Abstract
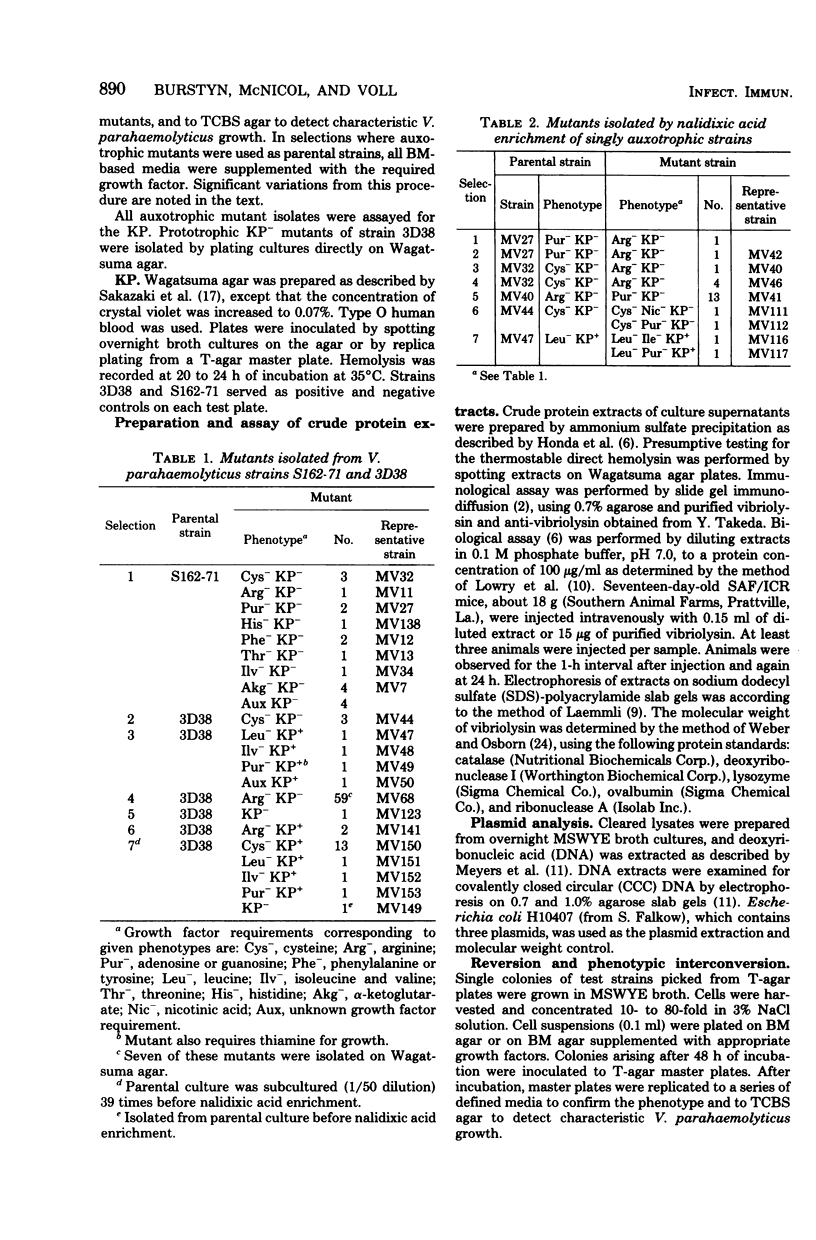
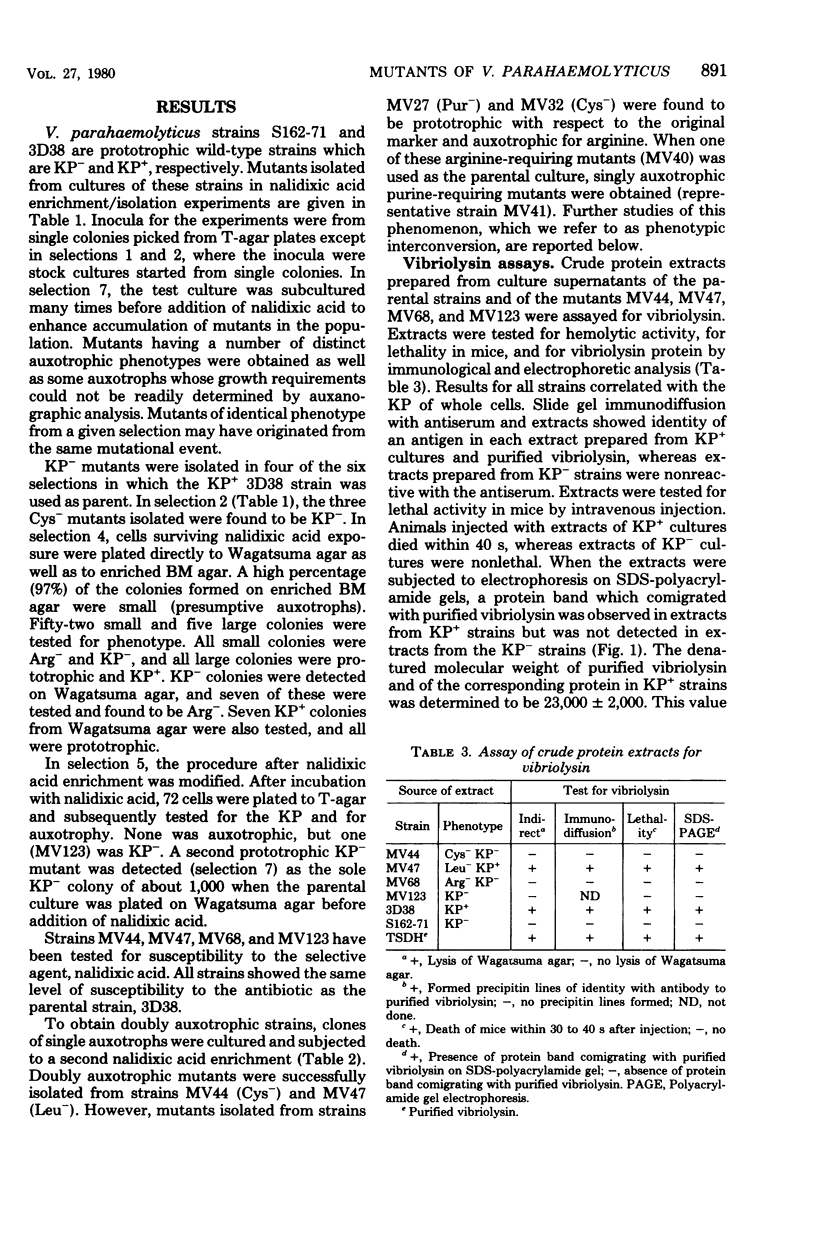
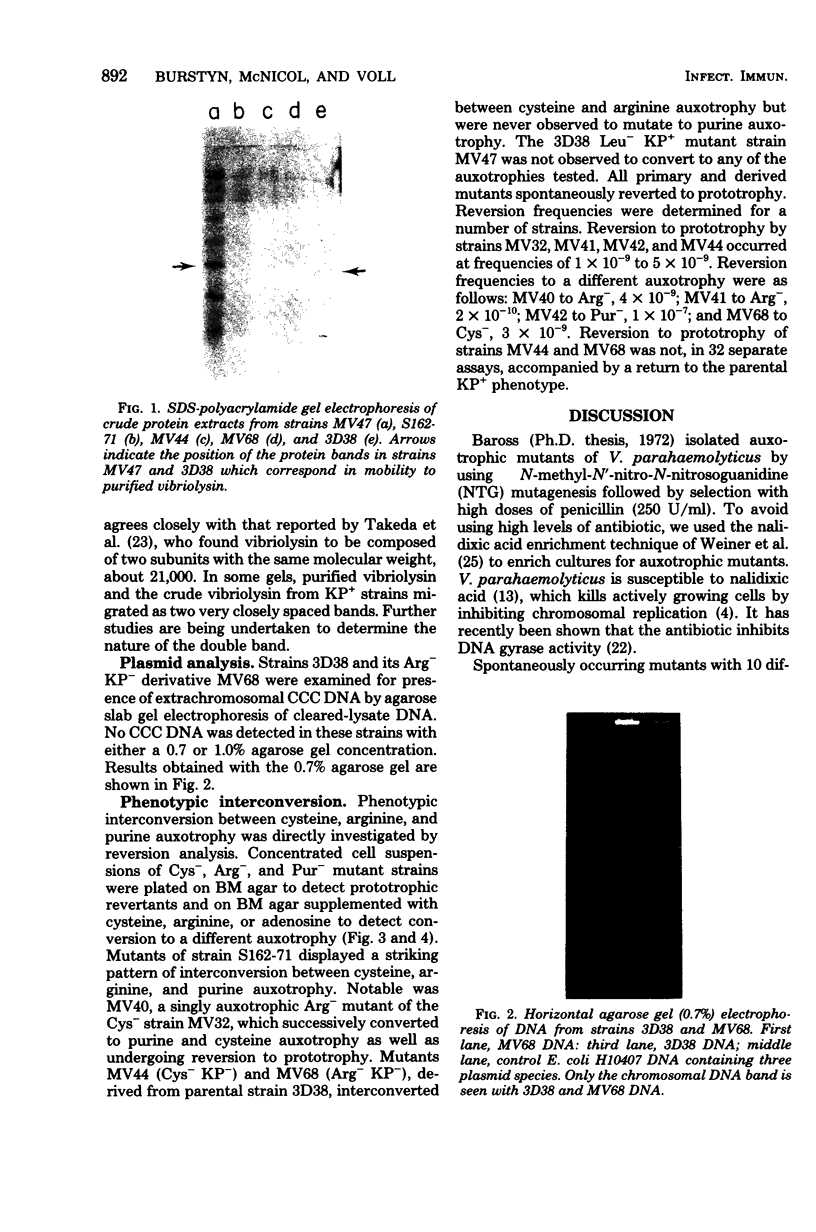
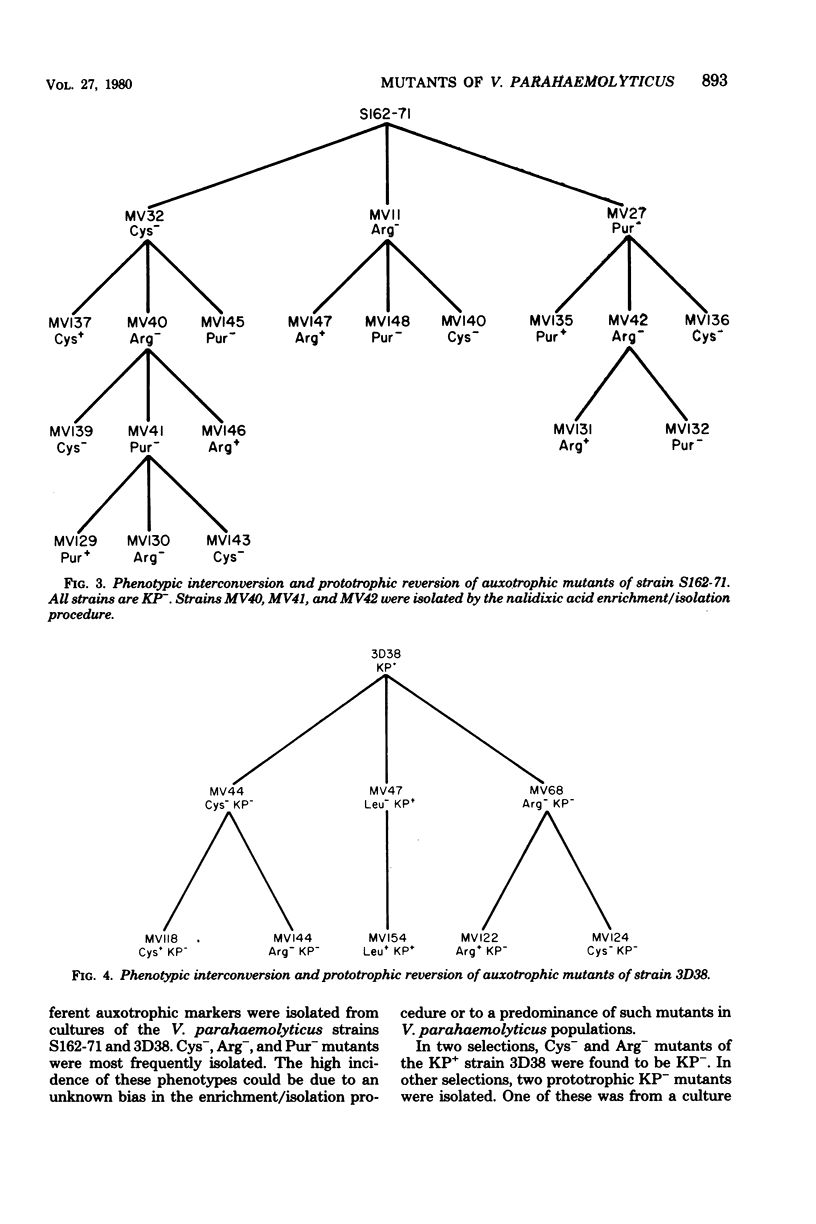
As a first step toward developing a system of genetic exchange between Vibrio parahaemolyticus strains, spontaneously arising auxotrophic and Kanagawa phenomenon-negative (KP-) mutants were isolated and characterized. Auxotrophic mutants were selected by nalidixic acid enrichment of parental cultures. Some Cys- and Arg- mutants of a KP+ strain were found to be KP-. Reversion to prototrophy by these strains was not accompanied by a return to the parental KP+ phenotype. Additionally, two prototrophic KP- mutants were isolated. No detectable levels of vibriolysin were found in supernatant extracts of KP- mutants by slide gel immunodiffusion analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, or assay for lethal activity in mice. All Cys-, Arg-, and Pur- mutants tested reverted to a different auxotrophy (phenotypic interconversion) as well as to prototrophy. The possible role of insertion sequence-like elements in vibriolysin production and phenotypic interconversion is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker W. H., Jr, Gangarosa E. J. Food poisoning due to Vibrio parahaemolyticus. Annu Rev Med. 1974;25:75–81. doi: 10.1146/annurev.me.25.020174.000451. [DOI] [PubMed] [Google Scholar]
- Fincham J. R., Sastry G. R. Controlling elements in maize. Annu Rev Genet. 1974;8:15–50. doi: 10.1146/annurev.ge.08.120174.000311. [DOI] [PubMed] [Google Scholar]
- Guerry P., Colwell R. R. Isolation of cryptic plasmid deoxyribonucleic acid from Kanagawa-positive strains of Vibrio parahaemolyticus. Infect Immun. 1977 Apr;16(1):328–334. doi: 10.1128/iai.16.1.328-334.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Taga S., Takeda T., Hasibuan M. A., Takeda Y., Miwatani T. Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus, and some physicochemical properties of the purified toxin. Infect Immun. 1976 Jan;13(1):133–139. doi: 10.1128/iai.13.1.133-139.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Kato T., Obara Y., Akiyama S., Takizawa K., Yamai S. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J Bacteriol. 1969 Nov;100(2):1147–1149. doi: 10.1128/jb.100.2.1147-1149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura S., Shigeno N., Tomita R. [Sensitivity of Vibrio parahaemolyticus to chemotherapeutic agents]. Nihon Saikingaku Zasshi. 1966 May;21(5):256–265. doi: 10.3412/jsb.21.256. [DOI] [PubMed] [Google Scholar]
- Saedler H., Reif H. J., Hu S., Davidson N. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 1974;132(4):265–289. doi: 10.1007/BF00268569. [DOI] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K., Kato T., Obara Y., Yamai S. Studies on the enteropathogenic, facultatively halophilic bacterium, Vibrio parahaemolyticus. 3. Enteropathogenicity. Jpn J Med Sci Biol. 1968 Oct;21(5):325–331. doi: 10.7883/yoken1952.21.325. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Matsuzaki A., Takeda Y., Miwatani T. Existence of two distinct hemolysins in Vibrio parahaemolyticus. Infect Immun. 1974 May;9(5):777–780. doi: 10.1128/iai.9.5.777-780.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Colwell R. R. Effect of hydrostatic pressure on growth and viability of Vibrio parahaemolyticus. Appl Microbiol. 1974 Dec;28(6):977–981. doi: 10.1128/am.28.6.977-981.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg R. L., Vincent R. D., Perlman P. S., Butow R. A. Asymmetric gene conversion at inserted segments on yeast mitochondrial DNA. Nature. 1978 Dec 7;276(5688):577–583. doi: 10.1038/276577a0. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner R. M., Voll M. J., Cook T. M. Nalidixic acid for enrichment of auxotrophs in cultures of Salmonella typhimurium. Appl Microbiol. 1974 Oct;28(4):579–581. doi: 10.1128/am.28.4.579-581.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]





