, McWilliams DF, Doherty M, et al. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19:1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Neuman P, Englund M, Kostogiannis I, et al. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36:1717–1725. doi: 10.1177/0363546508316770. [DOI] [PubMed] [Google Scholar]
- 9.Oiestad BE, Engebretsen L, Storheim K, et al. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 10.Oiestad BE, Holm I, Aune AK, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010;38:2201–2210. doi: 10.1177/0363546510373876. [DOI] [PubMed] [Google Scholar]
- 11.Kessler MA, Behrend H, Henz S, et al. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16:442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 12.Tourville TW, Johnson RJ, Slauterbeck JR, et al. Assessment of early tibiofemoral joint space width changes after anterior cruciate ligament injury and reconstruction: a matched case-control study. Am J Sports Med. 2013;41:769–778. doi: 10.1177/0363546513477838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tourville TW, Johnson RJ, Slauterbeck JR, et al. Relationship between markers of type II collagen metabolism and tibiofemoral joint space width changes after acl injury and reconstruction. Am J Sports Med. 2013;41:779–787. doi: 10.1177/0363546513476481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catterall JB, Stabler TV, Flannery CR, et al. Changes in serum and synovial fluid biomarkers after acute injury. Arthritis Res Ther. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sward P, Frobell R, Englund M, et al. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)-a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20:1302–1308. doi: 10.1016/j.joca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence JT, Birmingham J, Toth AP. Emerging ideas: prevention of posttraumatic arthritis through inter-leukin-1 and tumor necrosis factor-alpha inhibition. Clin Orthop Relat Res. 2011;469:3522–3526. doi: 10.1007/s11999-010-1699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker T, Martins TB, Hill HR, et al. Vitamins E and C modulate the association between reciprocally regulated cytokines after an anterior cruciate ligament injury and surgery. Am J Phys Med Rehabil. 2011;90:638–647. doi: 10.1097/PHM.0b013e318214e886. [DOI] [PubMed] [Google Scholar]
- 19.Misko TP, Radabaugh MR, Highkin M, et al. Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthritis Cartilage. 2013;21:151–156. doi: 10.1016/j.joca.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi H, Shirakura K, Kimura M, et al. Changes in biochemical parameters after anterior cruciate ligament injury. Int Orthop. 2006;30:43–47. doi: 10.1007/s00264-005-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–521. [PubMed] [Google Scholar]
- 22.Bigoni M, Sacerdote P, Turati M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31:315–321. doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 23.Kraus VB. Osteoarthritis year 2010 in review: biochemical markers. Osteoarthritis Cartilage. 2011;19:346–353. doi: 10.1016/j.joca.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus VB, Burnett B, Coindreau J, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19:515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu CR, Williams AA, Coyle CH, et al. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14:212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmander LS. Markers of altered metabolism in osteoarthritis. J Rheumatol Suppl. 2004;70:28–35. [PubMed] [Google Scholar]
- 27.Chu CR, Beynnon BD, Buckwalter JA, et al. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med. 2011;39:1569–1578. doi: 10.1177/0363546511411654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole AR. Biochemical/immunochemical biomarkers of osteoarthritis: utility for prediction of incident or progressive osteoarthritis. Rheum Dis Clin North Am. 2003;29:803–818. doi: 10.1016/s0889-857x(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 29.Larsson S, Lohmander LS, Struglics A. Synovial fluid level of aggrecan ARGS fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: a cross-sectional study. Arthritis Res Ther. 2009;11:R92. doi: 10.1186/ar2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson S, Englund M, Struglics A, et al. Association between synovial fluid levels of aggrecan ARGS fragments and radiographic progression in knee osteoarthritis. Arthritis Res Ther. 2010;12:R230. doi: 10.1186/ar3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Struglics A, Larsson S, Pratta MA, et al. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14:101–113. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Lohmander LS, Ionescu M, Jugessur H, et al. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42:534–544. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- 34.Sandy JD, Flannery CR, Neame PJ, et al. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Struglics A, Larsson S, Hansson M, et al. Western blot quantification of aggrecan fragments in human synovial fluid indicates differences in fragment patterns between joint diseases. Osteoarthritis Cartilage. 2009;17:497–506. doi: 10.1016/j.joca.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Struglics A, Hansson M, Lohmander LS. Human aggrecanase generated synovial fluid fragment levels are elevated directly after knee injuries due to proteolysis both in the inter globular and chondroitin sulfate domains. Osteoarthritis Cartilage. 2011;19:1047–1057. doi: 10.1016/j.joca.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Larsson S, Englund M, Struglics A, et al. The association between changes in synovial fluid levels of ARGS-aggrecan fragments, progression of radiographic osteoarthritis and self-reported outcomes: a cohort study. Osteoarthritis Cartilage. 2012;20:388–395. doi: 10.1016/j.joca.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A:58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 39.Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28:446–452. doi: 10.1177/03635465000280040201. [DOI] [PubMed] [Google Scholar]
- 40.Vincent TL. Fibroblast growth factor 2: good or bad guy in the joint? Arthritis Res Ther. 2011;13:127. doi: 10.1186/ar3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan D, Chen D, Cool SM, et al. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther. 2011;13:R130. doi: 10.1186/ar3441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Vincent T, Hermansson M, Bolton M, et al. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmark IC, Mikkelsen UR, Borglum J, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther. 2010;12:R126. doi: 10.1186/ar3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauerschnig M, Stolberg-Stolberg J, Schulze A, et al. Diverse expression of selected cytokines and proteinases in synovial fluid obtained from osteoarthritic and healthy human knee joints. Eur J Med Res. 2014;19:65. doi: 10.1186/s40001-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchida AI, Beekhuizen M, t Hart MC, et al. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res Ther. 2014;16:441. doi: 10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell. 2010;1:33–47. doi: 10.1007/s13238-010-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frobell RB, Roos HP, Roos EM, et al. The acutely ACL injured knee assessed by MRI: are large volume traumatic bone marrow lesions a sign of severe compression injury? Osteoarthritis Cartilage. 2008;16:829–836. doi: 10.1016/j.joca.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Frobell RB. Change in cartilage thickness, post-traumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93:1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]