Abstract
The polysaccharide-rich fungal cell wall provides pathogen-specific targets for antifungal therapy and distinct molecular patterns that stimulate protective or detrimental host immunity. The echinocandin antifungal caspofungin inhibits synthesis of cell wall β-1,3-glucan and is used for prophylactic therapy in immune suppressed individuals. However, breakthrough infections with fungal pathogen Aspergillus fumigatus are associated caspofungin prophylaxis. In this study, we report in vitro and in vivo increases in fungal surface chitin in A. fumigatus induced by caspofungin that was associated with airway eosinophil recruitment in immune competent and neutropenic mice with invasive pulmonary aspergillosis (IA). More importantly, caspofungin treatment of mice with IA resulted in a pattern of increased fungal burden and severity of disease that was reversed in eosinophil-deficient mice. In addition, the eosinophil granule proteins major basic protein and eosinophil peroxidase were more frequently detected in the bronchoalveolar lavage fluid of lung transplant patients diagnosed with IA that received caspofungin therapy when compared to azole-treated patients. Eosinophil recruitment and inhibition of fungal clearance in caspofungin-treated mice with IA required RAG1 expression and γδ T cells. These results identify an eosinophil-mediated mechanism for paradoxical caspofungin activity and support the future investigation of the potential of eosinophil or fungal chitin-targeted inhibition in the treatment of IA.
Keywords: Aspergillus fumigatus, lung immune responses, eosinophils, gammadelta T cells, chitin, echinocandin, caspofungin, invasive aspergillosis
Introduction
The ubiquitous filamentous fungus Aspergillus fumigatus is an opportunistic pathogen and aeroallergen source capable of inducing lung inflammation, colonization, and/or invasive infection contingent on the level of immune capability of the host (1–3). The incidence of invasive pulmonary aspergillosis (IA) has increased along with the use of immune suppressive and myeloablative therapies. Mortality in immune suppressed patients with IA may reach 90% depending on the population, and current options for antifungal therapy are not highly effective and often require prolonged administration (3–5). It is therefore of paramount importance for clinicians and researchers to increase their understanding of the underlying biological mechanisms that reduce antifungal efficacy in order to facilitate the development of new and improved therapies.
The cell wall/membrane of A. fumigatus is comprised of multiple structures distinct from mammalian cell membranes, thus offering pathogen-specific targets for antifungal therapy and host immunity (6). Voriconazole or other triazole drugs that inhibit synthesis of fungal cell membrane ergosterol are currently recommended for primary or empiric therapy (7, 8). However, fungal azole resistance in IA patients is increasing, possibly due to increased agricultural use of fungicidal azole compounds (9–12). In contrast to azoles, the echinocandin class of antifungals inhibit the synthesis of the fungal cell wall polysaccharide β-1,3-glucan (13). Administration of echinocandins is indicated for aspergillosis patients that do not respond to azole therapy alone (5, 14). In addition, echinocandins such as caspofungin may be used as a part of a prophylactic regimen for patients that are highly susceptible to fungal infections (15). However, among susceptible patients receiving prophylactic caspofungin, a commonly reported “breakthrough” fungal infection was aspergillosis (16–19). In a mouse model of IA, lung fungal burden was decreased at lower caspofungin doses, but paradoxically increased at the highest dose (20). Thus, in some patients caspofungin/echinocandin therapy may be less effective at preventing or treating A. fumigatus infection in comparison to other fungal pathogens.
The early lung immune response to inhalation of A. fumigatus conidia is shaped by innate recognition of fungal cell wall polysaccharides, particularly β-1,3-glucan and chitin (21–23). In dormant conidia, these covalently-linked sugars are masked by a hydrophobic rodlet layer that breaks down upon swelling and germination, thus initiating recognition via innate receptors on resident tissue macrophages and newly recruited inflammatory cells (24). Notably, early recognition of β-1,3-glucan and chitin in A. fumigatus conidia initiates distinct immune profiles (23). β-1,3-glucan recognition by dectin-1 promotes type 1 and IL-17-skewed immune responses while chitin promotes type 2 immunity that is detrimental in response to fungal pathogens but are otherwise protective for chitin-containing helminths (25–33). Notably, mouse inhalation of purified chitin induced lung accumulation of eosinophils and alternate activation of lung macrophages that was dependent on IL-5 and IL-13 secretion by type 2 innate lymphoid cells (31, 34). We previously observed that eosinophils in particular mediated type 2 pathology in a mouse model of IA; their absence resulted in increased fungal clearance (30). Although eosinophils were detrimental in this setting, the potential for eosinophil-mediated pathology in clinical invasive aspergillosis remains less clear.
A significant body of work has described the cell wall of A. fumigatus as exhibiting plasticity marked by structural changes in response to mutation and environmental stress (reviewed by Latge and Beauvais (35)). Furthermore, environmental stressors are not limited to outdoor and indoor environments, but also include the microenvironment encountered in vivo during host lung colonization and infection. Not surprisingly, antifungal drugs induce a significant amount of stress by directly inhibiting cell wall or membrane synthesis. For example, direct inhibition of the β-1,3-glucan synthesis pathway by in vitro growth in the presence of caspofungin resulted in a cell wall architecture characterized by increased levels of chitin in A. fumigatus (36, 37). However, a connection between increased caspofungin-mediated chitin exposure and increased eosinophil activation and pathology has not been described. Two clinical reports described eosinophilia in caspofungin-treated patients with IA, although a mechanism explaining these observations was neither proposed nor examined (38, 39). When considered together, these results suggest that caspofungin has the potential to increase detrimental eosinophilia in patients with IA, although direct evidence supporting this hypothesis remains lacking.
In this study, we demonstrate a clinical relevance for this relationship by demonstrating that caspofungin treatment increases eosinophil recruitment and pathology in a mouse model invasive aspergillosis. Furthermore, we identify a role for γδ T cells in this response. We also extend our findings to IA patients by comparing the levels of eosinophil activation markers in the bronchoalveolar lavage fluid (BALF) of patients treated with azole drugs with those that received a combination therapy that included caspofungin. Our results suggest a mechanism for caspofungin-mediated increases in fungal chitin and detrimental eosinophil recruitment in invasive aspergillosis.
Materials and Methods
Growth and handling of fungi
Aspergillus fumigatus (Af293) was purchased from the Fungal Genetics Stock Center. Fungi were cultured on malt extract agar (MEA). Conidia were isolated from culture plates kept at RT for 14 days by applying and gently shaking 1g of glass beads (0.5 mm, BioSpec Products), then placed in suspension by pouring the beads into a tube with sterile phosphate buffered saline (DPBS). For mouse aspiration, conidia were harvested using glass beads and resuspended in DPBS. The beads were then vortexed and the supernatant containing the conidia was removed, diluted and counted with a hemacytometer and used for aspiration. To determine the effect of capsofungin on conidial chitin exposure, the Af293 isolate was cultured on MEA plates containing 16ug/ml caspofungin diacetate (Sigma) and incubated at 37°C for 4 days. For flow cytometric analysis of conidia, harvested conidia were swollen in RPMI for 4 hours at 37°C and subsequently fixed with 4% paraformaldehyde. Swollen and fixed conidia were washed with ammonium chloride and DPBS and resuspended in DPBS for surface staining and flow cytometric analysis. For surface staining, swollen conidia was stained with carbohydrate binding lecithin, Wheat Germ Agglutinin (WGA; conjugated with APC) for surface chitin detection and analyzed on flow cytometry for quantification.
Mouse infection, sacrifice, histological staining, and collection of BALF
BALB/c or C57BL6/J mice were obtained from Envigo or Jackson Laboratory, while ΔdblGATA1, and TCRδ−/− mice aged 5 weeks were obtained from Jackson Laboratory. IL-4 GFP-reporter mice (4get) were previously obtained from Dr. Richard Locksley. Mice were allowed to rest 2–4 weeks prior to experiments. A subset of mice were bred at the IUSM-Terre Haute animal facility with offspring used in subsequent experiments at 7–10 weeks of age.
To induce invasive pulmonary aspergillosis in mice, neutrophils were depleted by intraperitoneal injection of 0.25 mg α-Ly6G (1A8; BioXCell) 24 hours before and after infection. Neutropenic mice were infected with 5x106 condia of A. fumigatus isolates by involuntary aspiration. Caspofungin was prepared in sterile DPBS at 5mg/Kg (high dose) or 1mg/Kg (low dose) (40) and was injected i.p. on a daily basis until mice were harvested. In some experiments, infected mice were monitored for survival or changes in disease using a five-point scale: 0) healthy 1) minimal disease (e.g. ruffled fur), 2) moderate disease (e.g. ungroomed, hunched), 3) severe disease (e.g. severely hunched, changes in eye color, low motility), and 4) moribund or deceased. Mice were sacrificed with sodium pentobarbitol, and lungs were perfused with 10ml phosphate buffered saline (PBS). Bronchoalveolar lavage fluid (BALF) was collected from the perfused lungs as previously described (41). For paraffin-embedded histological preparation, lungs were perfused with PBS followed by perfusion and inflation of the lungs with 10% buffered formalin phosphate (Fisher Scientific). To visualize lung infiltration by inflammatory cells, hematoxylin and eosin (H&E) stains were prepared and analyzed, and Gomori’s modified methanamine silver (GMS) stain was used for visualization of fungal germination in the lungs. Tissue processing, embedding, and staining was performed at Terre Haute Regional Hospital or at IUSM-TH. For frozen section preparation, lungs were perfused with 30% sucrose and inflated with 1:1 OCT (TissueTek) and immersed in 30% sucrose. After gradient freezing by embedding in OCT the tissue was kept frozen until sectioning. Lungs were cut in 5–10 μm using a cryostat (Avantik) and used for immunofluorescence microscopy. All animal procedures were approved by the Animal Care and Use Committee of Indiana State University, the host campus of IUSM-Terre Haute.
Flow cytometric analysis of bronchoalveolar lavage fluid and lung homogenates
BALF cell composition was determined by flow cytometric analysis of recovered lavage cells in suspension. BALF was centrifuged for 5 min at 1500 rpm, the supernatant removed, and the cell pellet resuspended and washed in 1ml of FACS buffer (Phosphate Buffered Saline, 5% fetal bovine serum, 0.05% sodium azide). The washed pellet was resuspended and stained in a solution containing FACS buffer with 10% rat serum, Fc-receptor blocking antibody (clone 24G2) and the following antibodies: rat-anti-mouse Ly-6G-FITC, rat-anti-mouse Siglec-F-PE, pan-leukocyte rat-anti mouse CD45-PerCP, and rat-anti-mouse CD11c-APC. For γδ T cell staining the following antibodies were used; rat-anti-mouse CD3-PeCy7 and TCRδ-PE (BD Biosciences). After staining, cells were washed and fixed with BD Cytofix, except for cells from 4get GFP reporter mice that were resuspended in FACS buffer and subsequently analyzed by flow cytometry. Flow cytometric data acquisition was performed on a Guava EasyCyte 8HT (EMD Millipore).
Immunofluorescence microscopy
6–8μm frozen sections of infected lungs were used for immunofluorescence staining. For fungal surface stain, calcofluor white (CFW; Sigma-Aldrich) was used to visualize surface chitin deposition in fungal hyphae. Rabbit anti-mouse CCR3 (Thermofisher) was used to visualize eosinophils with a goat anti-rabbit Dy-Light 488 (Abcam) secondary antibody. Briefly, sections were prepared by fixing the frozen sections in 4% paraformaldehyde at room temperature. Slides were stained with anti-CCR3 or CFW after blocking the sections with 10% goat serum for 1hr at 4°C followed by multiple washes with PBS and a 1 hour incubation with secondary antibody.
Total RNA processing and gene expression analysis
Lungs were removed and flash frozen in liquid nitrogen for RNA extraction. Total RNA was extracted from whole lungs homogenized in Trizol reagent (Invitrogen). Following the aqueous upper phase separation further RNA purification was performed using Qiagen RNEasy column with DNAse treatment per manufacturer’s recommendations. 2ug of total RNA was transcribed using High-capacity cDNA synthesis kit (Life Technologies) according to manufacturer’s protocol. For qPCR, Power-Up Sybr Green PCR Mastex Mix (Applied Biosystems) was used with Mxp3500 Real-time PCR system (Agilent). Select cytokine expression primers were obtained from SABiosciences.
Patient samples and ELISA
BALF was previously collected from lung transplant recipients at Royal Brompton and Harefiled NHS Foundation Trust (London, UK), with appropriate ethical approval (RBH/AS1) (42). Along with BALF, information regarding clinical diagnoses and antifungal pharmacotherapy were obtained. For detection of eosinophil peroxidase (EPO) and human major basic protein (MBP) in BAL of transplant patients, human EPO and MBP ELISA kits were purchased from NovateinBio (Woburn MA) and used according to manufacturer’s protocol. All samples were run in duplicates. Patients with a clinical diagnosis of fungal infection were further divided into two groups, those with caspofungin therapy alone or in combination with other antifungal drugs and patients that only received azole therapy.
Data analysis methods
Analysis of mouse flow cytometric data was performed with FlowJo software (TreeStar). GraphPad Prism was used for generation of graphs and Figures and for statistical analyses (GraphPad Software). Unpaired t-tests were used to measure statistical significance when two groups were compared, and one or two-way analysis of variance (ANOVA) tests were used along with Tukey’s or Sidak’s post-tests for multiple comparisons, respectively. Survival curves were analyzed with Mantel-Cox log-rank tests. Patient data from this study was analyzed by ANOVA using generalized estimating equation models, which allow the use of non-normal data by determining the best distribution of fit. Patient data were analyzed using SAS v9.4 (SAS Institute). Differences between experimental groups that resulted in a p-value of less than 0.05 were considered significant.
Results
Caspofungin increases fungal chitin exposure in vitro and in the lungs of mice with invasive aspergillosis
Previous studies have reported increased A. fumigatus chitin expression when β-1,3-glucan synthesis is inhibited by caspofungin (36, 37). To confirm if caspofungin increases chitin exposure in germinating conidia, we cultured and germinated the clinical isolate Af293 (low/normal chitin expressing (43)) in the presence or absence of caspofungin and subsequently stained with the chitin-binding wheat germ agglutinin (WGA) for flow cytometric analysis. We observed that growth in the presence of caspofungin increased WGA staining on germinating conidia (Figs. 1A and 1B). To determine if caspofungin therapy in invasive infection is associated with increased fungal chitin exposure in vivo, we infected neutropenic BALB/c mice with Af293 conidia and compared lung tissue sections of caspofungin-treated and untreated mice at day 3 post-infection (model timeline in Fig. 1C). Compared to untreated mice, fungi in the lungs of caspofungin-treated (5mg/Kg) mice with IA displayed dysmorphic growth with short, thickened hyphae and swollen or burst hyphal tips (Fig. 1D). Fluorescence staining of the lung tissues with the chitin-binding calcofluor white resulted in an increased intensity of tissue-invading hyphae in mice treated with caspofungin when compared to controls (Fig. 1E and inset, bottom left). In addition, CCR3+ cells were observed within areas of hyphal growth in drug-treated and untreated mice (Fig. 1E). These findings demonstrate that pharmacological targeting of β-1,3-glucan synthesis by caspofungin results in increased chitin exposure and/or deposition on the surface of A. fumigatus in vitro and in vivo.
FIGURE 1.
Caspofungin increases surface chitin exposure in A. fumigatus in vitro and in the lungs of mice with IA. (A and B) Af293 conidia were cultured and germinated (4h at 37°C) in the presence of caspofungin or control conditions, then fixed prior to WGA-APC staining. (A) Representative histogram overlay from 3 experiments. (B) Summary of median fluorescence intensity of WGA staining from 3 experiments (n=3/group). (C) Infection timeline. BALB/c mice were depleted of neutrophils and infected with 5x106 Af293 conidia, with a subset of mice treated with i.p. caspofungin daily until harvest at 72h post-infection. (D) Representative fungal morphology in GMS-stained control lung and caspofungin-treated mice. Red arrows highlight swollen or burst hyphal tips, blue arrows highlight short, thickened hyphae. Panels are representative of sections from 3 mice/group. (E) Representative immune fluorescence staining of d3 frozen lung sections from caspofungin treated or untreated mice with IA, stained for CCR3+ cells (green) and calcofluor white (red) to identify fungal chitin (n=3/group). Scale bars (D, E) are equivalent to 20 μm. ****p<0.0001.
Caspofungin treatment is associated with AMCase-sensitive eosinophil recruitment in mice with IA
Results of our previous work suggested that eosinophils are detrimental to protection in invasive aspergillosis in mice with Th2-skewed immunity (30). However, a high-chitin expressing isolate was used for exposures and infection that not only contained more chitin than commonly used clinical isolates such as Af293, but additionally displayed decreased virulence likely due to a decreased growth rate (43). We therefore aimed to determine if a pharmacologically mediated increase in chitin expression in the Af293 isolate in the lungs at the site of infection would result in a reciprocal increase in eosinophils in neutropenic BALB/c mice with IA. Seventy-two hours post-infection, we observed increased airway eosinophils in caspofungin-treated mice in comparison to untreated mice, while total leukocytes remained unchanged (Figs. 2A–C). Furthermore, airway eosinophil recruitment was partially decreased in caspofungin-treated mice that constitutively express acidic mammalian chitinase (AMCase; SPAM transgenic) when compared to non-transgenic littermates (Fig 2E). More specifically, a significant decrease was observed in the frequency of eosinophil in SPAM transgenic mice, while the total number of cells was not significant, possibly due to modulation of the number of total leukocytes in SPAM mice (Figs 2D, 2E). When considered together, these results suggest that caspofungin enhances chitin-mediated lung eosinophil recruitment in mice with IA.
FIGURE 2.
Airway eosinophil accumulation is increased with caspofungin treatment in mice with IA. A, B, and C, BALB/c mice were neutrophil-depleted, infected, and treated or untreated with caspofungin as described for Fig. 1 (timeline in Fig. 1C). BALF was analyzed for eosinophil recruitment as described in Materials and Methods. D and E, analysis of BALF from caspofungin-treated mice with IA that constititively express lung AMCase (SPAM+) compared to transgene-negative littermates (SPAM-). A, representative flow plots depicting gating of BALF CD45hiLy6G−SiglecF+CD11c− eosinophils (Eos) and CD45hiLy6G−SiglecF+CD11c+ alveolar macrophages (AM). B and D, total cells. C and E, frequency (left) and total number (right) of eosinophils. Data shown are a summary of 2–3 experiments. *p<0.05. **p<0.01.
Detection of eosinophil activation in caspofungin-treated patients with fungal infection
In patients with allergic or hypereosinophilic diseases, serum levels of eosinophil granule proteins were significantly increased and were correlated with inflammation and disease severity (44–47). We therefore appropriated this strategy to compare levels of the eosinophil granule proteins major basic protein (MBP) and eosinophil peroxidase (EPX) in bronchoalveolar lavage fluid (BALF) samples from lung transplant patients with or without a clinical diagnosis of Aspergillus infection (42). We further grouped these samples based on the associated antifungal therapy: those infected patients that received azole therapy alone compared to those that received caspofungin (alone or in combination with azoles). Despite a large range of activation among patients, MBP and EPX proteins were more frequently detected in the BALF of individuals that received caspofungin therapy in comparison to azole-treated patients (Figs. 3A and 3B). Therefore, our results in patients and mice with aspergillosis suggest that caspofungin therapy is associated with increased lung eosinophil recruitment and activation.
FIGURE 3.
Increased detection of eosinophil granule proteins in aspergillosis patients treated with caspofungin. Major basic protein (A) and eosinophil peroxidase (B) in the BALF of lung transplant patients with or without aspergillosis quantified by ELISA. Patients were further subdivided by antifungal therapy: those that received azoles (Infected) or those that received caspofungin alone or in combination with azoles (Inf+Caspo). Statistical analysis was performed as described in Materials and Methods.
Lack of effective caspofungin-mediated fungal clearance in invasive aspergillosis is reversed in eosinophil-deficient mice
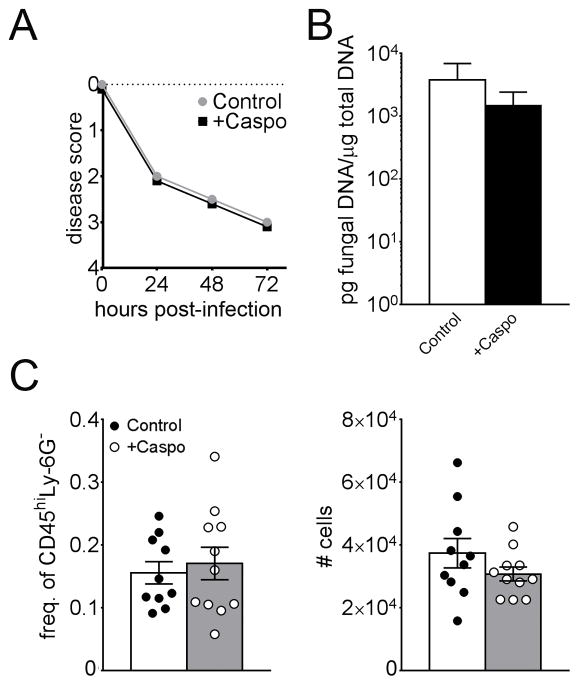
We next wanted to determine if eosinophils increased pathology and inhibited fungal clearance in caspofungin-treated mice with IA. By five days post-infection, all wild-type, caspofungin-treated BALB/c mice with IA had succumbed to infection, whereas half of caspofungin-treated eosinophil-deficient (ΔdblGATA1) mice survived to eight days (Fig. 4A). Similarly, disease severity was markedly increased in wild-type mice treated with caspofungin in comparison to untreated wild-type and eosinophil−/− mice, while significant improvement was observed with caspofungin in the absence of eosinophils (Fig. 4B). Furthermore, eosinophil-deficient mice exhibited improved fungal clearance with caspofungin treatment, while wild-type mice did not (Figs. 4C, D), and this phenotype was further confirmed with the observation and quantification of fungal staining in GMS histological sections that showed significant fungal clearance only in capsofungin-treated, eosinophil-deficient BALB/c mice (Figs. 4E, F). Decreased fungal burden was also observed in C57BL/6-background eosinophil-deficient ΔdblGATA1 mice when compared to wild-type B6 mice (Supplemental Fig. S1C), although total leukocyte and eosinophil recruitment were not significantly increased in B6 mice as in their BALB/c counterparts (Figs. S1A and S1B). A decrease in fungal burden or eosinophil recruitment was not evident in dectin-1-deficient mice (B6 background) treated with caspofungin, suggesting that β-glucan recognition does not play a major role in paradoxical caspofungin activity in neutropenic mice with IA (Supplemental Figs. S1D–F). Furthermore, BALB/c mice treated with a lower dose (1mg/Kg) of caspofungin did not exhibit increased eosinophil recruitment or increased fungal burden (Figs S1G–H). These results indicate that the increased disease severity and fungal burden in caspofungin-treated mice is dependent on antifungal dose and the presence of eosinophils.
FIGURE 4.
Increased disease severity and lack of fungal clearance in caspofungin-treated mice is eosinophil-dependent. Wild-type BALB/c or eosinophil-deficient (ΔdblGATA1) mice were infected and treated or left untreated with caspofungin as described for Fig. 1. (A) Survival. (B) Disease Score. (C) Fungal DNA (burden) (15–30 mice/group, summary of 3–6 experiments). (A–C) n=15–30 mice/group, data are a summary of 3–6 experiments. (D) Change in fungal DNA burden with caspofungin treatment calculated from results shown in Fig. 4A. (E) Fungal burden as measured by quantification of GMS staining of histological sections (n=3–4 mice/group). (F) Representative GMS staining of histological sections from wild-type (top) or eosinophil−/− mice (bottom). Scale bar is equivalent to 100 μm. *p<0.05. **p<0.01. ****p<0.0001.
Caspofungin-increased eosinophil recruitment requires RAG1 expression
Innate lung eosinophil recruitment in response to instillation of chitin particles was not dependent antigen receptor-rearranged lymphocytes that require expression of the recombinase activating gene 1 (RAG1) for development (31). Since we observed increased fungal chitin and eosinophil-mediated pathology in caspofungin-treated mice with IA, we aimed to determine if this phenotype in our model was also independent of RAG1 expression. In infected RAG1−/− (BALB/c background) mice, we observed no difference in disease severity or fungal burden between control and caspofungin-treated mice (Figs. 5A, 5B). Furthermore, airway eosinophil recruitment was not increased by caspofungin treatment (Fig. 5C). Thus, in contrast to results with chitin particles, RAG1 expression is required for the observed increase in eosinophil recruitment in response to A. fumigatus infection.
FIGURE 5.
Disease severity, fungal burden, and eosinophil recruitment are not increased in caspofungin-treated RAG1−/− mice. Mice deficient in RAG1 (BALB/c background) were infected with A. fumigatus as described for Fig. 1. (A) Disease severity and (B) Fungal burden (n=8). (C) Frequency (left) and total BALF eosinophils (right). Data shown are a summary of two experiments.
Modulation of CD3+TCRδ+ cells in caspofungin-treated mice with IA
The requirement for RAG1 expression for eosinophil-mediated pathology suggests a role for γδ T cells, iNKT cells, or conventional αβ T cells that are absent in RAG1-deficient mice. Of these subsets, γδ T cells were activated by inhalation of chitin particles and are capable of initiating early type 2 immune responses and eosinophil recruitment via secretion of IL-4 (34, 48, 49). Therefore, we wanted to determine if lung γδ T cells were increased in caspofungin-treated mice with IA or exhibited increased IL-4 activation at 48 hours post-infection when compared to infected, untreated mice. Unexpectedly, CD3+TCRδ+ cells were decreased in the lungs of infected mice that received caspofungin treatment (Figs. 6A and 6B). Furthermore, very few IL-4/GFP+ reporter activated (“4get” (50)) γδ T cells were detected regardless of caspofungin treatment (Fig. 6C and data not shown). These results suggest that γδ T cells are either decreased or decrease their surface TCR/CD3 expression, and do not display increased IL-4 gene activation in caspofungin-treated mice with IA.
FIGURE 6.
Modulation of CD3+TCRδ+ cells in caspofungin-treated mice with IA. Neutropenic mice were infected and harvested at 48 hours post-infection, with cell suspensions derived from lung homogenates analyzed by flow cytometry for expression of γδ T cell markers. A) Representative dot plots from two experiments. B) Frequency (left) and total numbers (right) of lung CD3+TCRδ+ cells. C) Representative histogram from two experiments depicting GFP fluorescence of CD3+TCRδ+ cells from IL4-GFP-reporter mice (4get). *p<0.05.
Eosinophil recruitment and fungal burden are decreased by caspofungin treatment in γδ T cell-deficient mice with invasive aspergillosis
Although we detected fewer CD3+TCRδ+ cells in the lungs of caspofungin-treated mice, this result does not preclude an involvement of γδ T cells in caspofungin-driven eosinophil pathology of IA, as T cells are known to decrease surface expression of the TCR/CD3 complex upon activation in order to prevent overstimulation (51–53). We therefore determined if γδ T cells were required for airway eosinophil recruitment during invasive infection. Interestingly, caspofungin-treated γδ T cell-deficient mice exhibited a marked increase in survival and decrease in fungal burden as measured by quantitative PCR of fungal DNA or quantification of fungal staining on histological sections in comparison to untreated mice (Figs. 7A–7C, with inset). Furthermore, in contrast to wild-type C57BL/6 and dectin-1 deficient mice (Figs. S1 A, B, D, and E), airway eosinophils were decreased in γδ T cell deficient mice that received caspofungin treatment, while the total number of airway cells remained unchanged (Figs 7D and 7E). These results suggest that γδ T cells act as regulators of eosinophil recruitment and pathology in invasive aspergillosis.
FIGURE 7.
Caspofungin-mediated eosinophil recruitment and pathology require γδ T cells. γδ T cell-deficient mice were infected with Af293, monitored and harvested as described for Fig. 1. (A) Survival (n=9–10, summary of two experiments). (B) Display of fungal burden determined by PCR quantification of fungal DNA (7–8mice/group, summary of two experiments). (C) Representative GMS staining of lung sections from γδ T cell-deficient mice with the indicated treatment. Scale bar is equivalent to 100 μM. X. Inset, bottom left of right panel, determination of fungal burden by quantification of GMS staining in treated and untreated mice. (D, E) BALF cell populations as determined by flow cytometry. (E) Frequency (left) and total number (right) of airway eosinophils in the indicated experimental groups. Data shown are a summary of two experiments. *p<0.05. **p<0.01. ***p<0.001.
Effects of caspofungin treatment and requirement for γδ T cells in lung expression of immunomodulatory genes in mice with IA
Our results demonstrate a role for γδ T cells in eosinophil recruitment and pathology of A. fumigatus infection. How γδ T cells promote IA pathology remains less clear. In an experimental model of allergy, IL-4 secretion by γδ T cells promoted eosinophil recruitment (48). However, our results with IL-4 reporter mice indicated very few IL-4+ γδ T cells in the lungs of mice with IA (Fig. 6C). Using quantitative RT-PCR on lung tissue, we aimed to determine if expression of other genes associated with type 2 immune, γδ T cell activation, or chitin responses were modulated in response to caspofungin treatment in the presence or absence of γδ T cells at 48 hours post-infection. In response to caspofungin treatment, wild-type mice with IA did not significantly modulate transcription of the cytokines/chemokines IL-4, IL-5, IL-17A, IL-22, CCL11, or CCL22 (Fig. 8A). Likewise, expression of the alternate activation marker of macrophages, Arginase-1, the chitinases AMCase and chitrosiadase (Chit1), and the chitinase-like proteins BRP39 and Ym1 were not significantly altered. Of the genes we examined, expression of the chitinase-like protein (CLP) Ym2 (Chitinase 3-like 4) was significantly increased in response to caspofungin. In contrast, none of the genes we examined in wild-type caspofungin-treated mice displayed significant changes in expression in the absence of γδ T cells (Fig. 8B). Therefore, caspofungin treatment and γδ T cells may influence eosinophil recruitment and pathology by an undescribed, novel pathway.
FIGURE 8.
Effects of caspofungin treatment and requirement for γδ T cells in lung expression of immunomodulatory genes in mice with IA. Neutropenic C57BL/6 (B6) wild-type or γδ T cell-deficient mice were infected with A. fumigatus, treated or untreated with caspofungin, and harvested for qRT-PCR analysis of the indicated genes in lung homogenate extracts at 48 hours post-infection. A) Wild-type B6 mice treated or untreated with caspofungin. B) Wild-type or TCRδ−/− mice infected and treated with caspofungin. *p<0.05.
Discussion
This study is the first to demonstrate a link between caspofungin-mediated increases in chitin exposure and detrimental eosinophil activation in mice and humans with aspergillosis. Previous studies detailed the in vitro “paradoxical effect” of fungal growth in the presence of high levels of caspofungin (20, 54), and a recent study demonstrated increased chitin synthase activity and fungal stress response activation in response to caspofungin that permits A. fumigatus growth despite the presence of drug-mediated cell wall remodeling (55). These in vitro results are supported by in vivo evidence from Moretti et al that demonstrated decreased caspofungin efficacy in mice with IA (20, 40). Similar to our results, they reported mouse strain-dependent differences in fungal burden, with BALB/c mice most susceptible to infection at any dose of caspofungin, and C57BL/6 mice exhibiting a drug dose-dependent response. In our study, only BALB/c mice displayed both increased fungal burden and eosinophil recruitment in response to caspofungin (Figs. 2 and 4). However, eosinophil-deficient mice from either background exhibited decreased fungal burden in response to caspofungin (Figs. 4 and S1). Therefore, despite differences in recruitment, eosinophils were detrimental to effective fungal clearance in caspofungin-treated BALB/c or C57BL/6 mice. Future studies will require consideration of these differences as mice with specific gene-targeted deficiencies are used from either background in order to elucidate mechanistic pathways of eosinophil-mediated pathology.
In addition to reporting increased mouse fungal burden in IA after caspofungin therapy, Moretti et al also provided mechanistic data that demonstrated a requirement for TLR2, TLR9, and Dectin-1 (40). Interestingly, TLR2 and TLR9 expression were required for macrophage secretion of IL-17A and IL-10 in response to purified chitin particles (56, 57). Other studies have shown roles for NOD2, mannose receptor, and FIBCD1 in chitin binding or chitin-mediated responses (56–58). In contrast to Moretti et al, our results did not show a significant decrease in fungal burden with high-dose (5 mg/Kg) caspofungin in dectin-1−/− mice (Supplemental Figs. S1D–F). Interestingly, Moretti et al reported increased neutrophils in the lungs of high-dose caspofungin-treated animals with IA (40), and these cells are known to express high levels of dectin-1 (59). However, in contrast to the cyclophosphamide induced immune suppression used in that study, we depleted neutrophils prior to and during infection. It is thus possible that neutrophils and dectin-1 recognition contribute to paradoxical caspofungin activity when present in mice with IA. We chose the neutropenic model of IA because it is considered one of the best methods to increase host susceptibility to IA while still preserving the ability to determine the contributions of specific cell populations (e.g. eosinophils) that may otherwise be affected by broad immune suppressants such as cyclophosphamide (60–63). It is therefore likely that co-recognition of β-glucan and chitin in paradoxical caspofungin activity and eosinophil pathology is a complex multivariate process that will require considerable resources to delineate, and will remain an active and important area of future investigation.
Although we observed that caspofungin-mediated airway eosinophil recruitment and pathology in IA was partly dependent on γδ T cells, the mechanism driving this phenotype remains unknown. The role of γδ T cells in lung eosinophil recruitment in our model is in contrast to the immune response to purified chitin particles that was mediated by type 2 innate lymphoid cell (ILC2) production of IL-4 and IL-13 (64). As noted in our discussion of pattern recognition receptors, it is possible that composite recognition of multiple fungal PAMPS also results in an increased role for γδ T cells in lung eosinophil recruitment and that these pathways are not sufficiently activated in response to chitin alone in the presence of ILC2s. Surprisingly, detection of lung γδ T cells was decreased in caspofungin-treated mice with IA when compared to untreated mice (Figs. 6A and 6B). It is possible that both CD3 and TCR levels were decreased to levels that rendered activated γδ T cells undetectable by flow cytometry (51–53). We did not detect any significant shift in median fluorescence intensity in lung γδ T cells in either the CD3 or TCRδ channels with caspofungin treatment (data not shown), although it is still possible that these cells remain despite a lack of detection. Furthermore, our results did not indicate a change in IL-4 activation in lung γδ T cells with caspofungin treatment, and no significant changes among a panel of effector cytokines, chitinases, and other markers of type 2 immunity (Figs. 6 and 8A). Rather, the chitinase 3-like 1/Ym2 was the only gene in our panel with increased expression with caspofungin therapy, and this pattern may be altered in the absence of γδ T cells (Fig. 8). Ym2 is a chitin binding protein that lacks chitinase activity and is thought to promote lung type 2 immunity (65). However, the understanding of the roles of this molecule in human infection and disease is preliminary. Future studies will require isolation of γδ T cells in untreated and caspofungin-treated mice with IA to compare changes in expression of these and other immune effectors, with the role of those identified pathways validated with knockout mice and adoptive transfer experiments.
Eosinophils have long been described as end-stage effector cells that secrete an array of cyototoxic proteins and lipid mediators that promote inflammation and collateral destruction of surrounding tissues. However, more recent studies have elucidated homeostatic and immunoregulatory roles for eosinophils (66, 67). It is unclear which role is most relevant in our caspofungin-increased pathology model or in patients with IA. Our analysis of eosinophil granule proteins in patients treated with caspofungin was limited by relatively low numbers of caspofungin-treated patients in the lung transplant cohort (n=6) and the large range and non-normal distribution of values of eosinophil activation markers in each group. These patients had a variety of disparate underlying conditions that necessitated lung transplant, the most common being cystic fibrosis and chronic obstructive pulmonary disease (COPD), two diseases with very distinct etiologies that develop at different stages of life. In addition, many of these patients were treated with multiple immune suppressive and antimicrobial drugs and/or may have exhibited sequelae of atopy or other infections that could influence levels of eosinophil activation independent of caspofungin. Despite potentially confounding factors inherent in human samples, we observed an increased detection of MBP and EPX in the BALF of fungal-infected patients that received caspofungin therapy when compared to those that received azoles. Future studies with paired samples (pre/post caspofungin treatment) will be necessary to determine the full contribution of these factors to eosinophil activation as well as the association of this activation with severity of disease in patients with IA or in other cohorts with marked eosinophil activation, such as allergic bronchopulmonary aspergillosis (ABPA) patients.
Since our results highlight the possibility that antifungal cell wall modulation could promote detrimental immunity in some patients, we encourage others to consider this potential host-pathogen relationship in studies that examine antimicrobial mechanisms of protection in susceptible hosts. It is likely that some combinations may prove complimentary. For example, caspofungin therapy could be combined with the chitin-synthesis inhibiting antifungal nikkomycin z to counteract increased chitin and detrimental eosinophilia induced by increased caspofungin (36, 68). However, in the absence of more specific clinical data, we do not believe that any risk of eosinophil pathology in caspofungin-treated patients currently outweighs the potential benefits of prophylactic or salvage therapy with echinocandins. The continued investigation of the potential of therapies that target eosinophil recruitment and activation along with fungal growth and dissemination in patients with fungal infection thus remains an important endeavor.
Supplementary Material
Acknowledgments
The authors would like to thank Amber Wilcox and Dylan Stolz for technical assistance and Joe Lewis for animal care.
Footnotes
Disclosure
This study was supported in part by an Indiana University School of Medicine Research Enhancement Grant and by NIH-NIAID 1R03AI122127-01. N.A. was partly supported during this period by a Careers in Immunology Fellowship from the American Association of Immunologists. The clinical studies were supported by the NIHR Respiratory Disease Biomedical Research Unit and the Imperial College Academic Health Science Centre.
References
- 1.Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryotic cell. 2007;6:1953–1963. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus--what makes the species a ubiquitous human fungal pathogen? PLoS pathogens. 2013;9:e1003743. doi: 10.1371/journal.ppat.1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20:156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010;9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 5.Dockrell DH. Salvage therapy for invasive aspergillosis. J Antimicrob Chemother. 2008;61(Suppl 1):i41–44. doi: 10.1093/jac/dkm426. [DOI] [PubMed] [Google Scholar]
- 6.Latge JP. The cell wall: a carbohydrate armour for the fungal cell. Molecular microbiology. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF A Infectious Diseases Society of. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 8.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA G. American Thoracic Society Fungal Working. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 9.Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(Suppl 6):42–48. doi: 10.1111/1469-0691.12513. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Current opinion in infectious diseases. 2013;26:493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 11.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 12.Denning DW, Bowyer P. Voriconazole resistance in Aspergillus fumigatus: should we be concerned? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:521–523. doi: 10.1093/cid/cit321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sucher AJ, Chahine EB, Balcer HE. Echinocandins: the newest class of antifungals. The Annals of pharmacotherapy. 2009;43:1647–1657. doi: 10.1345/aph.1M237. [DOI] [PubMed] [Google Scholar]
- 14.Koulenti D, Garnacho-Montero J, Blot S. Approach to invasive pulmonary aspergillosis in critically ill patients. Current opinion in infectious diseases. 2014;27:174–183. doi: 10.1097/QCO.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 15.Glockner A. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin:review of the literature. Eur J Med Res. 2011;16:167–179. doi: 10.1186/2047-783X-16-4-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendrup MC, Garcia-Effron G, Buzina W, Mortensen KL, Reiter N, Lundin C, Jensen HE, Lass-Florl C, Perlin DS, Bruun B. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrobial agents and chemotherapy. 2009;53:1185–1193. doi: 10.1128/AAC.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafaurie M, Lapalu J, Raffoux E, Breton B, Lacroix C, Socie G, Porcher R, Ribaud P, Touratier S, Molina JM. High rate of breakthrough invasive aspergillosis among patients receiving caspofungin for persistent fever and neutropenia. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2010;16:1191–1196. doi: 10.1111/j.1469-0691.2009.03050.x. [DOI] [PubMed] [Google Scholar]
- 18.Madureira A, Bergeron A, Lacroix C, Robin M, Rocha V, de Latour RP, Ferry C, Devergie A, Lapalu J, Gluckmana E, Socie G, Ghannoum M, Ribaud P. Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int J Antimicrob Agents. 2007;30:551–554. doi: 10.1016/j.ijantimicag.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Pang KA, Godet C, Fekkar A, Scholler J, Nivoix Y, Letscher-Bru V, Massias L, Kauffmann-Lacroix C, Elsendoorn A, Uzunov M, Datry A, Herbrecht R. Breakthrough invasive mould infections in patients treated with caspofungin. The Journal of infection. 2012;64:424–429. doi: 10.1016/j.jinf.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. The Journal of infectious diseases. 2004;190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 21.Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Current opinion in microbiology. 2011;14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Lenardon MD, Munro CA, Gow NA. Chitin synthesis and fungal pathogenesis. Current opinion in microbiology. 2010;13:416–423. doi: 10.1016/j.mib.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amarsaikhan N, Templeton SP. Co-recognition of beta-glucan and chitin and programming of adaptive immunity to Aspergillus fumigatus. Frontiers in microbiology. 2015;6:344. doi: 10.3389/fmicb.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 25.Bozza S, Gaziano R, Lipford GB, Montagnoli C, Bacci A, Di Francesco P, Kurup VP, Wagner H, Romani L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes and infection / Institut Pasteur. 2002;4:1281–1290. doi: 10.1016/s1286-4579(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 26.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. Journal of immunology. 2000;165:381–388. doi: 10.4049/jimmunol.165.1.381. [DOI] [PubMed] [Google Scholar]
- 27.Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, d’Ostiani CF, Mosci P, Bachmann M, Bistoni F, Kopf M, Romani L. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. Journal of Infectious Diseases. 1999;180:1957–1968. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 28.Chai LY, van de Veerdonk F, Marijnissen RJ, Cheng SC, Khoo AL, Hectors M, Lagrou K, Vonk AG, Maertens J, Joosten LA, Kullberg BJ, Netea MG. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130:46–54. doi: 10.1111/j.1365-2567.2009.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell host & microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Dea EM, Amarsaikhan N, Li H, Downey J, Steele E, Van Dyken SJ, Locksley RM, Templeton SP. Eosinophils are recruited in response to chitin exposure and enhance Th2-mediated immune pathology in Aspergillus fumigatus infection. Infection and immunity. 2014;82:3199–3205. doi: 10.1128/IAI.01990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med. 2011;208:369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. Journal of immunology. 2011;187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie AN, Krummel MF, Liang HE, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40:414–424. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latge JP, Beauvais A. Functional duality of the cell wall. Current opinion in microbiology. 2014;20:111–117. doi: 10.1016/j.mib.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Verwer PE, van Duijn ML, Tavakol M, Bakker-Woudenberg IA, van de Sande WW. Reshuffling of Aspergillus fumigatus cell wall components chitin and beta-glucan under the influence of caspofungin or nikkomycin Z alone or in combination. Antimicrobial agents and chemotherapy. 2012;56:1595–1598. doi: 10.1128/AAC.05323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker LA, Lee KK, Munro CA, Gow NA. Caspofungin Ttreatment of Aspergillus fumigatus Results in ChsG-dependent upregulation of chitin synthesis and the formation of chitin-rich microcolonies. Antimicrobial agents and chemotherapy. 2015;59:5932–5941. doi: 10.1128/AAC.00862-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anttila VJ, Salonen J, Ylipalosaari P, Koivula I, Riikonen P, Nikoskelainen J. A retrospective nationwide case study on the use of a new antifungal agent: patients treated with caspofungin during 2001–2004 in Finland. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2007;13:606–612. doi: 10.1111/j.1469-0691.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 39.Chua NG, Zhou YP, Lingegowda PB, Kwa AL, Lee W. Echinocandin-induced eosinophilia: a case report. Scandinavian journal of infectious diseases. 2014;46:809–812. doi: 10.3109/00365548.2014.938692. [DOI] [PubMed] [Google Scholar]
- 40.Moretti S, Bozza S, D’Angelo C, Casagrande A, Della Fazia MA, Pitzurra L, Romani L, Aversa F. Role of innate immune receptors in paradoxical caspofungin activity in vivo in preclinical aspergillosis. Antimicrobial agents and chemotherapy. 2012;56:4268–4276. doi: 10.1128/AAC.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Templeton SP, Buskirk AD, Law B, Green BJ, Beezhold DH. Role of germination in murine airway CD8+ T-cell responses to Aspergillus conidia. PloS one. 2011;6:e18777. doi: 10.1371/journal.pone.0018777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, Armstrong-James D, Schelenz S. The serum opsonin L-ficolin is detected in lungs of human transplant recipients following fungal infections and modulates inflammation and killing of Aspergillus fumigatus. The Journal of infectious diseases. 2015;212:234–246. doi: 10.1093/infdis/jiv027. [DOI] [PubMed] [Google Scholar]
- 43.Amarsaikhan N, O’Dea EM, Tsoggerel A, Owegi H, Gillenwater J, Templeton SP. Isolate-dependent growth, virulence, and cell wall composition in the human pathogen Aspergillus fumigatus. PloS one. 2014;9:e100430. doi: 10.1371/journal.pone.0100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonnkvist K, Hellman C, Lundahl J, Hallden G, Hedlin G. Eosinophil markers in blood, serum, and urine for monitoring the clinical course in childhood asthma: impact of budesonide treatment and withdrawal. The Journal of allergy and clinical immunology. 2001;107:812–817. doi: 10.1067/mai.2001.114246. [DOI] [PubMed] [Google Scholar]
- 45.Makiya MA, Herrick JA, Khoury P, Prussin CP, Nutman TB, Klion AD. Development of a suspension array assay in multiplex for the simultaneous measurement of serum levels of four eosinophil granule proteins. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Condjella RM, Bersoux S, Helmers RA, Moqbel R, Lacy P, Kelly EA, Jarjour NN, Kern R, Peters A, Schleimer RP, Furuta GT, Nair P, Lee JJ, Lee NA. A sensitive high throughput ELISA for human eosinophil peroxidase: a specific assay to quantify eosinophil degranulation from patient-derived sources. J Immunol Methods. 2012;384:10–20. doi: 10.1016/j.jim.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto H, Ninomiya H, Yoshimatsu K, Uchiyama Y, Shibasaki M, Enokihara H, Tachibana S, Abe T. Serum levels of major basic protein in patients with or without eosinophilia: measurement by enzyme-linked immunosorbent assay. Br J Haematol. 1994;86:490–495. doi: 10.1111/j.1365-2141.1994.tb04778.x. [DOI] [PubMed] [Google Scholar]
- 48.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 49.Born WK, Huang Y, Jin N, Huang H, O’Brien RL. Balanced approach of gammadelta T cells to type 2 immunity. Immunol Cell Biol. 2010;88:269–274. doi: 10.1038/icb.2009.105. [DOI] [PubMed] [Google Scholar]
- 50.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bachmann MF, Oxenius A, Speiser DE, Mariathasan S, Hengartner H, Zinkernagel RM, Ohashi PS. Peptide-induced T cell receptor down-regulation on naive T cells predicts agonist/partial agonist properties and strictly correlates with T cell activation. European journal of immunology. 1997;27:2195–2203. doi: 10.1002/eji.1830270912. [DOI] [PubMed] [Google Scholar]
- 52.Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. Journal of immunology. 1999;162:2073–2080. [PubMed] [Google Scholar]
- 53.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 54.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrobial agents and chemotherapy. 2008;52:321–328. doi: 10.1128/AAC.00699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrobial agents and chemotherapy. 2010;54:1555–1563. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS pathogens. 2014;10:e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. Journal of immunology. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlosser A, Thomsen T, Moeller JB, Nielsen O, Tornoe I, Mollenhauer J, Moestrup SK, Holmskov U. Characterization of FIBCD1 as an acetyl group-binding receptor that binds chitin. Journal of immunology. 2009;183:3800–3809. doi: 10.4049/jimmunol.0901526. [DOI] [PubMed] [Google Scholar]
- 59.Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad, Kontoyiannis DP. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. The Journal of infectious diseases. 2008;198:186–192. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infection and immunity. 2005;73:114–125. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003;8:481–495. doi: 10.1023/a:1025590308147. [DOI] [PubMed] [Google Scholar]
- 62.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. Journal of immunology. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 63.Su YC, Rolph MS, Cooley MA, Sewell WA. Cyclophosphamide augments inflammation by reducing immunosuppression in a mouse model of allergic airway disease. The Journal of allergy and clinical immunology. 2006;117:635–641. doi: 10.1016/j.jaci.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 64.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annual review of immunology. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothenberg ME, Hogan SP. The eosinophil. Annual review of immunology. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 68.Ganesan LT, Manavathu EK, Cutright JL, Alangaden GJ, Chandrasekar PH. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2004;10:961–966. doi: 10.1111/j.1469-0691.2004.00996.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.