Abstract
BACKGROUND
Effective medical therapies are lacking for the treatment of neurofibromatosis type 1– related plexiform neurofibromas, which are characterized by elevated RAS–mitogen-activated protein kinase (MAPK) signaling.
METHODS
We conducted a phase 1 trial of selumetinib (AZD6244 or ARRY-142886), an oral selective inhibitor of MAPK kinase (MEK) 1 and 2, in children who had neurofibromatosis type 1 and inoperable plexiform neurofibromas to determine the maximum tolerated dose and to evaluate plasma pharmacokinetics. Selumetinib was administered twice daily at a dose of 20 to 30 mg per square meter of body-surface area on a continuous dosing schedule (in 28-day cycles). We also tested selumetinib using a mouse model of neurofibromatosis type 1–related neurofibroma. Response to treatment (i.e., an increase or decrease from baseline in the volume of plexiform neurofibromas) was monitored by using volumetric magnetic resonance imaging analysis to measure the change in size of the plexiform neurofibroma.
RESULTS
A total of 24 children (median age, 10.9 years; range, 3.0 to 18.5) with a median tumor volume of 1205 ml (range, 29 to 8744) received selumetinib. Patients were able to receive selumetinib on a long-term basis; the median number of cycles was 30 (range, 6 to 56). The maximum tolerated dose was 25 mg per square meter (approximately 60% of the recommended adult dose). The most common toxic effects associated with selumetinib included acneiform rash, gastrointestinal effects, and asymptomatic creatine kinase elevation. The results of pharmacokinetic evaluations of selumetinib among the children in this trial were similar to those published for adults. Treatment with selumetinib resulted in confirmed partial responses (tumor volume decreases from baseline of ≥20%) in 17 of the 24 children (71%) and decreases from baseline in neurofibroma volume in 12 of 18 mice (67%). Disease progression (tumor volume increase from baseline of ≥20%) has not been observed to date. Anecdotal evidence of decreases in tumor-related pain, disfigurement, and functional impairment was observed.
CONCLUSIONS
Our early-phase data suggested that children with neurofibromatosis type 1 and inoperable plexiform neurofibromas benefited from long-term dose-adjusted treatment with selumetinib without having excess toxic effects. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01362803.)
Neurofibromatosis type 1 is a common genetic disorder that is characterized by multiple manifestations including tumors of the nervous system.1,2 Plexiform neurofibromas develop in 20 to 50% of persons with neurofibromatosis type 1 and can cause substantial complications including pain, functional impairment, disfigurement, and malignant transformation.3–7 Most plexiform neurofibromas are diagnosed in early childhood and grow most rapidly during this period.8,9 Complete surgical resection of these tumors is often not feasible, and regrowth of the tumor after incomplete surgical resection has been observed.10,11
The NF1 product neurofibromin functions as a negative regulator of RAS activity. Lack of functional neurofibromin in patients with neurofibromatosis type 1 leads to dysregulated RAS and tumorigenesis.12–14 The availability of agents that target RAS signaling and other pathways implied in the pathogenesis of plexiform neurofibromas15,16 has led to phase 2 clinical trials of tipifarnib,17 pirfenidone,18 sirolimus,19,20 pegylated interferon alfa-2b,21 and imatinib,22 in which the primary objectives were to increase the rate of progression-free survival or to decrease plexiform neurofibroma volumes. In these trials, tumor volume decreases from baseline of at least 20% were identified in only 4 of 83 patients (5%) who participated in the pegylated interferon alfa-2b trial21 and in only 5 of 36 patients (14%) who participated in the imatinib trial.22
The development of genetically engineered mouse models of neurofibromatosis type 1–related tumors has allowed for the conduct of preclinical trials of targeted agents.23–26 In a mouse model of neurofibromatosis type 1–deficient acute myeloid leukemia, mitogen-activated protein kinase (MAPK) kinase (MEK) inhibition induced objective tumor regression.26 In the DhhCre;Nf1fl/fl model of neurofibromatosis type 1– related neurofibromas, the specific MEK inhibitor PD0325901 resulted in tumor shrinkage in the majority of mice, which supported the evaluation of specific MEK inhibitors in patients with neurofibromatosis type 1–related neurofibromas.27
Selumetinib (AZD6244 or ARRY-142886) is an oral selective inhibitor of MEK 1 and 2 that has shown activity against several advanced adult cancers.28–30 We report here the results of a phase 1 trial of selumetinib, as well as preclinical data on the activity of selumetinib in the animal model of neurofibroma.
Methods
Trial Oversight
This trial was developed by investigators at the National Cancer Institute (NCI) in collaboration with the NCI Cancer Therapy Evaluation Program (CTEP), was sponsored by CTEP, and was coordinated by the NCI Pediatric Oncology Branch. Four institutions (the NCI Pediatric Oncology Branch, Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital, and Children’s National Health System) participated in patient recruitment and data collection. Data analysis was performed by NCI investigators. AstraZeneca provided selumetinib for the trial, approved the trial protocol (available with the full text of this article at NEJM.org), and provided financial support for the analysis of selumetinib in plasma samples. AstraZeneca did not have a role in patient recruitment, data analysis, or manuscript preparation but participated in the review and approval of the manuscript for submission. The protocol was approved by the institutional review board at each participating site. All patients or their legal guardians provided written informed consent. The authors vouch for the accuracy and completeness of the data reported and for the fidelity of the trial to the protocol.
Patients
Children 3 to 18 years of age who had received a clinical diagnosis of neurofibromatosis type 131 and who had inoperable, measurable plexiform neurofibromas32 that had the potential to cause substantial complications were eligible for participation in the trial. A complete list of the inclusion and exclusion criteria is provided in Table S1 in the Supplementary Appendix, available at NEJM.org.
Trial Design
Selumetinib (10-mg and 25-mg capsules) was administered approximately every 12 hours on a continuous dosing schedule (in 28-day cycles). The dose of selumetinib was calculated on the basis of body-surface area with the use of a dosing nomogram. The starting dose was 20 mg per square meter of body-surface area (approximately 50% of the recommended fixed dose of 75 mg for adults), with potential dose escalations to 50 mg per square meter. A standard 3 + 3 phase 1 dose escalation design33 was followed (details are provided in the Supplementary Methods section in the Supplementary Appendix).
Patient assessments included clinical examinations, laboratory evaluations, ophthalmologic examinations, echocardiography, and electrocardiography at baseline and at regular intervals during the trial (Table S2 in the Supplementary Appendix). Adherence to the dosing schedule was assessed by administration of the Responsibility for Medication Questionnaire,34 by review of a patient diary, and by capsule counts. Evaluations of response to treatment were performed centrally at the NCI by review of magnetic resonance imaging (MRI) studies obtained after cycles 5 and 10 and thereafter after every 6 cycles.32,35 The use of standard response criteria (Response Evaluation Criteria In Solid Tumors [RECIST] and World Health Organization [WHO] criteria)36,37 to evaluate the responses of plexiform neurofibromas to treatment has limited applicability because of the complex shapes and slow growth rates of plexiform neurofibromas. Therefore, a more sensitive and reproducible volumetric MRI analysis, which has been established as the definitive assessment method, was used in this trial.8,15,32,35 Patients who had documented disease progression at trial entry (i.e., a tumor volume increase of ≥20% within approximately 1.5 years before enrollment) could continue treatment with no limitation of duration if there were no unacceptable toxic effects of the treatment and if no further progression was documented. Patients who did not have documented disease progression at trial entry could continue treatment for a maximum of 2 years unless a partial response was observed, in which case treatment could continue beyond 2 years.
Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. An algorithm was developed for the assessment and management of cardiac toxic effects of selumetinib. The maximum tolerated dose was defined as the highest dose level at which one third of the patients or fewer had dose-limiting toxic effects during cycles 1 to 3. With specific exceptions, a dose-limiting toxic effect was defined as any selumetinib-related toxic effect of grade 3 or higher or a persistent (≥7 days) selumetinib-related toxic effect of grade 2 that the patient considered to be unacceptable. Administration of selumetinib was interrupted if a dose-limiting toxic effect occurred. If the toxic effect resolved to within protocol-defined trial specifications, treatment could resume at a dose that was 30% lower than the patient’s previous dose. Treatment with selumetinib was permanently discontinued if the toxic effect did not resolve to meet protocol specifications within the required time frame. A second reduction in dose according to the same criteria was allowed (details of the trial design are provided in the Supplementary Methods section in the Supplementary Appendix).
Pharmacokinetic Evaluations
The pharmacokinetics of selumetinib in plasma were evaluated during cycle 1 among patients for whom consent was provided. Blood samples were obtained as follows: on day 1 before the first dose was administered and 0.5, 1, 2, 3, 5, 8, 10 to 12, 24, and 30 to 36 hours after administration of that dose; in addition, a blood sample was obtained on day 27 before the first dose was administered. Concentrations of selumetinib and its active metabolite, N-desmethyl selumetinib, were analyzed by Covance Bioanalytical Services.38 Pharmacokinetic variables were calculated by means of noncompartmental methods.39
Response Criteria
A partial response was defined as a tumor volume decrease from baseline of at least 20% for at least 4 weeks. Disease progression was defined as a tumor volume increase from baseline of at least 20%. Stable disease was defined as a tumor volume change from baseline of less than 20%.
Preclinical Trial of Selumetinib
Selumetinib was evaluated in the DhhCre;Nf1fl/fl genetically engineered mouse model of neurofibroma with the use of an intermittent dosing schedule. Mice received selumetinib twice daily at a dose of 10 mg per kilogram through oral gavage on a 5-days-on, 2-days-off schedule over a period of 8 weeks. Volumetric MRI analysis of neurofibromas was performed at the NCI to assess response to selumetinib (further details are provided in the Supplementary Methods section in the Supplementary Appendix).
Results
Patient Characteristics
From September 21, 2011, to February 27, 2014, 24 patients (11 girls and 13 boys; median age, 10.9 years [range, 3.0 to 18.5]) were enrolled in this ongoing trial. Data that were available as of January 4, 2016, are included in this article. Table 1 provides a summary of demographic, clinical, and baseline disease characteristics, and details are listed for each patient in Table S3 in the Supplementary Appendix. The median volume of the target plexiform neurofibromas at baseline was 1205 ml (range, 29 to 8744). A total of 19 patients had received one to six previous medical therapies for the neurofibromas, and 11 patients had undergone one to six previous surgeries to debulk the neurofibromas. Of 17 patients for whom previous volumetric data were available, 9 had progressive disease at enrollment. A retrospective chart review identified neurofibroma-related complications that were present at baseline, including disfigurement (18 patients), pain (13 patients), and impairment of motor function (9 patients).
Table 1.
Demographic, Clinical, and Baseline Disease Characteristics.
| Characteristic | Value |
|---|---|
| Number of patients enrolled | 24 |
| Median age at enrollment (range) — yr | 10.9 (3.0–18.5) |
| Sex — no. | |
| Male | 13 |
| Female | 11 |
| Median performance status score (range)* | 90 (70–100) |
| No. of previous medical interventions for treatment of plexiform neurofibroma | 41 |
| No. of patients who had previous medical interventions | 19 |
| Median no. of previous medical interventions per patient (range) | 2 (1–6) |
| No. of previous plexiform neurofibroma debulking surgeries | 25 |
| No. of patients who underwent previous debulking surgeries | 11 |
| Median no. of previous debulking surgeries per patient (range) | 1 (1–6) |
| Predominant target location of plexiform neurofibroma — no. | |
| Face | 4 |
| Both head and neck | 1 |
| Both neck and chest | 6 |
| Trunk | 4 |
| Both trunk and extremity (upper or lower) | 8 |
| Whole body | 1 |
| Median target plexiform neurofibroma volume (range) — ml | 1205 (29–8744) |
| Progression status of target plexiform neurofibroma at enrollment — no. (%) | |
| Progressive | 9 (38) |
| Nonprogressive | 8 (33) |
| Insufficient information | 7 (29) |
| Documented plexiform neurofibroma–related complication at baseline — no. (%) | 21 (88) |
| Disfigurement | 18 (75) |
| Pain | 13 (54) |
| Motor dysfunction | 9 (38) |
| Vision loss | 1 (4) |
Karnofsky performance status was assessed in patients who were older than 16 years of age, and Lansky performance status was assessed in patients who were 16 years of age or younger. Both the Karnofsky performance status scores and the Lansky performance status scores range from 10 to 100, with higher scores indicating better functioning.
Dose Escalations, Toxic Effects, and Duration of Treatment
The 24 patients received selumetinib at three dose levels: 12 patients at 20 mg per square meter of body surface area (the 20-mg group), 6 patients at 25 mg per square meter (the 25-mg group), and 6 patients at 30 mg per square meter (the 30-mg group) (Table 2, and Tables S3 and S4 in the Supplementary Appendix). During cycles 1 through 3, the mean percentage of patients who were considered to have adhered to the dosing schedule was 99% (range, 91 to 100) on the basis of patient diaries and 98% (range, 96 to 100) on the basis of capsule counts. Dose-limiting toxic effects that were counted toward the determination of the maximum tolerated dose were grade 3 cellulitis (1 patient), grade 3 urticaria (1 patient), and grade 3 creatine kinase elevation (1 patient), all of which occurred during cycles 1 through 3; in addition, grade 3 asymptomatic decreased left ventricular ejection fraction, which occurred during cycle 5 (1 patient), was included as a dose-limiting toxic effect because of the potential clinical significance of this event and the fact that the first echocardiogram that is performed during treatment occurs after 5 cycles.
Table 2.
Dose-limiting Toxic Effects of Selumetinib and Response Evaluation.*
| Variable | 20-mg Group (N = 12) |
25-mg Group (N = 6) |
30-mg Group (N = 6) |
|---|---|---|---|
| Dose-limiting toxic effects of selumetinib† | |||
| Elevated creatine kinase | Grade 3 (1 patient) | Grade 4 (1 patient) | Grade 3 (2 patients)‡ |
| Cellulitis | Grade 3 (2 patients) | ||
| Urticaria | Grade 3 (1 patient) | ||
| Decreased left ventricular ejection fraction | Grade 3 (1 patient) | ||
| Mucositis | Grade 2 (1 patient) | Grade 2 and 3 (1 patient)§ | |
| Rash | Grade 3 (1 patient) | ||
| Response evaluation by volumetric MRI analysis | |||
| Partial response — no. (%) | 9 (75) | 5 (83) | 3 (50) |
| Median best response (range) — % volume change from baseline | −31 (−6 to −47) | −34 (−16 to −44) | −19 (−13 to −34) |
| Median time to best response (range) — mo | 22 (5 to 42) | 18 (9 to 22) | 8 (5 to 24) |
| Response according to progression status at trial entry | |||
| Progressive disease at trial entry | |||
| No. of patients | 4 | 4 | 1 |
| No. with response | 2 | 3 | 0 |
| Nonprogressive disease at trial entry | |||
| No. of patients | 4 | 0 | 4 |
| No. with response | 4 | 0 | 2 |
| Insufficient information on progression status at entry | |||
| No. of patients | 4 | 2 | 1 |
| No. with response | 3 | 2 | 1 |
| Median no. of completed cycles (range)¶ | 30 (6 to 56) | 25 (23 to 26) | 32 (18 to 40) |
| Off treatment — no. | 1 | 0 | 2 |
| Off trial — no. | 1 | 0 | 1 |
| Anecdotal Report of Amelioration of Complications of Plexiform Neurofibroma | |||
| Disfigurement | |||
| No. of patients with condition at baseline | 7 | 6 | 5 |
| No. of patients with improvement | 2 | 3 | 0 |
| Pain | |||
| No. of patients with condition at baseline | 7 | 3 | 3 |
| No. of patients with improvement | 4 | 3 | 1 |
| Motor dysfunction | |||
| No. of patients with condition at baseline | 3 | 3 | 3 |
| No. of patients with improvement | 2 | 3 | 0 |
For detailed data on toxic effects and responses per patient, see Table S3 in the Supplementary Appendix. MRI denotes magnetic resonance imaging.
Data are shown for 741 completed cycles. Toxic events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
One of these patients had two dose-limiting events of creatine kinase elevation.
This patient had three dose-limiting events of mucositis.
“Off treatment” refers to the number of patients who were permanently discontinued from trial treatment but continued to be followed for resolution of toxic effects. “Off trial” refers to the number of patients who were permanently withdrawn from the trial.
Thirty milligrams of selumetinib per square meter exceeded the maximum tolerated dose, and 20 mg per square meter was defined initially as the maximum tolerated dose. However, on the basis of a trial of selumetinib for low-grade gliomas that was being conducted concurrently by the Pediatric Brain Tumor Consortium, in which the maximum tolerated dose was determined to be 25 mg per square meter,40 we amended our trial to evaluate this additional dose level with the intention of harmonizing pediatric dosing. In our trial, a grade 3 dose-limiting maculopapular rash developed in only 1 of 6 patients in the 25-mg group (Patient 24). Thus, 25 mg per square meter was considered to have an acceptable side-effect profile and was determined to be the maximum tolerated dose. Dose-limiting toxic effects in later cycles were creatine kinase elevation (4 patients), oral mucositis (3 patients), and cellulitis (1 patient).
Throughout all treatment cycles, doses were reduced as a result of dose-limiting toxic effects in 4 of 12 patients in the 20-mg group, in 4 of 6 patients in the 30-mg group, and in 3 of 6 patients in the 25-mg group. All dose-limiting toxic effects were reversible. Most selumetinib-related adverse events were mild (grade 1). The most common adverse events of any grade included creatine kinase elevation, which was asymptomatic in all but 1 patient in whom transient arm pain developed; gastrointestinal toxic effects; acneiform rash in postpubertal patients; and maculopapular rash in prepubertal patients. Creatine kinase was fractionated in 17 patients and was found to be of skeletal muscle (CK-MM) origin rather than cardiac (CK-MB) origin. Early treatment of acneiform rash with topical clindamycin or glucocorticoids and oral tetracycline (5 patients) allowed for the successful management of rash. Toxic effects that were most common in later cycles included mucositis, aminotransferase elevation, decreased neutrophil count, and paronychia (Table S4 in the Supplementary Appendix).
Serial ophthalmologic examinations revealed no abnormalities, with the exception of a grade 1 asymptomatic cataract at cycle 16 in 1 patient. This patient had previously received imatinib and retinoic acid for treatment of his neurofibromas; he continues to receive selumetinib and has stable ophthalmologic findings. A reversible, asymptomatic decrease in the left ventricular ejection fraction of greater than 10 percentage points to less than the lower limit of the normal range (from 65% to 50%) was observed in 1 patient at the evaluation visit after cycle 5 (Patient 7). Selumetinib dosing was suspended and administration of lisinopril was initiated. The left ventricular ejection fraction recovered to 55% within 3 months. Selumetinib was restarted at a reduced dose; the left ventricular ejection fraction has remained normal and the patient continues to receive selumetinib (cycle 30). No measurable effect of selumetinib on height or weight was observed during treatment (see the Supplementary Results section in the Supplementary Appendix).
The 24 patients received a median of 30 cycles of selumetinib (range, 6 to 56). A total of 19 patients continue to receive treatment after a median of 30 cycles (range, 23 to 56). Treatment with selumetinib was discontinued in 5 patients; treatment was stopped because of dose-limiting toxic effects in only 1 of these patients (details are provided in Table S3 in the Supplementary Appendix).
Pharmacokinetics
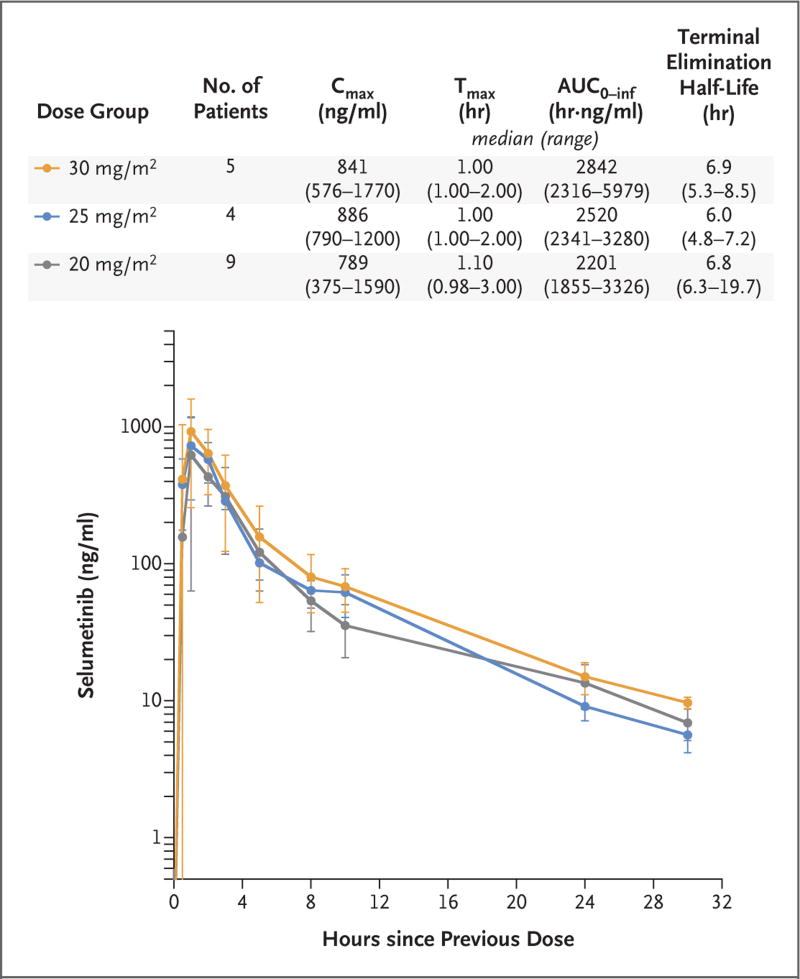
The mean plasma concentration–time profiles of selumetinib and the results of pharmacokinetic studies in the 18 patients for whom consent was provided showed limited variability (Fig. 1, and the Supplementary Results section and Table S5 in the Supplementary Appendix). Selumetinib was absorbed rapidly. Selumetinib drug exposures increased with increasing dose but were less than dose-proportional (Fig. S1 in the Supplementary Appendix). Selumetinib concentrations exceeded those of the N-desmethyl metabolite at all time points (Table S6 in the Supplementary Appendix).
Figure 1. Mean Plasma Concentration–Time Profiles of Selumetinib and Median Pharmacokinetic Results for Selumetinib for the Three Dose Levels Evaluated.
The pharmacokinetics of selumetinib in plasma were evaluated during cycle 1. Blood samples were obtained as follows: on day 1 before the first dose was administered and 0.5, 1, 2, 3, 5, 8, 10 to 12, 24, and 30 to 36 hours after administration of that dose; in addition, a blood sample was obtained on day 27 before the first dose was administered (data not shown). The mean plasma concentration–time profiles of selumetinib and the pharmacokinetic results for the 18 patients for whom consent was provided showed limited variability. Selumetinib was rapidly absorbed. I bars indicate standard deviations. AUC0–inf denotes the area under the plasma concentration–time curve from time 0 to infinity, Cmax the observed maximum plasma concentration after drug administration, and Tmax the time from drug administration to Cmax.
Preclinical Pharmacokinetics and Pharmacodynamics
Pharmacokinetic results in the mouse were similar to those in humans. Mouse tumor samples showed a transient decrease in phosphorylated extracellular signal-regulated kinase (ERK) (Fig. S2 in the Supplementary Appendix). Selumetinib was associated with decreases from baseline in neurofibroma volume in 12 of 18 mice (67%) in the animal model of neurofibromatosis type 1– related neurofibroma; in contrast, neurofibroma volume increase from baseline was observed in 14 of the 15 vehicle-treated control animals (Fig. S3 in the Supplementary Appendix).
Response Evaluation
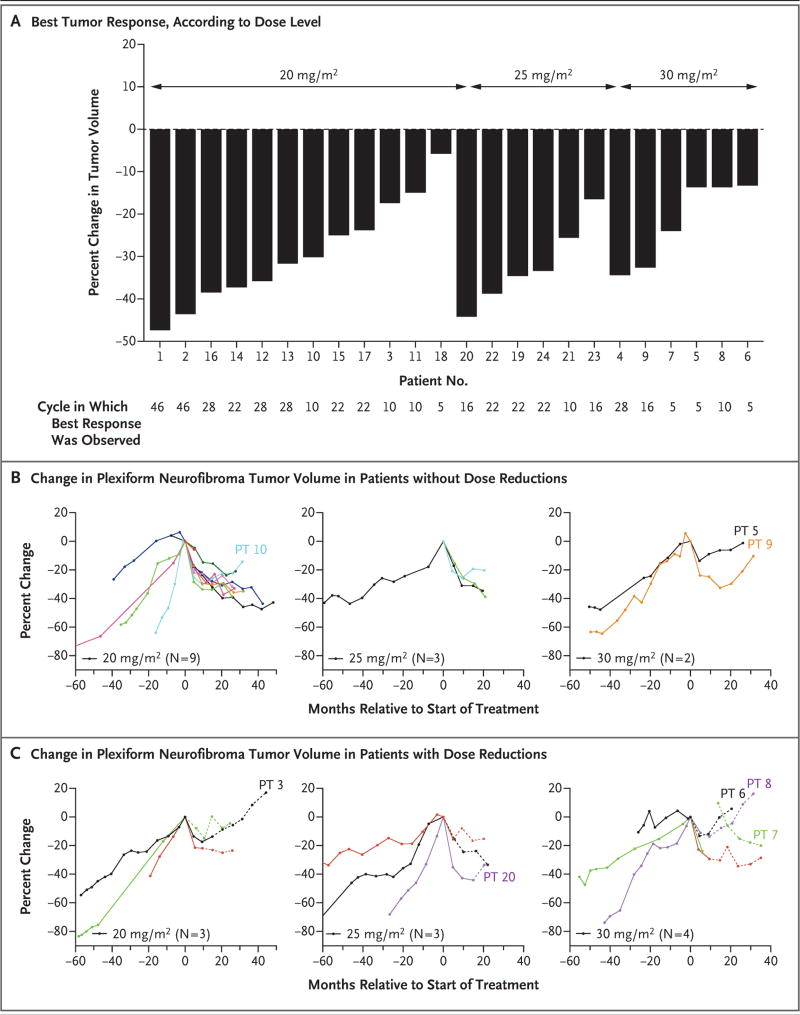
A decrease from baseline in plexiform neurofibroma volume was observed in all patients (median change, −31%; range, −5.8 to −47). The maximum response to selumetinib was reached after a median of 20 cycles (range, 5 to 42) (Table 2 and Fig. 2). A total of 17 of the 24 patients (71%) met the criteria for confirmed partial response, including 9 of 12 patients in the 20-mg group, 5 of 6 patients in the 25-mg group, and 3 of 6 patients in the 30-mg group; an example of the response in 1 of the patients with confirmed partial response is shown in Figure 3A and 3B. Partial responses to selumetinib were observed in 5 of 9 patients (56%) who had documented progressive disease at enrollment, in 4 of 6 patients (67%) who had growing tumors at enrollment but did not meet the criteria for progressive disease as defined in the protocol, in 2 of 2 patients who had tumors that were assessed as stable at enrollment, and in 6 of 7 patients (86%) who did not have previous volumetric data available at enrollment (Table 2, and Table S3 in the Supplementary Appendix).
Figure 2. Best Response to Selumetinib According to Dose Level and According to Dose Reduction Status.
Panel A shows the best response to selumetinib as assessed by the percent change from baseline in plexiform neurofibroma tumor volume, according to dose level. Panels B and C show the change in plexiform neurofibroma volumes before (when available) and after initiation of treatment with selumetinib among patients who did not have dose reductions (Panel B) and among those who had dose reductions (Panel C) during the trial. Separate graphs are presented for the three dose levels (20, 25, and 30 mg per square meter of bodysurface area). The time periods during which a patient had no dose reductions are depicted with solid lines, and periods during which a patient had a dose reduction are depicted with dashed lines. Patient 5 had a plexiform neurofibroma with a large nodular component that did not respond to treatment with selumetinib. Patient 7, who remains in the trial, had a prolonged interruption in selumetinib dosing as a result of a toxic effect. Patients 9 and 10, who also remain in the trial, are the only patients who did not have a dose reduction of selumetinib but had a slow increase in plexiform neurofibroma volume after maximal response had been observed. For Patients 3 and 20 (who remain in the trial) and Patients 8 and 6 (who permanently discontinued trial treatment), an increase in plexiform neurofibroma volume was observed after at least one selumetinib dose reduction as a result of a toxic effect (details are provided in Table S3 in the Supplementary Appendix). PT denotes patient number.
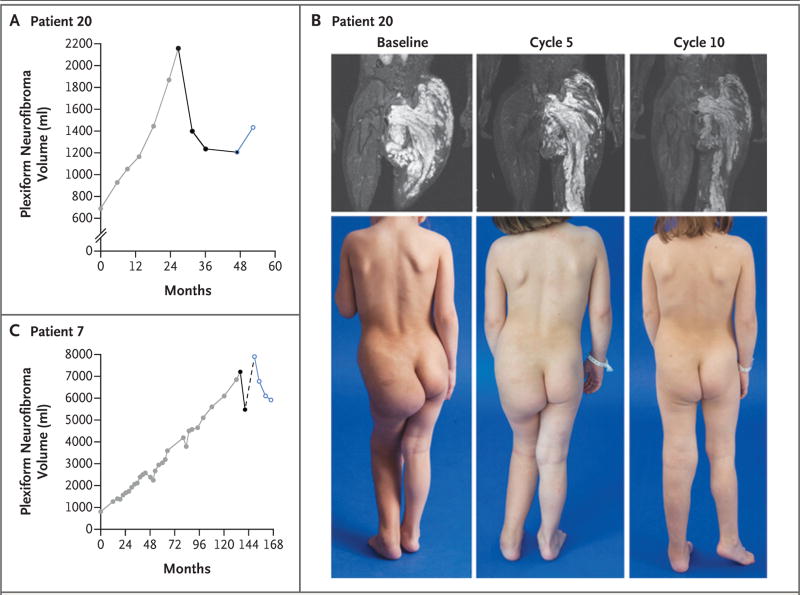
Figure 3. Examples of Response to Selumetinib.
Panels A and B show data from Patient 20 (25-mg group). In Panel A, results from the volumetric magnetic resonance imaging (MRI) analysis of plexiform neurofibroma growth show the tumor volume before treatment (gray symbols), tumor shrinkage during treatment with selumetinib (black symbols), and the increase in plexiform neurofibroma volume after dose reduction (blue open symbol). In Panel B, the visible reduction in plexiform neurofibroma burden is depicted in the MRI results and photographs before treatment as compared with those at the end of cycles 5 and 10 during treatment. Panel C shows an example of the need for prolonged administration of selumetinib to elicit a sustained response. Patient 7 (30-mg group) had progressive plexiform neurofibroma growth during six treatment regimens directed at his plexiform neurofibroma before treatment with selumetinib (gray symbols). He had a partial response at the evaluation visit after cycle 5 (black symbols), but selumetinib was stopped because of a decrease in the left ventricular ejection fraction (LVEF) (broken line). When selumetinib was restarted at a reduced dose after recovery of the LVEF, the plexiform neurofibroma volume had increased by 44% from the best response value and by 9.6% from baseline. Subsequent disease evaluations (blue open symbols) show responsive disease with a tumor volume reduction of 25% since the time of reinitiation of selumetinib at a reduced dose.
Partial responses were durable, in that they were sustained for a median of 23 cycles (range, 6 to 42), and 15 of the 17 patients with partial response maintain their response status to date. No patients have had disease progression to date, and the 9 patients with confirmed tumor growth before enrollment are free of progression after a median of 26 cycles (range, 23 to 46). Slow regrowth of tumors has been observed in some cases, most notably after dose reductions or interruptions of selumetinib dosing as a result of toxic effects (Fig. 2B and 2C and Fig. 3A and 3C). Anecdotal evidence of clinical improvement included decreases in tumor-related pain, increases in motor function, and decreases in disfigurement (Table 2 and Fig. 3B, and Table S7 in the Supplementary Appendix).
Discussion
In this phase 1 trial involving children with inoperable plexiform neurofibroma, selumetinib had acceptable rates of dose-limiting toxic effects when administered on a long-term basis and was associated with a sustained reduction in tumor volumes in the majority of patients. The maximum tolerated dose of 25 mg per square meter every 12 hours (approximately 60% of the recommended fixed dose of 75 mg for adults38) is identical to the maximum tolerated dose in the selumetinib trial that was conducted by the Pediatric Brain Tumor Consortium.40 Skin and gastrointestinal toxic effects were the most common events — a side-effect profile similar to that of selumetinib in adults.38,41,42 A previously unreported effect, elevation of the creatine kinase level, was observed in most patients in our trial and was largely asymptomatic. Few patients had potentially chronic or cumulative toxic effects such as oral mucositis or paronychia. No ophthalmologic toxic effects of concern were observed, and a reversible decrease in left ventricular function that required a dose reduction of selumetinib occurred in only one patient. In addition, no effect on height or weight was observed. The results of the pharmacokinetic evaluations of selumetinib among the children in this trial (Table S5 in the Supplementary Appendix) were similar to those in adults.38
Of the 24 patients in the trial, 17 (71%) had confirmed partial responses, and all the patients had some decrease in tumor volume (Table 2 and Fig. 2A, and Fig. S3 in the Supplementary Appendix). In previous phase 2 trials of treatment for progressive plexiform neurofibromas, in which tumor volume was evaluated with the use of centralized volumetric analysis at the NCI (Table S8 in the Supplementary Appendix), tumor volume decreases of at least 20% were observed in few patients who received pegylated interferon alfa-2b.17–19,21 In a phase 2 trial of imatinib, in which volumetric analysis was performed at the University of Indiana, volume decreases of at least 20% were limited to small tumors of less than 20 ml, which were substantially smaller than the majority of tumors evaluated in our trial.22
To date, tumor responses have been maintained in 15 of 17 patients, and no patient has had disease progression. Patients who had documented progressive disease at enrollment received 23 to 46 cycles of selumetinib (median, 26 cycles). These results compare favorably with the median time-to-progression values that were reported in previous phase 2 trials involving patients with progressive neurofibromas, which ranged from 13.2 to 29.4 months (Table S8 in the Supplementary Appendix). MEK inhibitors also showed the greatest activity among the targeted agents that have been tested in the neurofibroma animal model,27 which supports the predictive value of this model in selecting agents for clinical evaluation in patients with plexiform neurofibroma.
Slow tumor regrowth after the maximum response had been reached has been observed in several patients, most of whom had required at least one dose reduction as a result of toxic effects (Fig. 2B and 2C, and Table S3 in the Supplementary Appendix). This finding suggests a dose-dependent effect of MEK inhibition on neurofibroma growth. The clinical course of Patient 7 (Fig. 3B), who had disease that was responsive to treatment with selumetinib but who subsequently had tumor growth during a period of drug interruption due to toxic effects, highlights the fact that prolonged administration of selumetinib will be necessary among patients with progressive tumors. Although similar responses to selumetinib were observed at the 20-mg and the 25-mg dose levels, we selected 25 mg per square meter as the recommended phase 2 dose.
It is of interest that in the mouse model, intermittent dosing was efficacious, even though target inhibition of MEK in tumor tissue was modest and transient. Thus, even limited inhibition of MEK may be sufficient to shrink tumors that have no mutations beyond the loss of NF1 itself.43 In the future, evaluation of intermittent dosing in patients may be considered as a way to minimize toxic effects while retaining efficacy. In an ongoing phase 2 trial of selumetinib involving adults with neurofibromatosis type 1–related plexiform neurofibromas (ClinicalTrials.gov number, NCT02407405), we are performing serial tumor biopsies to assess the relationship between inhibition of phosphorylated extracellular-signal-regulated kinase (pERK) and tumor response.
A limitation of our trial is that the potential clinical benefit of decreases in plexiform neurofibroma volume was not assessed prospectively. However, the anecdotal decreases in pain, decreases in the degree of disfigurement, and increases in motor function among patients in our trial suggest that even small decreases in tumor volumes may result in clinical benefit. The absence of emergence of drug resistance is also of interest. Further trials are warranted to characterize tumors that no longer respond to selumetinib; however, such cases have not yet been observed.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health; the Center for Cancer Research of the National Cancer Institute (NCI); the NCI Cancer Therapy Evaluation Program; the Children’s Tumor Foundation (Clinical Trial Award to Dr. Fisher for support of participating sites other than the NCI); and AstraZeneca (provision of selumetinib and funding for the pharmacokinetic analysis); and by grants from the Children’s Tumor Foundation and the Neurofibromatosis Therapeutic Acceleration Program (to Dr. Ratner for the mouse preclinical trials).
We thank the patients and their families who participated in the trial for their commitment; Mi-Ok Kim (Cincinnati Children’s Hospital) for statistical analysis of the mouse preclinical studies; and R. Scott Dunn (Cincinnati Children’s Hospital) for conducting the mouse MRI studies.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–51. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 2.Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834–43. doi: 10.1016/S1474-4422(14)70063-8. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim A, Gillespie A, Dombi E, et al. Characteristics of children enrolled in treatment trials for NF1-related plexiform neurofibromas. Neurology. 2009;73:1273–9. doi: 10.1212/WNL.0b013e3181bd1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89:31–7. doi: 10.1002/(sici)1096-8628(19990326)89:1<31::aid-ajmg7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10:593–8. doi: 10.1215/15228517-2008-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. J Pediatr. 2011;159(4):652–5. e2. doi: 10.1016/j.jpeds.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68:643–7. doi: 10.1212/01.wnl.0000250332.89420.e6. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75. doi: 10.1186/1750-1172-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canavese F, Krajbich JI. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J Pediatr Orthop. 2011;31:303–11. doi: 10.1097/BPO.0b013e31820cad77. [DOI] [PubMed] [Google Scholar]
- 11.Needle MN, Cnaan A, Dattilo J, et al. Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974–1994. J Pediatr. 1997;131:678–82. doi: 10.1016/s0022-3476(97)70092-1. [DOI] [PubMed] [Google Scholar]
- 12.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 13.Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015;15:290–301. doi: 10.1038/nrc3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss B, Bollag G, Shannon K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am J Med Genet. 1999;89:14–22. [PubMed] [Google Scholar]
- 15.Gutmann DH, Blakeley JO, Korf BR, Packer RJ. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013;22:443–62. doi: 10.1517/13543784.2013.772979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Packer RJ, Gutmann DH, Rubenstein A, et al. Plexiform neurofibromas in NF1: toward biologic-based therapy. Neurology. 2002;58:1461–70. doi: 10.1212/wnl.58.10.1461. [DOI] [PubMed] [Google Scholar]
- 17.Widemann BC, Dombi E, Gillespie A, et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16:707–18. doi: 10.1093/neuonc/nou004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61:1598–602. doi: 10.1002/pbc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss B, Widemann BC, Wolters P, et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015;17:596–603. doi: 10.1093/neuonc/nou235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss B, Widemann BC, Wolters P, et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: an NF clinical trials consortium phase II study. Pediatr Blood Cancer. 2014;61:982–6. doi: 10.1002/pbc.24873. [DOI] [PubMed] [Google Scholar]
- 21.Jakacki RI, Dombi E, Steinberg SM, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2016 Aug 10; doi: 10.1093/neuonc/now158. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13:1218–24. doi: 10.1016/S1470-2045(12)70414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Dombi E, Jousma E, et al. Preclinical testing of sorafenib and RAD001 in the Nf(flox/flox);DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr Blood Cancer. 2012;58:173–80. doi: 10.1002/pbc.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Williams JP, Rizvi TA, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–16. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−– and c-kit–dependent bone marrow. Cell. 2008;135:437–48. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauchle JO, Kim D, Le DT, et al. Response and resistance to MEK inhibition in leukaemias initiated by hyperactive Ras. Nature. 2009;461:411–4. doi: 10.1038/nature08279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123:340–7. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–32. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 30.Robert C, Dummer R, Gutzmer R, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol. 2013;14:733–40. doi: 10.1016/S1470-2045(13)70237-7. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Vol. 1. Bethesda, Md., USA: Jul 13–15, 1987. pp. 172–8. Neurofibromatosis 1988. [PubMed] [Google Scholar]
- 32.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81:S33–40. doi: 10.1212/01.wnl.0000435744.57038.af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith M, Bernstein M, Bleyer WA, et al. Conduct of phase I trials in children with cancer. J Clin Oncol. 1998;16:966–78. doi: 10.1200/JCO.1998.16.3.966. [DOI] [PubMed] [Google Scholar]
- 34.Riekert K. Ph.D. thesis. Cleveland: Case Western Reserve University; 2001. Health beliefs and medication adherence. [Google Scholar]
- 35.Solomon J, Warren K, Dombi E, Patronas N, Widemann B. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28:257–65. doi: 10.1016/j.compmedimag.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Estey E, Hoth D, Simon R, Marsoni S, Leyland-Jones B, Wittes R. Therapeutic response in phase I trials of antineoplastic agents. Cancer Treat Rep. 1986;70:1105–15. [PubMed] [Google Scholar]
- 37.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 38.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–23. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 39.Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24:507–16. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee A, Jakacki R, Onar-Thomas A, et al. A phase 1 study of AZD6244 in children with recurrent or refractory low-grade gliomas: a Pediatric Brain Tumor Consortium report. J Clin Oncol. 2014;32(Suppl):5s. abstract. [Google Scholar]
- 41.Balagula Y, Barth Huston K, Busam KJ, Lacouture ME, Chapman PB, Myskowski PL. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886) Invest New Drugs. 2011;29:1114–21. doi: 10.1007/s10637-010-9567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–81. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 43.Beert E, Brems H, Daniëls B, et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer. 2011;50:1021–32. doi: 10.1002/gcc.20921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.