Abstract
In yeast, the interaction of General Control Non-derepressible 1 (GCN1) with GCN2 enables GCN2 to phosphorylate eIF2α (the alpha subunit of eukaryotic translation initiation factor 2) under a variety of stresses. Here, we cloned AtGCN1, an Arabidopsis homologue of GCN1. We show that AtGCN1 directly interacts with GCN2 and is essential for the phosphorylation of eIF2α under salicylic acid (SA), ultraviolet (UV), cold stress and amino acid deprivation conditions. Two mutant alleles, atgcn1-1 and atgcn1-2, which are defective in the phosphorylation of eIF2α, showed increased sensitivity to cold stress, compared with the wild type. Ribosome-bound RNA profiles showed that the translational state of mRNA was higher in atgcn1-1 than in the wild type. Our result also showed that cold treatment reduced the tendency of the tor mutant seedlings to produce purple hypocotyls. In addition, the kinase activity of TOR was transiently inhibited when plants were exposed to cold stress, suggesting that the inhibition of TOR is another pathway important for plants to respond to cold stress. In conclusion, our results indicate that the AtGCN1-mediated phosphorylation of eIF2α, which is required for inhibiting the initiation of protein translation, is essential for cold tolerance in Arabidopsis.
Keywords: amino acid deprivation, cold stress, eIF2α phosphorylation, GCN1, GCN2, TOR
INTRODUCTION
Mammals contain four eIF2α kinases, including the general control nonderepressible-2 kinase (GCN2), the double-stranded-RNA-dependent protein kinase R (PKR), the PKR-like endoplasmic reticulum kinase (PERK) and the heme-regulated eIF2α inhibitor kinase (HRI) (Baird and Wek 2012; Wek et al. 2006). GCN2 is activated by nutritional stress; PKR participates in antiviral responses; PERK is involved in the response to endoplasmic reticulum stress (ER stress) and HRI is activated by heme deprivation. Although these kinases are activated by different stresses, all of them can phosphorylate eIF2α (Rowlands et al. 1988; Scorsone et al. 1987). The phosphorylation of eIF2α increases the affinity of eIF2α for eIF2B. As a result, eIF2-GTP cannot be released from the complex of the phosphorylated eIF2α and eIF2B to initiate translation, thereby preventing protein translation.
In Saccharomyces cerevisiae, GCN2 is the only kinase involved in the phosphorylation of eIF2α in response to amino acid deficiency (Dever et al. 1992), glucose limitation (Yang et al. 2000), salt stress and ultraviolet (UV) irradiation (Narasimhan et al. 2004; Wu et al. 2004). Under stress, GCN2-mediated phosphorylation of eIF2α inhibits global protein translation.
Like S. cerevisiae, Arabidopsis contains only one GCN2 kinase. Upon amino acid deprivation, GCN2 phosphorylates eIF2α, which in turn inhibits translation initiation (Lageix et al. 2008). These results indicate that the GCN2-mediated inhibition of protein translation in response to amino acid starvation is conserved in yeast and Arabidopsis. In plants, GCN2 is known to be activated to phosphorylate eIF2α in response to wounding, salicylic acid (SA), methyl jasmonate (JA) and cold stress (Lageix et al. 2008; Zhang et al. 2003; Zhang et al. 2008).
In mammals, phosphorylation of eIF2α, inhibition of Target of Rapamycin Complex 1 (TORC1) and other signals function in parallel to keep cells alive under low temperature and ER stress by inhibiting protein synthesis (Duan et al. 2010; Hofmann et al. 2012). In perk mutants, or in eIF2α-S51A (A/A cells; eIF2α-phosphorylation-deficient), or 4ebp1/4ebp2 (the eIF4E-binding protein 1 and 2, downstream of TORC1) mammalian cells, in which the phosphorylation of eIF2α or the TOR pathway is blocked, protein synthesis is still inhibited under cold stress. Protein translation is also inhibited in yeast gcn2Δmutants under cold stress. In these mutants, moreover, cell growth and cell survival were not affected under cold stress. All of these results suggest that neither the phosphorylation of eIF2α nor the TOR pathway is essential for the inhibition of translation under cold stress in yeast and mammals.
In yeast, GCN1 is required for the activation of GCN2, which affects the occupancy of uncharged tRNA on ribosomal decoding sites under amino acid starvation (Marton et al. 1993; Sattlegger and Hinnebusch 2005). Here, we isolated a mutant that produced yellow leaves when grown in soil. In addition, the mutant was more sensitive to cold stress than the wild type. Using map-based cloning, we found that AtGCN1, which encodes a homolog of yeast GCN1, is mutated. We also investigated how AtGCN1 is involved in cold tolerance in Arabidopsis.
MATERIALS AND METHODS
Plant growth conditions
Plants were grown in pots with pH-balanced peat moss and vermiculite (3:1) in a greenhouse at 23 °C with a 16/8 h light/dark photoperiod. The sterilized seeds were sown on half-strength Murashige and Skoog (MS) medium supplemented with 1% sucrose and 1.0% agar (for seeding growth) or 0.8% agar (for seed germination).
For treatment with the herbicide chlorsulfuron, 6-day-old seedlings were submerged in 50 mL of 0.5 mM chlorsulfuron containing 0.01% (v/v) Silwet L-77 (a surfactant) for 1 min, and 0.5 mM solutions containing amino acids of leucine, isoleu-cine and valine were added to reduce the impairment of chlorsulfuron. After these treatments, the solutions were removed, and the seedlings were grown in a chamber for further observation.
For SA treatments, 0.6 mM SA was sprayed on 1-week-old seedlings.
For UV treatments, seedlings were irradiated by 500 mJ/cm2 UV (254 nm) and then incubated for 1 h under light.
For cold stress treatments, seedlings that had been vertically grown on plates were kept at 4 °C for the indicated number of days.
Chlorophyll (Chl) and anthocyanin measurement
Chl content was measured as reported previously (Lolle et al. 1997) with minor modifications. Four-week-old seedlings grown in soil were submerged in 95% ethanol for 24 h. Absorption values at 663 and 645 nm were recorded to calculate Chl content. Anthocyanin content was also measured as described previously (Matsui et al. 2004). In brief, seedlings for anthocyanin extraction were submerged in 45% methanol and 5% acetic acid at 4 °C for 16 h. The relative levels of anthocyanin were calculated by absorbance at 530 nm and 637 nm.
Screening of atgcn1-1 and map-based cloning
The atgcn1-1 mutant was crossed with Landsberg erecta (Ler), and F2 plants with the mutant phenotype were selected for map-based cloning. Genomic DNA of the selected plants was extracted, and polymerase chain reaction (PCR) was performed using primers that were designed according to Simple Sequence Repeat (SSR) or Derived Cleaved Amplified Polymorphic Sequences (dCAPS) markers from the information in the Cereon Arabidopsis (Jander et al. 2002). The mutation was mapped to chromosome I. SSR markers in BAC clones F1N19, F13O11, F16G16 and T23K8, and a dCAPS marker in F15H21 were used in fine mapping. All primers used in this study are listed in Table S1.
Yeast two-hybrid assay
The partial sequences of AtGCN1 were amplified and inserted into the prey plasmid pGADT7, and the full-length of GCN2 was cloned into the bait plasmid pGBKT7 by homologous recombination according to the kit protocol (NR001, Novoprotein). Different combinations of plasmids were transformed into yeast strain AH109 (Gietz and Woods 2002). Transformant cells were gradually diluted and dropped on SD medium without leucine and tryptophan (Leu−Trp−) or without leucine, tryptophan, histidine and adenine (Leu−Trp−His−Ade−), and supplemented with 2% (g/v) glucose. The empty vector pGADT7 was used as a negative control.
Firefly luciferase complementation imaging assay
The luciferase images were obtained as previously described (Chen et al. 2008). The fragment of AtGCN1 was fused to pCAMBIA-C-Luc, and GCN2 was fused to pCAMBIA-N-Luc. The plasmids were transformed into Nicotiana benthamiana leaves by Agrobacterium (GV3101 strain)-mediated transformation. LUC images were captured using a CCD imaging apparatus (CHEMIPROHT 1300B/LND, Roper Scientific).
Protein extraction and immunoblot analysis
Proteins were extracted and immunoblotting was performed as described previously (Lageix et al. 2008). After stress treatment, 1-week-old seedlings were ground in extraction buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 0.2% Triton X-100 and 1 mM DTT) containing both complete protease inhibitor and PhosSTOP phosphatase inhibitor (Roche). Extracted proteins were centrifuged, and the suspensions were incubated at 95 °C for 5 min. Proteins were separated by SDS-PAGE and then transferred to PVDF membranes for immunoblotting.
The membranes were probed using a monoclonal antibody of phospho-eIF2a (Ser51) (Catalogue no. 9721, Cell Signalling, 1/1000 dilution), a monoclonal antibody phosphor-S6K1-2 (AS132664, Agrisera, 1/5000 dilution) or an anti-HA monoclonal antibody (AS152921, Agrisera, 1/5000 dilution). After incubation with peroxidase-coupled secondary antibody (Proteintech 1/5000 dilution), the membranes were immersed in ECL Plus Western Blotting detection reagents (GE Healthcare Bio-Sciences) and finally exposed to X-film for visualization. The membranes were re-probed with anti-alpha-Actin-2 (Abmart, M20009, 1/10 000 dilution) or anti-Tubulin (AS10 681, Agrisera, 1/5000 dilution) as a loading control.
Quantitative real-time PCR (qRT-PCR)
Seedlings were ground in liquid nitrogen, and total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The concentration of RNAwas quantified using a NanoDrop ND-1000. A 1 μg quantity of DNase-treated RNA (Turbo DNA-free kit, Ambion) was transcribed into the first-strand cDNA using oligo dTand reverse transcriptase (GoScript Reverse Transcription System). An IQ5 Multi-color Real-Time PCR Detection system was used to perform qRT-PCR, according to the following programme: 95 °C for 3 min; 35 cycles at 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 30 s; 95 °C for 10 s; and finally melt-curve was analysed with the procedure of 65 °C to 95 °C, 0.5 °C increment per each read. Experiments were performed three times with similar results. Actin was used as the reference gene. The primers used are listed in Table S1.
Labelling of newly synthesized proteins with 35S amino acids
Newly synthesized proteins were labelled with 35S amino acids as described previously (Guo et al. 2002).
Polysome profile analysis
Polysomes were extracted as described previously (Sormani et al. 2011). A 200 mg quantity of 10-day-old seedlings was ground into powder in liquid nitrogen and was suspended in 1 mL of lysis buffer (100 mM Tris–HCl pH 8.4, 50 mM KCl, 25 mM MgCl2, 5 mM EGTA, 50 μg/mL cycloheximide, 50 μg/mL chloramphenicol, 0.5% Nonidet P40, Diethy pyrocarbonate treated). Cell debris was eliminated by centrifugation at 9000 g for 15 min. The supernatant was loaded on an 11 mL 0.8–1.5 M sucrose gradient. The sample was centrifuged at 175 000 g in a Beckman SW41 rotor for 150 min, and the OD (260 nm) of fraction from the bottom of the gradients was recorded using an ISCO gradient fractionator. Twenty-two fractions (0.5 mL/fraction) were collected from the top to the bottom of the sucrose gradients. RNA from each fraction was isolated using TRIzol reagent for RNA gel analysis.
RESULTS
Map-based cloning of AtGCN1
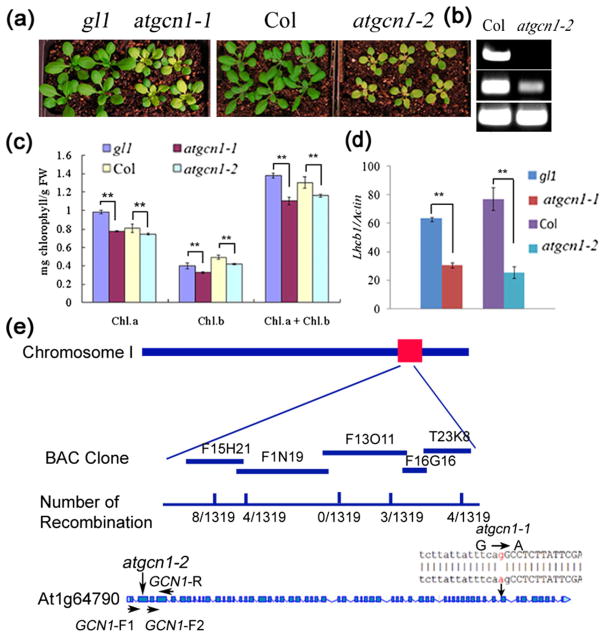
By screening EMS-mutagenized M2 progeny of wild-type plants (Columbia gl1 background) grown in soil, we identified a mutant whose new leaves were yellow (Fig. 1a). We crossed the mutant with the corresponding wt plants of gl1 background and found that 129 of 409 plants showed the yellow phenotype in the F2 generation (1:3 ratio, χ2 = 0.30, P = 0.58), which indicates that the yellow phenotype of the mutant is controlled by a single recessive gene. To clone the mutated gene, we crossed the mutant with wild-type Ler ecotype and collected the seeds of the F2 population. Plants of the F2 population whose new leaves were yellow were selected for map-based cloning.
Figure 1.
Map-based cloning of AtGCN1. (a) The new leaves of 4-week-old seedlings of atgcn1-1 and atgcn1-2 grown in soil are yellow. (b) Transcription of AtGCN1 in atgcn1-2. PCR products were amplified by primers across the T-DNA insertion site of atgcn1-2 (AtGCN1-F1 and AtGCN1-R) (top) and downstream of the T-DNA insertion site of atgcn1-2 (AtGCN1-F2 and AtGCN1-R) (middle). Actin was used as the control (bottom). (c) Chl content in atgcn1-1 and atgcn1-2. A t-test was performed between atgcn1-1, atgcn1-2, and the wild type. Double asterisks indicate p <0.01. (d) Quantitative analysis of the expression of LHCB1 in atgcn1-1 and atgcn1-2 grown in soil. Double asterisks indicate p <0.01. (f) Map-based cloning of AtGCN1. The mutation was mapped to the bottom of chromosome I. Fine mapping was performed in the BAC clones F15H21, F1N19, F13O11, F16G16 and T23K8, and the numbers of recombinations at the corresponding BAC clones are listed. A mutation from G to A was identified in the 3'-splicing site of the 52nd intron of AT1G64790.1. In atgcn1-2 (SALK_063702C), a T-DNA was inserted in the second exon of AT1G64790.1.
By using the SSLP markers developed by Cereon Genomics (http://www.Arabidopsis.org), the mutation was mapped to the bottom of chromosome I, between the BAC clones F15H21 and T23K8 (Fig. 1e). Fine mapping was performed in BAC clones F1N19, F13O11 and F16G16. Genes between BAC clones F1N19 and F16G16 were amplified and sequenced. A single-nucleotide mutation from G to A was identified in AT1G64790, which is a homolog of the yeast GCN1. The mutation site is located in the 3' splicing site of the 52nd intron of AT1G64790, in which AGG (the ‘AG’ should be spliced out in the wild type) was mutated to AAG (the new ‘AG’ was spliced out in atgcn1-1), leading to the loss of one G in the 53rd exon of AT1G64790.1 (Fig. 1e and S1). As a result, a frame-shift occurs in the coding region of AT1G64790, leading to a premature stop codon and hence a truncated protein. The mutant was named atgcn1-1.
We ordered the T-DNA insertion line SALK_063702C of AtGCN1 from the Arabidopsis Stock Center (ABRC). In SALK_063702C, designated atgcn1-2, a T-DNA was inserted in the second exon of AT1G64790.1 (Fig. 1e). Like atgcn1-1, atgcn1-2 produced yellow leaves when grown in soil (Fig. 3a). RT-PCR showed that the expression of the AtGCN1 fragment downstream of the T-DNA insertion site was down-regulated in atgcn1-2, while the expression of the fragment across the insertion site was not detectable (Fig. 1b).
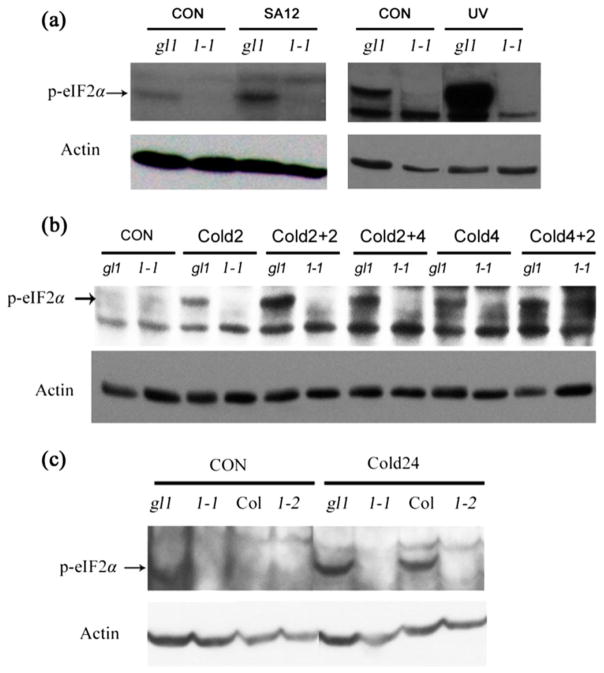
Figure 3.
The phosphorylation of eIF2α is abolished in atgcn1-1 and atgcn1-2. (a) The phosphorylation level of eIF2α was examined in the wild type and atgcn1-1 after SA treatment or UV stress. Seedlings were treated with 0.6 mM SA for 12 h or with UV (500 mJ/cm2 UV irradiation) and then with 1 h light. Immunoblotting assays were performed using phospho-specific antibody of eIF2α. (b) The phosphorylation level of eIF2α was assessed in the wild type and atgcn1-1 after cold shock. Cold2, 2 h of cold treatment; Cold2 + 2, 2 h of recovery after 2 h of cold treatment; Cold2 + 4, 4 h of recovery after 2 h of cold treatment; Cold4, 4 h of cold treatment; Cold4 + 2, 2 h of recovery after 4 h of cold treatment. Immunoblotting assays were performed using phospho-specific antibody of eIF2α. (c) The phosphorylation level of eIF2α in atgcn1-1, atgcn1-2 and gcn2 after 24 h of cold treatment. 1-1, atgcn1-1; 1-2, atgcn1-2. Upper and lower bands are the results of immunoblotting assays using the antibodies against phosphorylated eIF2α and Actin, respectively.
To determine whether the production of yellow leaves in atgcn1 mutants when grown in soil is because of a deficiency in chloroplast development, we measured the contents of chlorophyll (Chl) A and B and also measured the expression of Light harvesting complex b1 (Lhcb1), which encodes a light-harvesting Chl A/B-binding protein in Photosystem II. The levels of Chl were significantly lower in atgcn1-1 and atgcn1-2 than in the wild type (Fig. 1c), and the expression of the Lhcb1 gene was significantly down-regulated in atgcn1-1 and atgcn1-2, relative to the wild type (Fig. 1d).
AtGCN1 was previously isolated and named ILITYHIA (ILA) (Monaghan and Li 2010). In that study, ILA was found to be required for plant immunity, because ila mutants were more sensitive to pathogens than the wild type. Moreover, the new leaves of ila mutants were yellow when ila mutants were grown in soil, the phenotype of which is as same as atgcn1-1 and atgcn1-2, supporting that AtGCN1 is the gene required for chloroplast development.
AtGCN1 interacts with GCN2 and is required for the phosphorylation of eIF2α
We analysed the protein sequence of AtGCN1 by BLAST search in the NCBI database and found that AtGCN1 has a high degree of similarity with the predicted translational activator GCN1 in other plants: the identity between AtGCN1 and the GCN1 in Brassica napus, Glycine max and Oryza sativa is 90%, 71% and 65%, respectively. Protein alignment indicated that AtGCN1 shares relatively low identity with the GCN1 from both yeast (36% identity) and mammals (43% identity) (Fig. S2a, b). In yeast, GCN1, a member of the ATP-binding cassette (ABC) family, is required for the activation of GCN2, which in turn phosphorylates eIF2α under amino acid-deprived conditions (Marton et al. 1993; Sattlegger and Hinnebusch 2005). In Arabidopsis, GCN2 has been reported to phosphorylate eIF2α upon stress (Lageix et al. 2008; Zhang et al. 2003). To date, the role of GCN1 in the phosphorylation of eIF2α has not been reported in plants. Because AtGCN1 is the homolog of GCN1 in yeast, we hypothesized that AtGCN1 might also interact with GCN2 and trigger GCN2-mediated phosphorylation of eIF2α in Arabidopsis.
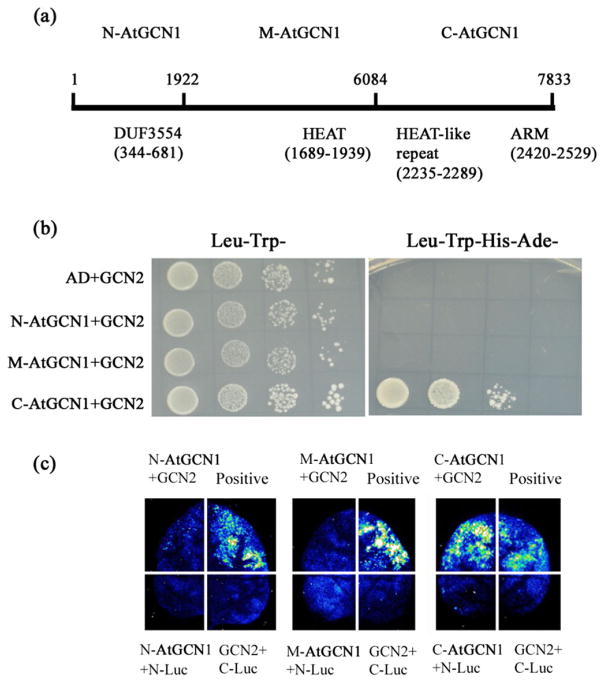
The large size of the AtGCN1 cDNA (7833 bp) makes cloning of full length AtGCN1 difficult, and we therefore chose to clone the N-terminal, middle and C-terminal regions of AtGCN1 (Fig. 2a) and to test the interaction of each of these fragments with GCN2. Only the C-terminal region of AtGCN1 interacted with GCN2 (Fig. 2b), which is consistent with the result reported in yeast (Sattlegger and Hinnebusch 2000). The firefly luciferase complementation assay further confirmed the interaction between the C-terminal region of AtGCN1 and GCN2 (Fig. 2c). Upon UV, cold or SA-induced stress, the phosphorylation level of eIF2α was greatly increased in the wild type but was nearly absent in atgcn1-1 (Fig. 3a,b). These data demonstrate that AtGCN1 interacts with GCN2 and suggest that this interaction is required for GCN2-mediated phosphorylation of eIF2α in Arabidopsis.
Figure 2.
Interaction between AtGCN1 and GCN2. (a) The AtGCN1 CDS sequence was divided into three fragments: N-AtGCN1 (1–1922 nt), M-AtGCN1 (1923–6084 nt) and C-AtGCN1 (6085–7833 nt). The motifs identified in AtGCN1 are described below the black line. (b) The C-terminal of AtGCN1 interacts with GCN2 in yeast. AD, the empty pGADT7 vector; N-AtGCN1, the N-terminal region of AtGCN1 fused with AD; M-AtGCN1, the middle part of AtGCN1 fused with AD; C-AtGCN1, the C-terminal region of AtGCN1 fused with AD. GCN2 was fused with pGBDT7. Leu− Trp− represents SD medium without Leu and Trp, and Leu−Trp− His−Ade− represents SD medium without Leu, Trp, His and Ade. (c) The firefly luciferase (LUC) images of N. benthamiana leaves expressing the fusion genes. N-Luc, N-terminal fragment of Luc; C-Luc, C-terminal fragment of Luc; N-AtGCN1, N-terminal region of AtGCN1 fused with C-Luc; M-AtGCN1, the middle region of AtGCN1 fused with C-Luc; C-AtGCN1, C-terminal region of AtGCN1 fused with C-Luc. GCN2 was fused with N-Luc. The positive interaction between OST1-C-Luc and ABI1-N-Luc was used as a control.
atgcn1-1 and atgcn1-2 are hypersensitive to the herbicide chlorsulfuron
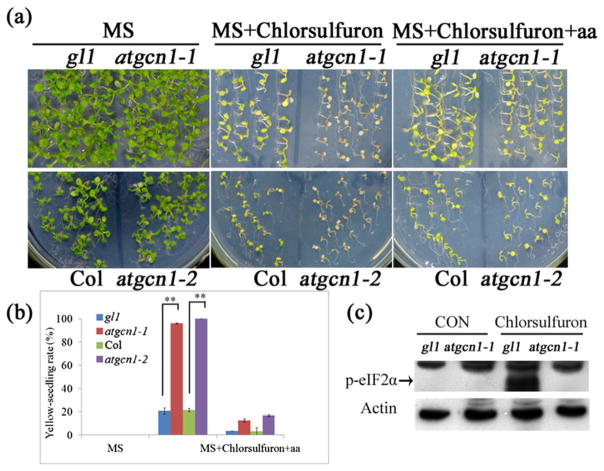
Because it interferes with the biosynthesis of isoleucine, leucine and valine, the herbicide chlorsulfuron can be used to induce amino acid starvation in plants (Ray 1984). Chlorsulfuron treatment induced the phosphorylation of eIF2α in the wild type, but the phosphorylation was absent in atgcn1-1 (Fig. 4c). After chlorsulfuron treatment, many more atgcn1-1 and atgcn1-2 seedlings became yellow than their wild-type counterparts (Fig. 4a,b). The result showed that atgcn1 mutants were more sensitive to amino acid deficiency, induced by chlorsulfuron, than the wild type. Supplementing plants with leucine, isoleucine and valine, the frequency of yellow-seedlings in atgcn1-1 and atgcn1-2 was restored to the wild-type level, suggesting that amino acid complementation alleviated the hypersensitivity of these mutant lines to chlorsulfuron. These results indicate that AtGCN1 plays an important role in the response to amino acid-starvation in Arabidopsis.
Figure 4.
atgcn1-1 and atgcn1-2 are sensitive to the herbicide chlorsulfuron. (a) The atgcn1-1 and atgcn1-2 mutants displayed higher sensitivity to chlorsulfuron than the wild type. MS, MS medium; MS + chlorsulfuron, MS medium supplemented with chlorsulfuron; MS + chlorsulfuron + aa, MS medium supplemented with chlorsulfuron and amino acids including isoleucine, leucine, and valine. (b) Quantification of yellow plants induced by chlorsulfuron. t-tests were performed between atgcn1-1, atgcn1-2 and the wild type (gl1 background). Double asterisks indicate p <0.01. (c) Immunoblotting assay shows the phosphorylation level of eIF2α in atgcn1-1 after chlorsulfuron treatment.
atgcn1-1 and atgcn1-2 are hypersensitive to cold stress
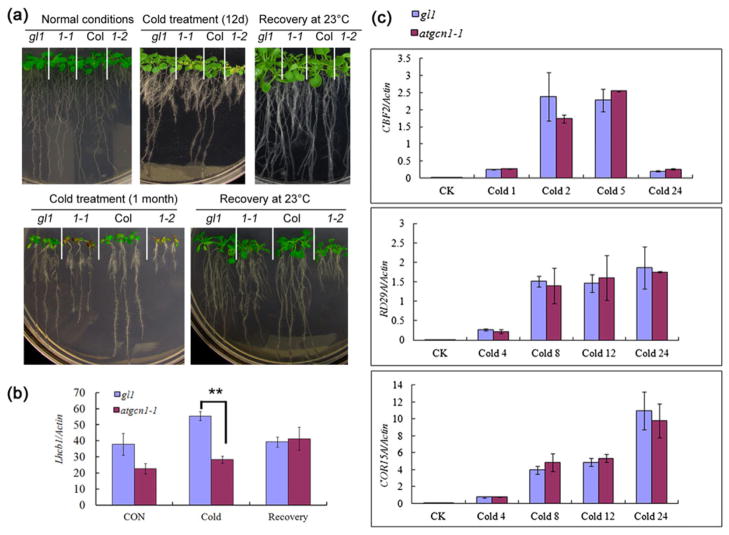
Cold shock or prolonged cold stress induced the phosphorylation of eIF2α in the wild type but not in atgcn1 mutants (Fig. 3b,c), suggesting that AtGCN1 is involved in the plant response to cold stress. We subjected atgcn1-1 and atgcn1-2 to low temperature and found that atgcn1-1 and atgcn1-2 were hypersensitive to cold stress (Fig. 5a). The new leaves of atgcn1-1 and atgcn1-2 were green in plates at 23 °C but were yellow with incubation at 4 °C for 12 d and were purple after a 30 d cold treatment. However, the yellow leaves became green when the mutants were transferred to 23 °C for 3 d. In contrast, the leaves of the wild type did not become yellow after cold stress for even 1 month. These results suggest that eIF2α phosphorylation mediated by AtGCN1 plays important roles in cold tolerance.
Figure 5.
atgcn1-1 and atgcn1-2 are hypersensitive to cold stress. (a) The new leaves of atgcn1-1 and atgcn1-2 were yellow or purple under cold stress. One-week-old seedlings were transferred to 4 °C for 12 d, or 5-day-old seedlings were transferred to 4 °C for 1 month. Seedlings began to produce green leaves 3 d after they were transferred from cold to warm conditions. (b) Quantitative analysis of the expression of Lhcb1 in atgcn1-1 under cold stress. For cold treatment, the seedlings were transferred to 4 °C for 12 d. For recovery, the seedlings were moved to 23 °C for 3 d after cold treatment. t-tests were performed between atgcn1-1 and the wild type (gl1 background). Double asterisks indicate p <0.01. (c) Quantitative analysis of the expression of cold-responsive genes CBF2, RD29A and COR15A in atgcn1-1 after cold treatment.
Because Lhcb1 is associated with chloroplast development (Jansson 1994), we investigated whether the expression of Lhcb1 is altered in the atgcn1-1 mutant under cold stress (4 °C). The expression of Lhcb1 was down-regulated in atgcn1-1 after cold treatment for 12 d but recovered to the wild-type level when the mutants were transferred to 23 °C (Fig. 5b). This result is consistent with the phenotype of atgcn1-1 under cold stress, suggesting that the cold-induced yellow leaves of the atgcn1 mutant are caused, at least partially, by the down-regulation of Lhcb1 expression.
In plants, low temperatures rapidly induce the expression of CBF genes, which encode AP2/ERF (APETALA2/Ethylene-Responsive Factor) transcription factors, and downstream cold-responsive (COR) genes, such as RD29A and COR15A (Jaglo-Ottosen et al. 1998). We investigated whether the expression of these cold-responsive genes was altered in the atgcn1-1 mutant, and we found that the expression levels of CBF2, CBF1 and CBF3 did not significantly differ between atgcn1-1 and the wild type after cold treatment (Fig. 5c and Fig. S3). The expression of the downstream COR genes RD29A and COR15A was also not significantly affected in atgcn1-1 mutant plants (Fig. 5c). These results suggest that AtGCN1 is involved in the cold response via a CBF-independent pathway.
AtGCN1 is required for the inhibition of protein translation
Upon amino acid-starvation stress, global protein synthesis is shut down through the phosphorylation of eIF2α in yeast (Rowlands et al. 1988; Scorsone et al. 1987). Because atgcn1-1 and atgcn1-2 lack eIF2α phosphorylation (Fig. 3c), we hypothesized that the protein translation rate would be higher in atgcn1-1 and atgcn1-2 than in the wild type. However, by labelling newly synthesized proteins with [35S]Met and [35S]Cys, we found that the rate of the de novo protein synthesis was decreased in both the wild type and atgcn1 mutants under cold stress and that the rate of protein synthesis did not significantly differ between the wild type and the atgcn1 mutants under either warm or cold conditions (Fig. S4).
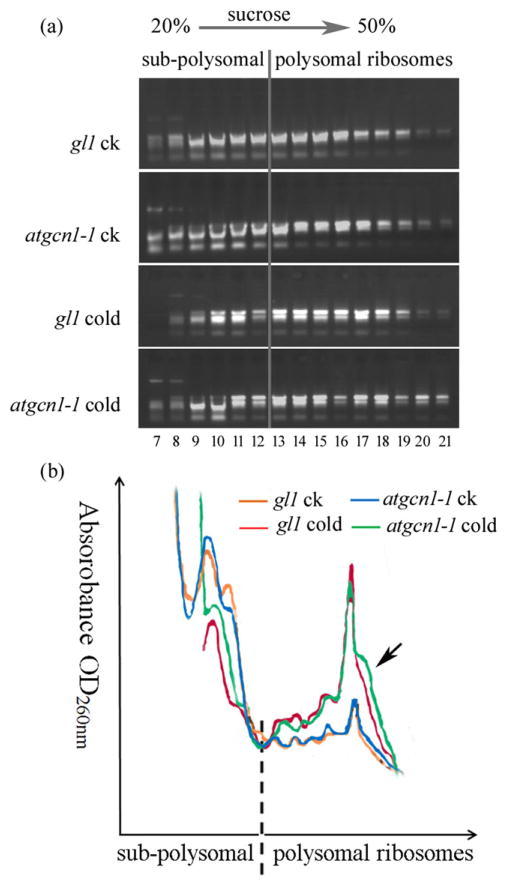
Ribosomal profiles, representing the translation state of mRNA, can be used to determine translation levels (del Prete et al. 2007). Ribosomes of the wild type and atgcn1-1 were extracted and loaded on sucrose gradients for centrifugation. The sucrose gradients were divided into 22 fractions, and each was collected for RNA extraction. Under cold stress, more mRNAs with polysomes towards fractions of 50% sucrose (20–21st fractions) were accumulated in atgcn1-1 than in the wild type (Fig. 6a), indicating that the translation rate was higher in atgcn1-1 than in the wild type. In addition, ribosomal extraction and RNA profiling at A260 after centrifugation in sucrose gradients also showed that mRNAs with polysomes towards fractions of 50% sucrose were more abundant in atgcn1-1 than in the wild type under cold stress (Fig. 6b).
Figure 6.
Ribosome-bound mRNA profiles under cold stress. (a) Ribosome-bound mRNA analysis in gel. The extracted polysomes were loaded to 11 mL 20%–50% sucrose gradients and then centrifuged at 175 000 g for 150 min at 4 °C. After centrifugation, 22 fractions (0.5 mL/fraction) were harvested from the top to the bottom of the sucrose gradients. RNA from each fraction was isolated for RNA gel analysis. The 1–6th and 22nd fractions, in which no mRNA accumulated, were removed. (b) Polysome profiles of atgcn1-1 plants. After centrifugation, OD260 values from the bottom of the sucrose gradients were recorded. CON, control condition; Cold24, 24 h of cold treatment. Arrow shows the increased mRNA content in ribosomes enriched in 50% sucrose in atgcn1-1 under cold stress. The highest peak pointed to 50% sucrose should be the interference of chlorophyll, which occurred in the green fractions of sucrose gradients. Experiments were repeated three times with similar results.
In brief, although newly synthesized proteins decreased in both atgcn1 mutants and the wild type under cold stress, ribosome-bound RNA profiling showed that AtGCN1 is required for the inhibition of protein translation under cold stress.
The TOR pathway is involved in cold response
Previous study has shown that the phosphorylation level of eIF2α is decreased in the gcn2 mutant, which causes the gcn2 mutant to be hypersensitive to amino acid deficiency (Zhang et al. 2008). We also checked de novo protein synthesis in gcn2 and found no difference between gcn2 and the wild type under both warm temperature and cold stress conditions (Fig. S4). The absence of a significant difference in de novo protein synthesis between atgcn1-1, gcn2 and their wild-type counterparts (Fig. S4) suggests that eIF2α phosphorylation mediated by AtGCN1 and GCN2 is not the only pathway triggering the inhibition of protein translation under cold stress.
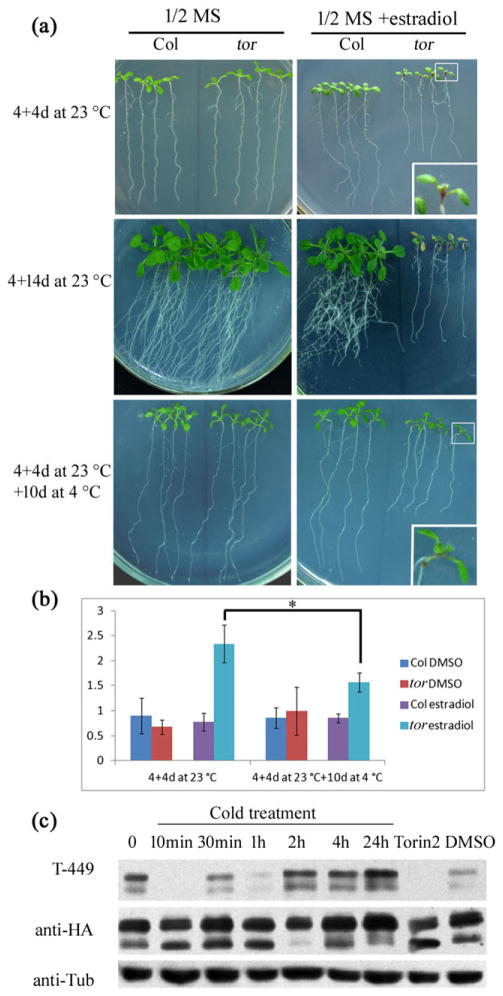
In mammals, phosphorylation of eIF2α and inhibition of the TOR pathway work in parallel to inhibit protein translation in response to low temperature and ER stress (Duan et al. 2010; Hofmann et al. 2012). We next investigated whether the TOR pathway is also involved in the cold response in Arabidopsis. The estradiol-induced tor mutant, which overcomes the embryo lethality of the null tor mutants, was used to examine the cold response. The tor seedlings grew well on MS medium, but the upper hypocotyls of the tor seedlings became purple after they were transferred to a medium containing estradiol for 4 d, and the plants were nearly dead after an additional 10 d (Fig. 7a). This symptom is caused by the decreased translation in tor seedlings after treatment with estradiol. However, when estradiol-induced tor mutant seedlings with purple hypocotyls were transferred to cold conditions for an additional 10 d, the purple hypocotyls became green. The increased quantity of anthocyanin in the estradiol-induced tor mutant was also partially recovered under prolonged cold stress (Fig. 7b). We propose that the decreased translation level is one of the responses under cold stress in Arabidopsis, so the low translation level in tor seedlings increases their ability to survive under cold stress.
Figure 7.
The TOR pathway is involved in the cold response. (a) The production of purple hypocotyls by estradiol-induced tor seedlings is reduced by cold treatment. Four-day-old seedlings were transferred to MS medium with or without estradiol (10 μM) for 4 d, and seedlings were then kept at 23 °C or at 4 °C for 10 d with light. The squared seedling was magnified and shown at the bottom. (b) Anthocyanin content in tor mutant seedlings. t-tests compared the contents in the mutants before and after prolonged cold treatment. Single asterisk indicates p <0.05. (c) The phosphorylation level of S6K1 after cold treatment. Total proteins were analysed by immunoblotting using anti-Thr (P)-T449 (T-449) of S6K1, anti-HA (for detecting S6K1-HA) or anti-Tubulin (anti-Tub) antibody. As a control, plants were treated with Torin2, an inhibitor of TOR activity and the solvent DMSO at 0.1% for 1 h.
Because the purple hypocotyls of the tor mutant were partially recovered under cold condition (Fig. 7a,b), we inferred that TOR is inactivated and thereby inhibits protein translation under cold stress in Arabidopsis as was shown to occur in mammalian cells (Hofmann et al. 2012). Under normal conditions, active TOR kinase phosphorylates its target AtS6K1 to initiate protein translation of uORF-mRNA in Arabidopsis (Schepetilnikov et al. 2013). Using a phospho-specific antibody against phosphorylated AtS6K1, we found that the phosphorylation level of AtS6K1 was significantly decreased after cold treatment for 10, 30 and 60 min (Fig. 7c). As a control, Torin 2, which is an inhibitor of TOR (Xiong and Sheen 2012), inhibited the phosphorylation of AtS6K1 (Fig. 7c). This result indicates that TOR kinase activity was suppressed under cold stress. However, the phosphorylation of AtS6K1 was restored to the original level after a 2 h cold treatment and even increased after a 24 h cold treatment, suggesting that TOR activity is suppressed at the early stage of cold treatment but recovers and even increases after prolonged cold treatment.
DISCUSSION
In yeast, GCN2-mediated phosphorylation of eIF2α has been extensively studied under a variety of stresses, including nutrient deprivation (Dever et al. 1992; Yang et al. 2000), UV damage and osmotic stress (Narasimhan et al. 2004; Wu et al. 2004). The interaction of GCN1 with GCN2 is essential for the activation of GCN2 (Marton et al. 1993; Sattlegger and Hinnebusch 2005). Here, we cloned Arabidopsis AtGCN1, which is a homologue of the yeast GCN1. Our results show that AtGCN1 interacts with GCN2 and that the interaction is required for the phosphorylation of eIF2α under UV damage, SA treatment, amino acid deprivation and cold stress. The atgcn1-1 and atgcn1-2 mutants were sensitive to amino acid starvation and cold stress, suggesting that AtGCN1 is required for plants to cope with amino acid starvation and cold stress in Arabidopsis.
AtGCN1 was previously isolated and named as ILITYHIA (ILA) (Monaghan and Li 2010). Mutant ila plants produced yellow leaves and had reduced immunity against pathogens. The function of AtGCN1 in the phosphorylation of eIF2α and cold tolerance had not been reported. In this study, we found that the phosphorylation of eIF2α was abolished in the atgcn1-1 mutant not only under SA induction but also under cold stress, suggesting that AtGCN1 is involved both inthe cold response and in SA-mediated plant immunity. Moreover, the absence of SA-induced eIF2α phosphorylation in atgcn1 suggests that the hypersensitivity of ila mutants to bacterial pathogens may result from the loss of eIF2α phosphorylation.
Arabidopsis GCN2 showed a high degree of similarity with the protein kinase domain of yeast GCN2 and with human PKR, PERK, and HRI kinases (Lageix et al. 2008; Zhang et al. 2003). Previous studies have shown that the phosphorylation level of eIF2α was decreased in the gcn2 mutant. Here, we also found that the phosphorylation level of eIF2α was decreased but still detectable in the gcn2 mutant (Fig. S5a), indicating that the function of GCN2 is affected but not eliminated in gcn2. In the gcn2 mutant, a DS transposon was inserted in the first intron of GCN2, which probably leads to a weak mutation. This weak mutation may explain why the gcn2 mutant grew well both in soil and on plates either with or without cold treatment (Zhang et al. 2008 and Fig. S5b).
The mutants of translation elongation factors show higher sensitivity to cold stress than the wild type, suggesting that translation elongation factors are required for expression of COR genes (Calderon-Villalobos et al. 2007; Guo et al. 2002). In atgcn1-1, however, the expression of CBFs and of the downstream COR genes RD29A and COR15A was not altered in response to cold stress, suggesting that inhibition of the phosphorylation of the translation initiation factor eIF2α does not affect the expression of cold-responsive genes, and that the cold hypersensitive phenotype of atgcn1-1 is not caused by the impairment of the CBF-mediated pathway.
Phosphorylation of eIF2α in response to stress reduces global protein translation because the complex of phosphorylated eIF2α and GDP occupies eIF2B so that no eIF2B is available to convert eIF2α-GDP into eIF2α-GTP for translation initiation (Rowlands et al. 1988; Scorsone et al. 1987). However, the responses of the gcn2Δ mutant in yeast and of the perk mutant or eIF2α-S51A cells in mammals to cold stress are similar to those of the wild type, indicating that eIF2α phosphorylation does not appear to be required for the cold-induced inhibition of protein translation in either yeast or mammalian cells (Hofmann et al. 2012). In the current study, ribosome-bound RNA profiling after centrifugation in a sucrose gradient showed that the state of protein translation is higher in atgcn1-1 than in the wild type, not only under warm temperatures but also under cold stress. The mutation of AtGCN1 impairs chloroplast development under cold conditions and causes plants to be hypersensitive to cold stress. These results indicate that eIF2α phosphorylation is essential for the inhibition of protein translation under cold stress, which in turn contributes to the cold tolerance in Arabidopsis.
When growing on plates at warm temperatures, atgcn1-1 and atgcn1-2 produce new leaves that are as green as those of the wild type. When growing in soil at warm temperatures, however, atgcn1-1 produces new leaves that are yellow. The reason for this discrepancy in phenotypes for seedlings growing on plates versus in soil is unclear. The humidity of plates in chambers at 23 °C and 4 °C was 80% and 84%, respectively, while the humidity in the greenhouse, where the seedlings were grown in soil, was 47%. Covering atgcn1-1 seedlings in the greenhouse with a transparent film maintained the humidity at 80% and resulted in a partial recovery of the yellow new leaves and the restoration of Chl content to wild-type levels (Fig. S6a,b). This suggests that atgcn1-1 produces yellow new leaves in the greenhouse because of low humidity and that AtGCN1 plays important roles in chloroplast development not only in response to cold stress but also in response to low humidity.
In yeast and mammalian cells, the inhibition of global protein translation in response to cold stress is associated with a decreased polysomal level (Hofmann et al. 2012). In Arabidopsis, although protein translation was inhibited under cold stress (Fig. S4), polysomes still accumulated. The discrepancy between ribosome density and protein production rate under warm temperatures and cold stress is consistent with a previous study with wheat in which polysomes increased while protein translation was inhibited at low temperatures (Fehling and Weidner 1986). One possible explanation for the accumulation of polysomes is that cold stress, as well as cadmium intoxication, may inhibit translation elongation or translation termination, which causes ribosomes to remain longer than normal on mRNAs (Sormani et al. 2011). Another possible explanation is that low temperatures affect the biogenesis of ribosomal RNAs or proteins, which as a feedback triggers the formation of ribosomes in plants (Laroche and Hopkins 1987).
The ribosome-bound RNA profiling is used to track the real-time translational state in vivo while the labelling of newly synthesized proteins with 35S amino acids is used to detect the amount of proteins that are newly synthesized during a certain period, so the former method is more sensitive and accurate than the latter one to determine the translational level at a specific time. Therefore, because of the different sensitivity, the difference in translational level between atgcn1-1 mutants and the wild type can be detected by the method of ribosome-bound RNA profiling, but not by the method of labelling of newly synthesized proteins (Fig. S4). Based on the result from ribosome-bound RNA profiling, we can conclude that the translational level is higher in atgcn1-1 mutants than in the wild type.
In both yeast and mammalian cells, protein synthesis is inhibited when eIF2α phosphorylation or the TOR pathway is blocked, and the mutants that are disrupted in eIF2α phosphorylation or in the TOR pathway grew as well as the wild type under cold stress (Hofmann et al. 2012). These results showed that eIF2α phosphorylation is not required for cold tolerance in either yeast or mammalian cells. The current study showed that atgcn1-1, which was defective in the phosphorylation of eIF2α and had a higher translational level than the wild type under cold stress, was more sensitive to cold stress than the wild type, suggesting that the inhibition of the translation initiation caused by eIF2α phosphorylation is required for cold tolerance in Arabidopsis.
Although the ribosome-bound RNA profiling showed that the translation level is higher in atgcn1-1 than in the wild type, the de novo protein synthesis levels decreased in both atgcn1 mutants and the wild type under cold stress (Fig. S4), suggesting that the AtGCN1-mediated phosphorylation of eIF2α is not the only pathway for the cold-induced inhibition of protein translation in Arabidopsis. In mammalian cells, (mammalian TOR complex 1 (mTORC1) mediates the suppression of translation during cold shock and prolonged ER stress through a pathway independent of eIF2α phosphorylation (Duan et al. 2010; Hofmann et al. 2012). Cold treatment caused the purple hypocotyls of tor seedlings to become partially green, and the phosphorylation of S6K1 was decreased upon cold stress, indicating that TOR is inactivated under cold stress in order to reduce protein translation and to promote cell growth in Arabidopsis.
Inhibition of AMPK reduces the inhibition of translation and reduces cell survival during cold shock, indicating that translation inhibition is required for cold tolerance in mammals (Hofmann et al. 2012). AMPK works upstream of TORC1, and TORC1 is required for the dephosphorylation of GCN2 at Ser577, which subsequently affects eIF2α phosphorylation in mammals and yeast (Cherkasova and Hinnebusch 2003; Hofmann et al. 2012). These results indicate that AMPK, the TOR pathway and eIF2α phosphorylation communicate with each other in mammals and yeast. However, neither the tor mutant nor transgenic plants overexpressing TOR interfered with eIF2α phosphorylation in Arabidopsis (Lageix et al. 2008), suggesting that the TOR pathway and eIF2α phosphorylation work in parallel in plants.
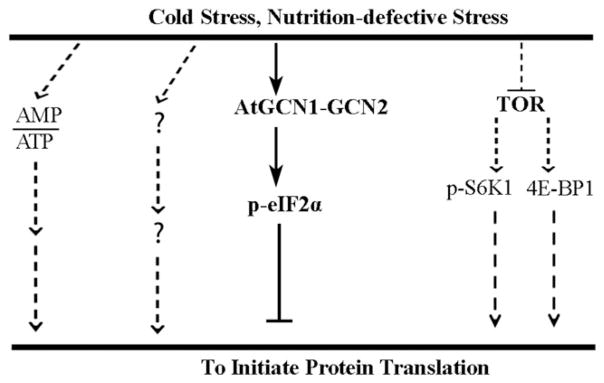
Protein synthesis, which costs 22–30% of total energy, is very sensitive to ATP supply (Buttgereit and Brand 1995). Therefore, upon cold stress or nutrient deprivation, at least three pathways, including those involving the ratio of AMP/ATP, GCN1-GCN2-p-eIF2 and the inactivation of TOR kinase, contribute to the inhibition of protein translation in Arabidopsis (Fig. 8). Upon stress, the inhibition of translation initiation enhances cell survival by reducing ATP consumption. Our results showed that the absence of eIF2α phosphorylation in the atgcn1 mutant impairs chloroplast development in new leaves under prolonged cold stress, which is at least partially caused by a reduced ability to inhibit translation initiation.
Figure 8.
Model of GCN1-GCN2-p-eIF2α in cold response. Upon cold stress or nutrient deprivation, at least three cellular signalling pathways, including those involving the ratio of AMP/ATP, GCN1-GCN2-p-eIF2 and TOR kinase, contribute to the inhibition of protein translation in Arabidopsis. In this study, GCN1-GCN2 and the TOR pathways, indicated by bold characters, were found to be required for cold tolerance. Dotted arrows indicate the pathways that are known to be involved in the cold tolerance in mammals, but the functions of these pathways in the cold tolerance in plants are still unknown. The functions of GCN1-GCN2-p-eIF2α TOR pathways in response to nutrient deficiency, such as amino acid or glucose starvation, have been well documented in Arabidopsis.
Although our results demonstrate that eIF2α phosphorylation and TOR inactivation are involved in the response to cold stress in Arabidopsis, many questions remain, including the following: Is there a gene similar to GCN4 or ATF4 that can activate the expression of stress-responsive genes in plants? Why does the phosphorylation level of S6K1 increase under prolonged cold stress? Moreover, the mechanism regulating the AMP/ATP ratio in the cold response requires study. Our understanding of the cold response in plants also requires more information on the communication among eIF2α phosphorylation, TOR kinase and AMP/ATP ratio.
In conclusion, our results demonstrate that AtGCN1 interacts with GCN2 and suggest that the interaction is required for the phosphorylation of the translation initiation factor eIF2α. Compared with the wild type, atgcn1-1 and atgcn1-2, which are defective in the phosphorylation of eIF2α, are hypersensitive to cold stress. The ribosome-bound RNA profiling showed that the translation state was higher in atgcn1-1 than in the wild type, supporting the inference that AtGCN1-mediated phosphorylation of eIF2α increases the cold tolerance of Arabidopsis by inhibiting the initiation of protein translation. Finally, cold treatment partially relieved the purple hypocotyl phenotype of the tor mutant, and the phosphorylation of S6K1 was decreased when tor plants were exposed to cold stress, suggesting that the inactivation of TOR kinase, in addition to eIF2α phosphorylation, is also involved in the inhibition of protein translation in response to cold stress in Arabidopsis.
Supplementary Material
Figure S1 Diagram of the atgcn1-1 mutation. (a) Partial sequence of AtGCN1, including the atgcn1-1 mutation. Upper-case letters represent exon sequences; lowercase ones represent intron sequences. The sequence in atgcn1-1 mutant is marked as blue. The red letters indicate the mutation from G to A in atgcn1-1, which leading to the splicing of the next G. (b) The partial protein sequence of AtGCN1, including the mutation site. The sequence in atgcn1-1 mutant is marked as blue. The red letters show the mutated site in which the sequence of atgcn1-1 is frame-shifted, leading to a premature stop when translated.
Figure S2 Multiple protein alignment of AtGCN1 with its homologues by Clustal Omega. (a) Alignment results of AtGCN1 with its homologues in other species. (b) Phylogenetic tree of AtGCN1 and its homologues. AtGCN1, AT1G64790; Saccharomyces cerevisiae, CAA96907.1; Homo sapiens, NP_006827.1; Oryza sativa, XP_015630606.1; Brassica napus, XP_013683657.1; Glycine max, XP_006577327.1.
Figure S3 Quantitative analysis of the expression levels of cold-responsive genes CBF1 and CBF3 in atgcn1-1. There was no significant difference in the expression of CBF1 and CBF3 between atgcn1-1 and gl1 after the cold treatments.
Figure S4 De novo protein synthesis of gcn mutants at both warm temperature and cold conditions. One-week-old plants grown on MS medium were cold-acclimated at 4 °C for 4 d and then labelled with [35S]Met and [35S]Cys in the chamber at either 23 °C (CON) or 4 °C (Cold) for 36 h. 1-1, atgcn1-1; 1-2, atgcn1-2. The total amount of proteins were extracted and analysed by running in a 10% SDS-PAGE gel. Coomassie Blue stained gel (bottom) was exposed for autoradiography (top). Experiments were repeated twice with similar results.
Figure S5 Phenotypic analysis of gcn2 under prolonged cold stress. (a) The phosphorylation level of eIF2α was reduced but detectable in gcn2 mutant. (b) New leaves of gcn2 did not become yellow after 12-day prolonged cold stress.
Figure S6 The yellow leaves of atgcn1-1 are partially recovered in high humidity condition. (a) The yellow leaves of atgcn1-1 are partially recovered in high humidity condition. Three-week plants grown in soil were covered by a transparent membrane for 5 d. (b) Quantification of Chl contents in atgcn1-1 after growing in high humidity condition for 5 d. Double asterisks indicate p-value <0.01. Experiments were repeated for three times with similar results.
Table. S1 Primers used in the study.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China to Hairong Zhang (31270309 and U1204303) and by grants from National Institutes of Health (R01GM070795 and R01GM059138) and the Chinese Academy of Sciences to Jian-Kang Zhu. We also thank the China Scholarship Council for financial support to Hairong Zhang.
We acknowledge the Arabidopsis Biological Resource Center (Columbus, Ohio) for providing the T-DNA seeds, Cold Spring Harbor Laboratory (New York, http://genetrap.cshl.org) for providing the gene trap line GT8359 of mutant gcn2 and Dr. Yan Xiong (Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences) for providing the tor mutant and the At-S6K1-HA transgenic seeds.
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Advances in Nutrition. 2012;3:307–321. doi: 10.3945/an.112.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochemistry Journal. 1995;312:163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Villalobos LI, Nill C, Marrocco K, Kretsch T, Schwechheimer C. The evolutionarily conserved Arabidopsis thaliana F-box protein AtFBP7 is required for efficient translation during temperature stress. Gene. 2007;392:106–116. doi: 10.1016/j.gene.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiology. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova VA, Hinnebusch AG. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes and Development. 2003;17:859–872. doi: 10.1101/gad.1069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Prete MJ, Vernal R, Dolznig H, Mullner EW, Garcia-Sanz JA. Isolation of polysome-bound mRNA from solid tissues amenable for RT-PCR and profiling experiments. RNA. 2007;13:414–421. doi: 10.1261/rna.79407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen HC, Zhang Y. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytologist. 2010;186:681–695. doi: 10.1111/j.1469-8137.2010.03207.x. [DOI] [PubMed] [Google Scholar]
- Fehling E, Weidner M. Temperature characteristics and adaptive potential of wheat ribosomes. Plant Physiology. 1986;80:181–186. doi: 10.1104/pp.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods in Enzymology. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xiong L, Ishitani M, Zhu JK. An Arabidopsis mutation in translation elongation factor 2 causes superinduction of CBF/DREB1 transcription factor genes but blocks the induction of their downstream targets under low temperatures. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7786–7791. doi: 10.1073/pnas.112040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S, Cherkasova V, Bankhead P, Bukau B, Stoecklin G. Translation suppression promotes stress granule formation and cell survival in response to cold shock. Molecular Biology of the Cell. 2012;23:3786–3800. doi: 10.1091/mbc.E12-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. Arabidopsis map-based cloning in the post-genome era. Plant Physiology. 2002;129:440–450. doi: 10.1104/pp.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson S. The light-harvesting chlorophyll a/b-binding proteins. Biochimica et Biophysica Acta. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Lageix S, Lanet E, Pouch-Pelissier MN, Espagnol MC, Robaglia C, Deragon JM, Pelissier T. Arabidopsis eIF2alpha kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC Plant Biology. 2008;8:134. doi: 10.1186/1471-2229-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche A, Hopkins WG. Polysomes from winter rye seedlings grown at low temperature: I. Size class distribution, composition, and stability. Plant Physiology. 1987;85:648–654. doi: 10.1104/pp.85.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter WD, Pruitt RE. Developmental regulation of cell interactions in the Arabidopsis fiddlehead-1 mutant: a role for the epidermal cell wall and cuticle. Developmental Biology. 1997;189:311–321. doi: 10.1006/dbio.1997.8671. [DOI] [PubMed] [Google Scholar]
- Marton MJ, Crouch D, Hinnebusch AG. GCN1, a translational activator of GCN4 in Saccharomyces cerevisiae, is required for phosphorylation of eukaryotic translation initiation factor 2 by protein kinase GCN2. Molecular Cell Biology. 1993;13:3541–3556. doi: 10.1128/mcb.13.6.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Tanaka H, Ohme-Takagi M. Suppression of the biosynthesis of proanthocyanidin in Arabidopsis by a chimeric PAP1 repressor. Plant Biotechnology Journal. 2004;2:487–493. doi: 10.1111/j.1467-7652.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- Monaghan J, Li X. The HEAT repeat protein ILITYHIA is required for plant immunity. Plant Cell Physiology. 2010;51:742–753. doi: 10.1093/pcp/pcq038. [DOI] [PubMed] [Google Scholar]
- Narasimhan J, Staschke KA, Wek RC. Dimerization is required for activation of eIF2 kinase Gcn2 in response to diverse environmental stress conditions. Journal of Biological Chemistry. 2004;279:22820–22832. doi: 10.1074/jbc.M402228200. [DOI] [PubMed] [Google Scholar]
- Ray TB. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiology. 1984;75:827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AG, Panniers R, Henshaw EC. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. Journal of Biological Chemistry. 1988;263:5526–5533. [PubMed] [Google Scholar]
- Sattlegger E, Hinnebusch AG. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO Journal. 2000;19:6622–6633. doi: 10.1093/emboj/19.23.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger E, Hinnebusch AG. Polyribosome binding by GCN1 is required for full activation of eukaryotic translation initiation factor 2{alpha} kinase GCN2 during amino acid starvation. Journal of Biological Chemistry. 2005;280:16514–16521. doi: 10.1074/jbc.M414566200. [DOI] [PubMed] [Google Scholar]
- Schepetilnikov M, Dimitrova M, Mancera-Martinez E, Geldreich A, Keller M, Ryabova LA. TOR and S6K1 promote translation reinitiation of uORF-containing mRNAs via phosphorylation of eIF3h. EMBO Journal. 2013;32:1087–1102. doi: 10.1038/emboj.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorsone KA, Panniers R, Rowlands AG, Henshaw EC. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. Journal of Biological Chemistry. 1987;262:14538–14543. [PubMed] [Google Scholar]
- Sormani R, Delannoy E, Lageix S, Bitton F, Lanet E, Saez-Vasquez J, … Deragon JM. Sublethal cadmium intoxication in Arabidopsis thaliana impacts translation at multiple levels. Plant Cell Physiology. 2011;52:436–447. doi: 10.1093/pcp/pcr001. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochemical Society Transactions. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wu S, Tan M, Hu Y, Wang JL, Scheuner D, Kaufman RJ. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. Journal of Biological Chemistry. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Sheen J. Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. Journal of Biological Chemistry. 2012;287:2836–2842. doi: 10.1074/jbc.M111.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Wek SA, Wek RC. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Molecular Cell Biology. 2000;20:2706–2717. doi: 10.1128/mcb.20.8.2706-2717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dickinson JR, Paul MJ, Halford NG. Molecular cloning of an arabidopsis homologue of GCN2, a protein kinase involved in co-ordinated response to amino acid starvation. Planta. 2003;217:668–675. doi: 10.1007/s00425-003-1025-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Kanyuka K, Parry MA, Powers SJ, Halford NG. GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2alpha in Arabidopsis. Journal of Experimental Botany. 2008;59:3131–3141. doi: 10.1093/jxb/ern169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Diagram of the atgcn1-1 mutation. (a) Partial sequence of AtGCN1, including the atgcn1-1 mutation. Upper-case letters represent exon sequences; lowercase ones represent intron sequences. The sequence in atgcn1-1 mutant is marked as blue. The red letters indicate the mutation from G to A in atgcn1-1, which leading to the splicing of the next G. (b) The partial protein sequence of AtGCN1, including the mutation site. The sequence in atgcn1-1 mutant is marked as blue. The red letters show the mutated site in which the sequence of atgcn1-1 is frame-shifted, leading to a premature stop when translated.
Figure S2 Multiple protein alignment of AtGCN1 with its homologues by Clustal Omega. (a) Alignment results of AtGCN1 with its homologues in other species. (b) Phylogenetic tree of AtGCN1 and its homologues. AtGCN1, AT1G64790; Saccharomyces cerevisiae, CAA96907.1; Homo sapiens, NP_006827.1; Oryza sativa, XP_015630606.1; Brassica napus, XP_013683657.1; Glycine max, XP_006577327.1.
Figure S3 Quantitative analysis of the expression levels of cold-responsive genes CBF1 and CBF3 in atgcn1-1. There was no significant difference in the expression of CBF1 and CBF3 between atgcn1-1 and gl1 after the cold treatments.
Figure S4 De novo protein synthesis of gcn mutants at both warm temperature and cold conditions. One-week-old plants grown on MS medium were cold-acclimated at 4 °C for 4 d and then labelled with [35S]Met and [35S]Cys in the chamber at either 23 °C (CON) or 4 °C (Cold) for 36 h. 1-1, atgcn1-1; 1-2, atgcn1-2. The total amount of proteins were extracted and analysed by running in a 10% SDS-PAGE gel. Coomassie Blue stained gel (bottom) was exposed for autoradiography (top). Experiments were repeated twice with similar results.
Figure S5 Phenotypic analysis of gcn2 under prolonged cold stress. (a) The phosphorylation level of eIF2α was reduced but detectable in gcn2 mutant. (b) New leaves of gcn2 did not become yellow after 12-day prolonged cold stress.
Figure S6 The yellow leaves of atgcn1-1 are partially recovered in high humidity condition. (a) The yellow leaves of atgcn1-1 are partially recovered in high humidity condition. Three-week plants grown in soil were covered by a transparent membrane for 5 d. (b) Quantification of Chl contents in atgcn1-1 after growing in high humidity condition for 5 d. Double asterisks indicate p-value <0.01. Experiments were repeated for three times with similar results.
Table. S1 Primers used in the study.