Abstract
Recent antihypertensive trials show conflicting results on blood pressure (BP) targets in patient populations with different metabolic profiles, with lowest benefit from tight BP control observed in patients with type 2 diabetes mellitus. This paradox could arise from the heterogeneity of study populations and underscores the importance of precision medicine initiatives towards understanding and treating hypertension. Wnt signaling pathways and genetic variations in its signaling peptides have been recently associated with metabolic syndrome, hypertension and diabetes, generating a breakthrough for advancement of precision medicine in the field of hypertension.
We performed a review of PubMed for publications addressing the contributions of Wnt to BP regulation and hypertension. In addition, we performed a manual search of the reference lists for relevant articles, and included unpublished observations from our laboratory.
There is emerging evidence for Wnt’s role in BP regulation and its involvement in the pathogenesis of hypertension. Wnt signaling has pleiotropic effects on distinct pathways that involve vascular smooth muscle plasticity, and cardiac, renal, and neural physiology.
Hypertension is a heterogeneous disease with unique molecular pathways regulating its response to therapy. Recognition of these pathways is a prerequisite to identify novel targets for drug development and personalizing medicine. A review of Wnt signaling reveals its emerging role in BP regulation and as a target for novel drug development that has the potential to transform the therapy of hypertension in specific populations.
Keywords: Wnt, β-catenin, hypertension, blood pressure, diabetes, metabolic syndrome
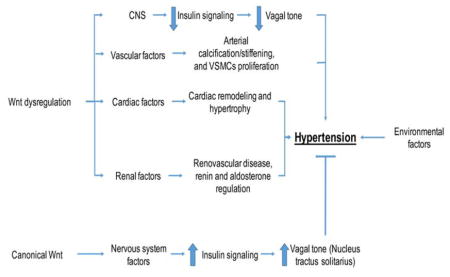
Graphical Abstract
I. Introduction
Canonical Wnt signaling is a highly conserved pathway named after the first gene discovered in the cascade; the “W”ingless gene in drosophila and its mouse homologue “INT” (1). The Wnt cascade is ubiquitous in metazoans and is active in most tissue types throughout the life cycle. During embryogenesis it has been implicated in controlling the mechanics of cell proliferation, migration, differentiation, apoptosis, establishment of cell polarity, and body axis determinations (2). Derangements in Wnt signaling have been associated with developmental disorders, cancers, fibrotic disorders and cardiovascular disease. The involvement of Wnt in this wide spectrum of biologic processes and human diseases has fueled research efforts into its components. The cascade is complex, involving an array of ligands, receptors, and multiple downstream effectors defining the canonical and non-canonical pathways. The newly uncovered molecular connections offer many challenges and new opportunities for novel classes of pharmacotherapies. Of special interest to this review is the link between Wnt and hypertension.
Hypertension affects approximately 30% of adults in the United States (3, 4) and is associated with serious complications that can be fatal. Worldwide, there are over 1 billion individuals suffering from the disease, and by 2025 this number is estimated to increase by 50% (5). Hypertension is a component of metabolic syndrome (MetS), which also encompasses truncal obesity, glucose intolerance, atherogenic dyslipidemias, a pro-thrombotic state, and a pro-inflammatory state. These heritable cardio-metabolic traits tend to cluster more than predicted by random chance, and significantly increase the risk for cardiovascular mortality (6). Hypertension has a high rate of heritability estimated at 30–68% (7, 8) and in rare cases is inherited as a single gene disorder (9–11). However, this disease is heterogeneous and is affected by the interplay of genetic and environmental factors that regulate or modify its determinants such as vascular tone, volume status, cardiac function, neural function, and endocrine function. This heterogeneity is also reflected in the distinct responses to pharmacotherapies and blood pressure targets observed in populations with different ethnicities (12) or metabolic profiles. The Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD-BP) trial (13) and the Systolic Blood Pressure Intervention Trial (SPRINT) trial (14) showed that strict blood pressure control reduces cardiovascular events in non-diabetic patients, however, there was no benefit in diabetics with MetS. This suggests different pathogenesis of hypertension in subjects with and without type 2 diabetes mellitus. Therefore, precision medicine initiatives might help provide optimum outcomes. The links between Wnt, hypertension and MetS are recent, exciting discoveries that may open a new front in cardiovascular risk stratification or risk modulation. Does targeting Wnt signaling satisfy the demands placed by precision medicine for the treatment of hypertension? What are the challenges and concerns associated with such intervention? In this review, we will summarize the current evidence and the potential for novel therapeutics targeting this pathway.
II. Methods
We used a comprehensive search of MEDLINE with PubMed interface for original studies in English that reported on hypertension and Wnt from January 1, 1900 to March 1, 2016. Search terms included keywords that referred to Wnt, and Hypertension. The search strategy was (“Wnt signaling”[MAJR] OR (WNT[TIAB] OR “ β-catenin” OR “beta catenin”)) AND (“Hypertension”[MAJR] OR (high blood pressure) OR “Blood pressure”[MAJR]). Overall, we screened 176 articles retrieved through this systematic search. Moreover, the reference lists were hand-searched for pertinent articles. We also discuss unpublished observations from our laboratory. Studies addressing pulmonary hypertension and Wnt were excluded.
III. What are the mechanisms of Wnt signaling?
Wnt ligands are a family of nineteen lipidated-glycoproteins. Different ligands preferentially activate the canonical or non-canonical Wnt pathways, which reciprocally inhibit each others (15). The Wnt receptors belong to the Frizzled (Fz) receptor family, which includes 10 different trans-membrane proteins in humans (16). In addition, there are several co-receptors such as LDL receptor-related proteins (LRP) 5 and 6, the Derailed/receptor tyrosine kinase (RYK) (17), and ROR trans-membrane tyrosine kinases. (18, 19) The LRP 5/6 co-receptors activate the canonical cascade whereas the RYK and ROR-transmembrane tyrosine kinase co-receptors activate the non-canonical pathways.
β-catenin is a key molecule in the canonical Wnt pathway. It functions as an adherens junction protein, but more importantly as a transcriptional activator. At baseline, cytoplasmic β-catenin is in constant turnover being synthesized and destroyed intracellularly. The β-catenin destruction complex is composed of APC (adenomatous polyposis coli), Axin, CK-1 (casein kinase 1), and GSK3 (glycogen synthase kinase 3) proteins. The destruction complex phosphorylates β-catenin and targets it for ubiquitination to keep the cytoplasmic levels within tight regulation and thus prevents β-catenin from translocating into the nucleus (20). Canonical Wnt signaling in essence overrides this inhibitory state, causes β-catenin cytoplasmic buildup, translocation into the nucleus, and activation of gene transcription.
The cascade is initiated when the Wnt ligand binds to the Fz receptor and LRP5/6 co-receptors (20). This binding activates Dishevelled, which inhibits the destruction complex and thus rescues β-catenin. Once β-catenin translocates to the nucleus it binds to a variety of transcription factors such as the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors, and many other co-activators (21, 22) as shown in Fig. 1A.
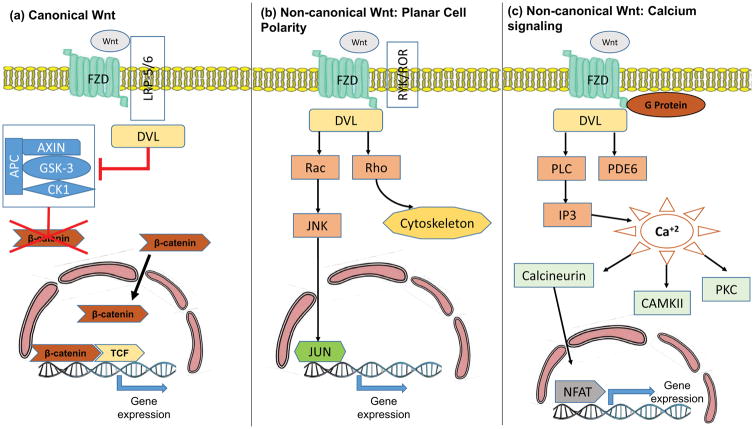
Fig. 1.
Wnt signaling pathways.
(A) The canonical pathway is activated once Wnt binds to the frizzled (FZD) receptor and LRP-5/6 co-receptor, thus recruiting disheveled (DVL) protein that inhibits the β-catenin destruction complex. Consequently, β-catenin accumulates in the cytoplasm and diffuses into the nucleus, binds to other transcription factors and activates gene expression. The non-canonical pathways (A and B) do not involve β-catenin. The planar cell polarity pathway (B) is activated once Wnt binds to FZD and the RYK/ROR co-receptor recruiting DVL and resulting in activation of the Rho and Rac signaling cascades. This causes cytoskeletal modifications and alteration in gene expression via the JNK pathway. The calcium signaling non-canonical pathway (C) involves Wnt binding to a G-protein coupled FZD that results in activation of phopholipase C (PLC) and phosphodiesterase 6, this leads to inositol triphosphate (IP3) production and stimulation of intracellular calcium release. The calcium cascade involves activation of CAMKII, PKC and alteration in gene transcription via the calcineurin-NFAT mechanism.
In contrast, non-canonical Wnt signaling involves at least two distinct pathways; the planar cell polarity pathway and the calcium-signaling pathway, both of which are independent from β-catenin (20). The cell polarity pathway regulates the actin cytoskeleton, determines cell polarity and migration. Binding of Wnt4, Wnt5a or Wnt11 to Fz and non-canonical Fz co-receptors such as RYK, and ROR2, activates the Rho and Rac GTPases, leading to cytoskeletal modifications (20). Activation of the calcium pathway causes the release of Ca+2 from the endoplasmic reticulum via G-protein signaling (23). Moreover, activation of the non-canonical Wnt pathway wields an antagonistic effect on the canonical pathway through the Nemo-like kinase (NLK)-mediated phosphorylation and ubiquitination of TCF7L2 (24) (Fig. 1B).
IV. What diseases are associated with Wnt signaling defects?
Mutations in the Wnt pathway have been associated with multiple developmental disorders such as Fuhrman syndrome (25), Tetra-amelia syndrome (26), caudal duplication syndrome (27), sclerosteosis (28), and familial exudative vitreo-retinopathy (29) among others. Moreover, mutations in pathway components such as APC and β-catenin are causally linked to the development of many forms of cancer (30, 31), including familial adenomatous polyposis (32) and Wilms’ tumor (33). Additionally, aberrant Wnt signaling has been detected in fibrotic diseases such as idiopathic pulmonary fibrosis (34), renal fibrosis (35), and systemic sclerosis (36). Contributions to vascular disease such as pulmonary arterial hypertension and atherosclerosis have also been noted.
Interestingly, genetic investigations of families with extreme forms of MetS, severe hypertension and type 2 diabetes have led to the discovery of several mutations in the Wnt signaling cascade. These included p.R611C, p.R473Q, p.R360H, and p.N433S variants in the LRP6 gene that are associated with autosomal dominant MetS with early onset coronary artery disease and atherosclerosis (37, 38). Common variants in the LRP6 gene have also been associated with arterial calcification in a genome wide association study (GWAS) of African Americans (39). A common (p.1062V) variant of LRP6 has been strongly associated with carotid artery atherosclerosis (CAA) in hypertensive patients (40). In addition, common genetic variants in the TCF7L2 gene have been associated with the risk for type 2 diabetes, hyperlipidemia and coronary artery disease (41), indicating the broader role of this downstream Wnt effector in common diseases. Single nucleotide polymorphisms (SNPs) of TCF7L2 such as rs7903146 and rs17685538 have been associated with elevated blood pressure (42).
V. Is there a relationship between Wnt signaling and hypertension?
Multiple lines of evidence suggest the existence of such a relationship. These include data from genome wide association studies, genetic kindred studies, in vivo mammalian experiments, in vitro experiments, in addition to collateral links to cardiac, renal and neural physiology.
1. Evidence from genome wide association studies
GWAS scans the genome for common single nucleotide polymorphisms (SNPs) in association with a disease and takes advantage of linkage disequilibrium between the SNPs and nearby polymorphisms. This means that identified SNPs can be the disease-causing mutations or more likely be linked to other disease causing polymorphisms in relevant genes, a phenomenon known as disequilibrium. Several GWAS studies have been performed in relation to blood pressure and hypertension.
Among the over 50 identified genes, there are two that fall in the Wnt signaling pathways. In a study of 76,064 Europeans, the WNT3 gene that encodes a canonical Wnt ligand was directly associated with pulse pressure and mean arterial pressure (43). There is ample experimental evidence implicating WNT3 in vascular disease such as arterial calcification, transforming growth factor (TGF) and vascular endothelial growth factor (VEGF) regulation (44).
The SOX-6 gene was associated with hypertension and blood pressure in a study of 1,017 African Americans (45). The SOX family of transcription factors has emerged as modulators of canonical Wnt/β-catenin signaling both in development and disease states. Recent evidence suggests that SOX proteins physically interact with β-catenin and modulate the transcription of Wnt-target genes. SOX-6 can directly bind to β-catenin in a region of the armadillo repeats, which overlaps with the site where TCF, another modulator of β-catenin, binds (46–49). Wnt signaling also regulates SOX expression in feedback regulatory loops that further calibrate cellular β-catenin/TCF activity. Interestingly, the Renin promoter is downregulated by SOX3, another member of the SOX family. This suggests that a direct effect of SOX on the renin angiotensin system could also underlie its contribution to BP regulation (50).
2. Evidence from outlier kindreds
Our group has identified an autosomal dominant form of metabolic syndrome and premature coronary artery disease caused by the Arg611Cys substitution in LRP6. Mutation carriers compared to non-mutation carriers had an average systolic BP of 168 ± 21 mmHg versus 116 ± 5 mmHg (p-value =8×10−5), and an average diastolic BP of 100 ±14 mmHg versus 81 ± 7 mmHg (p-value =0.0025), respectively. While obesity is a major component of metabolic syndrome and is strongly associated with hypertension, hypertension in most patients with LRP6 Arg611Cys variant preceded the obesity. In fact, the mean BMI of the patients at the onset of disease was 24.6, and none had a BMI greater than 26 (38). Functional characterization of these mutations has shown that they impair canonical (37) and activate non-canonical Wnt (21, 51).
3. Experimental evidence
In a series of experiments, Sumida et al. (52) created a physiological model of hypertension by infusing mice with angiotensin II. This resulted in about 70 mmHg elevation in BP. It was demonstrated that blood pressure elevation in response to angiotensin II infusions activates β-catenin and induces VSMC proliferation. VSMC proliferation was observed within 1 week of angiotensin II infusion into mice, and arterial remodeling within 6 weeks post transfusion (52, 53). Similarly, β-catenin signaling was activated early in the hypertensive state, at 1-week post infusion, and was preferentially activated in aortic VSMCs rather than endothelial cells. Furthermore, β-catenin signaling and VSMC proliferation were blunted when lowering BP using hydralazine during the same time period (52).
4. Wnt and BP regulation in the central nervous system
The nucleus tractus solitarius (NTS) located in the dorsal medulla of the brainstem is the main modulatory site of blood pressure (BP) and sympathetic nerve activity. Insulin has been implicated in the regulation of the baroreceptor reflex in the NTS as it easily crosses the blood brain barrier (54, 55). It has been shown to be involved in NTS mediated BP control via the PI3K-Akt-NO synthase cascade (56–58). Interestingly, Wnt signaling in the NTS is defective in hypertension, as evidenced by decreased phosphorylation of LRP6 (activation) and GSK-3β (deactivation) in genetic (spontaneously hypertensive rats or SHR) and dietary (fructose fed mice) models of hypertension. It has been shown that intraventricular infusion of Wnt3a into the brains of SHR rats lowers BP, decreases heart rate, and increases nitric oxide release (59). This effect was reversed with the administration of Wnt antagonist Dickkopf-1 (DKK1) (59, 60). Similar results were obtained in the fructose-fed–induced hypertension model. Therefore, increased Wnt3a and β-catenin signaling in the NTS of the brain might mitigate hypertension. In another set of experiments, rats exhibit reduced BP and heart rate in response to insulin injection into the NTS (56). Wnt3a administration significantly increased downstream insulin signaling in the NTS of SHR rats and fructose-fed hypertensive rats (59). Further studies showed that the interaction between GSK3-β and IRS1 plays a central role in Wnt3a-mediated hypotensive effects in the NTS (59). Overall, current evidence suggests that the neuronal regulation of blood pressure is dependent on coordinated interaction of insulin and Wnt signaling.
5. Wnt and cardiac function
Cardiac output is a determinant of systemic blood pressure. Wnt/β-catenin has been linked to adult cardiac function and remodeling. Wnt/β-catenin signaling plays a pivotal role during cardiac development via a biphasic signal. Initially, a strong Wnt signal activates mesenchymal to cardiomyocyte transition followed by a downregulated signal that leads to cardiomyocyte differentiation (61). In adult life, reduced Wnt signaling activation is associated with decreased cardiac hypertrophy (62). Activation of canonical Wnt leads to inhibition of the glycogen synthase GSK3-β, a potent inhibitor of cardiomyocyte hypertrophy, while simultaneously increasing the β-catenin mediated transcription of hypertrophy genes (62). β-catenin has been shown to redistribute and accumulate at higher levels in the nuclei of cardiomyocytes in hypertensive rats (63). In a recent study of pressure-induced left ventricular hypertrophy and heart failure elicited by transverse aortic constriction (TAC), activation of GSK3 was associated with decreased hypertrophy, fibrosis and left ventricular dysfunction (64). Interestingly, GSK3 was activated by 2,5-Dimethylcelecoxib, a derivative of celecoxib that inhibits Akt (a negative regulator of GSK3), but is unable to inhibit the cyclooxygnenase-2 (COX-2) (64). Suppression of prostacyclin production via inhibition of COX-2 has been previously associated with adverse thromboembolic cardiovascular outcomes (65, 66). On the other hand, decreased GSK3β activity in GSK3β hetero-deficient mice led to more hypertrophy, and interstitial fibrosis. Interestingly, the levels of miR29-α, which is positively linked to Wnt regulation (67, 68), has been found to be significantly higher in the serum of hypertensive patients with left ventricular hypertrophy compared to those with hypertension and without hypertrophy (69, 70). Accordingly, inhibition of miR29-α in a mouse model led to suppression of cardiomyocyte hypertrophy and the associated hypertrophy markers (69). Blocking Wnt signaling in hypertensive rats leads to inhibition of myofibroblast migration into the myocardium and its secretion of collagen 1 with reduction of the myocardial fibrosis (71–73)
6. Wnt and renal function
Wnt regulates formation of the renal medulla. The urine concentrating ability of the renal medulla is related to its length, and blunting of the medulla is a common cause of renal diseases. The medulla, composed of loop-of-henle, collecting ducts, interstitium, and vasa recta plays an important role in water and electrolyte homeostasis and BP control. Nephron quantity is related to susceptibility to hypertension and chronic kidney disease. Specific Wnt proteins such as Wnt 7b are highly expressed in the collecting ducts. Defective Wnt 7b signaling leads to change in the cell orientation along the collecting ducts, leading to duct dilation instead of elongation (74). Wnt 9b and Wnt 4 are also involved in nephron development (75).
The Pro-renin receptor, a receptor for renin and pro-renin molecules, acts as an accessory protein in the intracellular proton pump (H+ ATPase) (76) implicated in autophagy (77) and is a recent addition to the Renin-Angiotensin-Aldosterone system implicated in BP regulation (78). The pro-renin receptor (PRR) interacts with and activates the Wnt co-receptors LRP5 and 6 (79).
Notably, Wnt signaling and autophagy have been shown to be inter-related (80). Interestingly, PRR is involved in the planar-cell-polarity pathway of Wnt signaling in Drosophila (81). Mutations in the pro-renin receptor in human subjects are associated with hypertension (82, 83). Moreover, ablation of the pro-renin receptor in mice resulted in significantly decreased number of nephrons during development that was attributed to impaired response to the Wnt/β-catenin signaling (84). The Wnt/β-catenin canonical signaling seems to act downstream of PRR in initiating the mesenchyme to epithelial transition and is thought to regulate nephron progenitor cell self-renewal and differentiation in part via Six2 (85, 86).
Another line of investigation implicated Wnt signaling in regulation of aldosterone, volume status and blood pressure in mice. This effect seems to be mediated via the APC protein, a major component of the β-catenin destruction complex, and its downstream effector SGK-1 that increases adrenal release of aldosterone, and absolute renal Na+ absorption (87, 88). Moreover, there is strong evidence that Wnt signaling is activated in response to kidney injury and along with the Notch and Hedgehog pathways drive renal fibrosis (35).
VI. The potential role of Wnt signaling in hypertensive vasculopathy
Vascular smooth muscle cell (VSMC) loss of plasticity or de-differentiation has been associated with hypertension (89) and plays a critical role in arterial remodeling observed in hypertension (90). Wnt/β-catenin signaling is implicated in proliferation and differentiation of smooth muscle cells during embryonic and postnatal angiogenesis (91, 92). Moreover, Wnt signaling is involved in vascular smooth muscle plasticity in adults in response to injury such as acute arterial ligation or mechanical injury to the arterial lumen (93–95). Recent findings suggest three distinct mechanisms by which Wnt signaling components contribute to VSMC remodeling. These can be broken down into macrovascular effects, microvascular effects and immune mediated mechanisms.
1. Macrovascular
In a series of experiments, our group determined that altered LRP6 function contributes to pathological VSMC proliferation that is observed in coronary artery disease (96). We found that LRP6 is overexpressed in sub-intima and muscularis layers of atherosclerotic lesions. Overexpression of the LRP6R611C variant, which is associated with autosomal dominant MetS and hypertension, in aortic VSMCs resulted in reduced VSMC proliferation and cyclin D1 expression in response to Wnt3a stimulation compared to wild type LRP6 vector and uninfected cells (96). This is consistent with prior experiments showing reduced but not complete inhibition of canonical Wnt signaling among the LRP6 R611C mutants (38). This finding suggested that VSMC proliferation in the LRP6R611C mutants is independent of canonical Wnt. Further investigations revealed that platelet-derived growth factor β (PDGF- β) induced VSMC proliferation in cells expressing the R611C mutation compared to wild type or un-transfected controls (96). PDGF-β is a known mitogen that activates ERK1/2 (97) and STAT1 (98) pathways. Wildtype LRP6 forms complexes with the PDGF-β receptor and targets it to degradation, unlike the R611C mutant (96). Moreover, LRP6 inhibits ERK1/2 and STAT1 phosphorylation, whereas the R611C mutant increased ERK and STAT1 phosphorylation in response to PDGF-β (96).
Knock-in mice carrying the LRP6 R611C mutation in a homozygous state exhibited thickened aorta on chow diet and neointima formation and occlusive coronary arteries on high fat diet. There was increased expression of PDFG receptors, PDGF ligands, and insulin growth factor 1 (IGF1) in the aortic media (51). In addition, these mice exhibited increased expression of vascular progenitor cell markers vimentin and c-kit, and decreased expression of contractile proteins in isolated VSMCs (51). In addition, the latter expression profile was partially reversed with Wnt3a administration over a 3-week period, which correlated with reductions in aortic medial thickness. The altered expression profile in the knock-in mice is attributed to activation of the SP1 transcription factor (51), an established regulator of VSMC plasticity that is normally inhibited by canonical Wnt signaling (99–101). Wnt3a administration had little effect on β-catenin levels in the R611C mutant. However, there was a significant increase in TCF7L2 expression in VSMC following Wnt3a administration to LRP6R611C mice. This correlated with decreased SP1 expression and reversal of PDGF activity. We later showed that TCF7L2 inhibits SP1 by direct binding to the T-C-A-A-A-G motif downstream from its transcription initiation site (51).
Therefore, impaired function of the LRP6/TCF7L2 axis could induce VSMCs plasticity and initiate aberrant vascular wall remodeling. The importance of these findings is derived from the characterization of a causal mutation associated with diabetes, hypertension and premature coronary artery disease in human subjects. Interestingly, mice with VSMC-specific LRP6 knockout develop arterial calcification (102), which is also seen in human mutation carriers.
2. Microvascular
Wnt can directly stimulate VSMC proliferation via canonical pathway activation. This was demonstrated in vitro by Wnt stimulation of cultured human aortic smooth muscle cells and by addition of lithium chloride, which stabilizes β-catenin via inhibiting GSK3 (52). Interestingly, the VSMC proliferation was also independent of mitogen-activated protein kinases such as ERK (52). Inhibition of β-catenin by PKF115-584 or by genetic knock out in the same mouse model didn’t lower BP but inhibited remodeling (52). Therefore, it can be concluded that the β-catenin meditated VSMC remodeling is a consequence and not a mediator of hypertension in this model. These observations reflect early events in angiotensin II induced hypertension and hypertensive arterial remodeling.
3. Immune mediated
Another mechanism is a Wnt-independent, C1q-mediated arterial remodeling via activation of β-catenin signaling. The C1q complement protein exerts its effects without complement cascade activation. At the cellular level, the M2 macrophages, which exhibit an anti-inflammatory phenotype, move into the aortic adventitia in response to high BP and secrete C1q that activates β-catenin by enzymatic cleavage of LRP6 by C1s. The truncated LRP6 is constitutively activated and phosphorylates uncleaved LRP6 receptors and activates β-catenin (103). Recruitment of macrophages to the aortic adventitia has been shown to be critical for β-catenin signaling, as depletion of the monocyte lineage from these mice abolished the signal and VSMC proliferation (52). Study of macrophage humoral factors implicated interlukin 4 (IL-4) in promoting complement C1q production from M2 type macrophages, independent of the complement cascade (52). C1q then stimulates the β-catenin pathway in a dose-dependent manner, triggering VSMC proliferation and arterial remodeling in the early phase of hypertension. These effects were relatively suppressed but not completely inhibited with C1 inhibitors (52), which suggests the presence of other pathways regulating VSMC proliferation in hypertension.
VII. Pharmacology
The Wnt pathway is complex, delicately balanced, and might not lend itself to safe pharmacologic tinkering. There are over 27 compounds at different stages of clinical trials that are known to modulate Wnt signaling (104). Some of them are FDA approved such as; the anti-helmithic agent Pyrvinium targeting CK1α, Lithium targeting GSK3, Celecoxib targeting COX2, and Sulindac and aspirin targeting COX1 and 2. Others in phase I or II trials include; OMP-18R5 (mAb) an antibody targeting Frizzled, and OMP-54F28 (FZD8-Fc fusion) a decoy receptor targeting Wnt ligands and Romosozumab, a humanized anti-sclerostin monoclonal antibody used for osteoporosis. Inhibitors of β-catenin, TCF, Dishevelled, Transkyrase and other Wnt components are also under investigation (104). However, many of these agents are being developed as chemotherapeutic agents and are likely teratogenic as they target the crucial components in the Wnt pathway. Vitamins A retinoid derivatives and vitamin D interact with nuclear receptors that inhibit Wnt signaling by competing for nuclear β-catenin (Shah JBC 2003, Palmer JBC 2001) or activating the transcription of inhibitory genes. There are no current studies on Wnt pharmacotherapies for hypertension management. It remains to be seen whether current or newer agents that target downstream effectors of Wnt can safely be used to specifically treat hypertension or mitigate the deleterious cardiovascular remodeling.
VIII. Future directions and conclusions
Although blood pressure (BP) follows a normal distribution in the population, the cutoff value for hypertension is defined arbitrarily at a pressure above 140/85 mmHg, on the higher end of BP distribution at which point the benefits of medical intervention may exceed those of inaction (105). This one-size-fits-all stratification scheme has proven to be problematic. Although its simplicity lends it for easy use by clinicians, it falls short of capturing major variations in individuals or subgroups within the population. While the cardiovascular risk is present and incrementally increases at a systolic blood pressure as low as 115 mmHg (106), high quality trials have failed to show reduction of risk across the entire population by merely targeting a strict blood pressure numeral (13, 14, 107). Most recently, the Heart Outcomes Prevention Trial (HOPE-3) showed lack of overall benefit from blood pressure lowering in subjects with intermediate risk for cardiovascular events. The study population was demographically heterogeneous, and the metabolic profiles also varied significantly among the study participants (108). This invites a personalized medicine approach based on genetic and metabolic profiling for effective study and treatment of hypertension. Additionally, blood pressure is a complex emergent phenotype that is not controlled by any single cell line, tissue, or organ. Instead it is regulated by multiple organ systems. Nonetheless, complex phenotypes have oftentimes been successfully reduced to defects in single molecular pathways. The strong evidence from human studies associating Wnt defects with severe forms of MetS and hypertension; in addition to the cumulating data on Wnt’s entanglement in cardiovascular physiology offer bright prospects for therapeutics in an untapped pathway.
Extensive review of the current literature suggests a bidirectional relationship between Wnt and blood pressure regulation though more investigation is necessary to tease-out this “chicken versus egg” association. Some evidence of causation comes from animal models where changes of Wnt signaling in the brainstem caused alterations in systemic blood pressure. However, these findings still need to be meaningfully extrapolated to humans. Indirect evidence of causation comes from GWAS and genetic kindred studies of autosomal dominant MetS; where mutations in Wnt components segregated with severe hypertension in the members of the same family. Evidence of lesser weight emerges from indirect correlations of Wnt defects linked to organ dysfunction such as renal fibrosis and kidney disease, which in turn contribute to hypertension. On the other hand, animal models of induced hypertension showed that hypertension causes dynamic changes in Wnt signaling, which in turn drive pathologic vascular remodeling. This is also corroborated by human and animal studies on cardiac hypertrophic remodeling which seems to be worse in hypertensive subjects with aberrant Wnt signaling.
Therefore, focusing research efforts on decoding the Wnt pathway will lead to better understanding of hypertension and factors that underlie association of diverse MetS traits. The concept of a unifying molecular pathophysiology for hypertension and other traits observed in MetS is promising and may lead to more effective and novel classes of treatment for these prevalent burdensome disorders.
Nascent therapeutics in this domain will have to account for the complexity of Wnt signaling, and the multi-dimensional cross talk between Wnt and other pathways and between the different arms of the Wnt pathways.
Highlights.
Mutations in Wnt signaling components have been implicated in metabolic traits such as hypertension and hyperlipidemia.
Wnt signaling influences peripheral and central regulation of blood pressure.
Derangements in Wnt signaling cause vascular smooth muscle remodeling that may underlie arterial calcification, high blood pressure and vascular diseases.
Further dissection of Wnt signaling pathway in blood vessels may help with development of novel therapeutics against hypertension.
Acknowledgments
Financial support:
This manuscript was supported by grants from the National Institutes of Health (NIH) (1R01HL122830 and 1R01HL122822 to Arya Mani).
Footnotes
Conflict of interest:
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, et al. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64(2):231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 4.Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Report. 2011;(35):1–22. 4. [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 6.Abou Ziki MD, Mani A. Metabolic syndrome: genetic insights into disease pathogenesis. Curr Opin Lipidol. 2016 doi: 10.1097/MOL.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hottenga JJ, Boomsma DI, Kupper N, Posthuma D, Snieder H, Willemsen G, et al. Heritability and stability of resting blood pressure. Twin Res Hum Genet. 2005;8(5):499–508. doi: 10.1375/183242705774310123. [DOI] [PubMed] [Google Scholar]
- 8.Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJ. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 2005;45(1):80–5. doi: 10.1161/01.HYP.0000149952.84391.54. [DOI] [PubMed] [Google Scholar]
- 9.Garovic VD, Hilliard AA, Turner ST. Monogenic forms of low-renin hypertension. Nat Clin Pract Nephrol. 2006;2(11):624–30. doi: 10.1038/ncpneph0309. [DOI] [PubMed] [Google Scholar]
- 10.Hassan-Smith Z, Stewart PM. Inherited forms of mineralocorticoid hypertension. Curr Opin Endocrinol Diabetes Obes. 2011;18(3):177–85. doi: 10.1097/MED.0b013e3283469444. [DOI] [PubMed] [Google Scholar]
- 11.Simonetti GD, Mohaupt MG, Bianchetti MG. Monogenic forms of hypertension. Eur J Pediatr. 2012;171(10):1433–9. doi: 10.1007/s00431-011-1440-7. [DOI] [PubMed] [Google Scholar]
- 12.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 13.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group SR, Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusse R. Wnt signaling. Cold Spring Harb Perspect Biol. 2012;4(5) doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 17.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119(1):97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Kani S, Oishi I, Yamamoto H, Yoda A, Suzuki H, Nomachi A, et al. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase Iepsilon. J Biol Chem. 2004;279(48):50102–9. doi: 10.1074/jbc.M409039200. [DOI] [PubMed] [Google Scholar]
- 19.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Song K, Srivastava R, Dong C, Go GW, Li N, et al. Nonalcoholic fatty liver disease induced by noncanonical Wnt and its rescue by Wnt3a. FASEB J. 2015;29(8):3436–45. doi: 10.1096/fj.15-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, et al. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17(2):197–209. doi: 10.1016/j.cmet.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–46. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399(6738):798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 25.Woods CG, Stricker S, Seemann P, Stern R, Cox J, Sherridan E, et al. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Hum Genet. 2006;79(2):402–8. doi: 10.1086/506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, et al. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74(3):558–63. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oates NA, van Vliet J, Duffy DL, Kroes HY, Martin NG, Boomsma DI, et al. Increased DNA methylation at the AXIN1 gene in a monozygotic twin from a pair discordant for a caudal duplication anomaly. Am J Hum Genet. 2006;79(1):155–62. doi: 10.1086/505031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 29.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–95. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 30.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 31.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Kinzler KW, Nilbert MC, Vogelstein B, Bryan TM, Levy DB, Smith KJ, et al. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251(4999):1366–70. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 33.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316(5827):1043–6. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 34.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162(5):1495–502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edeling M, Ragi G, Huang S, Pavenstadt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12(7):426–39. doi: 10.1038/nrneph.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dees C, Distler JH. Canonical Wnt signalling as a key regulator of fibrogenesis - implications for targeted therapies? Exp Dermatol. 2013;22(11):710–3. doi: 10.1111/exd.12255. [DOI] [PubMed] [Google Scholar]
- 37.Singh R, Smith E, Fathzadeh M, Liu W, Go GW, Subrahmanyan L, et al. Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum Mutat. 2013;34(9):1221–5. doi: 10.1002/humu.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315(5816):1278–82. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wojczynski MK, Li M, Bielak LF, Kerr KF, Reiner AP, Wong ND, et al. Genetics of coronary artery calcification among African Americans, a meta-analysis. BMC Med Genet. 2013;14:75. doi: 10.1186/1471-2350-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarzani R, Salvi F, Bordicchia M, Guerra F, Battistoni I, Pagliariccio G, et al. Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. Nutr Metab Cardiovasc Dis. 2011;21(2):150–6. doi: 10.1016/j.numecd.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Muendlein A, Saely CH, Geller-Rhomberg S, Sonderegger G, Rein P, Winder T, et al. Single nucleotide polymorphisms of TCF7L2 are linked to diabetic coronary atherosclerosis. PLoS One. 2011;6(3):e17978. doi: 10.1371/journal.pone.0017978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Phillips CM, Williams CM, Gulseth HL, et al. Pleiotropic effects of TCF7L2 gene variants and its modulation in the metabolic syndrome: from the LIPGENE study. Atherosclerosis. 2011;214(1):110–6. doi: 10.1016/j.atherosclerosis.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43(10):1005–11. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Aly Z. Arterial calcification: a tumor necrosis factor-alpha mediated vascular Wnt-opathy. Transl Res. 2008;151(5):233–9. doi: 10.1016/j.trsl.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5(7):e1000564. doi: 10.1371/journal.pgen.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T, et al. SOX6 suppresses cyclin D1 promoter activity by interacting with beta-catenin and histone deacetylase 1, and its down-regulation induces pancreatic beta-cell proliferation. J Biol Chem. 2007;282(26):19052–61. doi: 10.1074/jbc.M700460200. [DOI] [PubMed] [Google Scholar]
- 47.Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4(4):487–98. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 48.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–87. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, et al. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27(22):7802–15. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araujo FC, Milsted A, Watanabe IK, Del Puerto HL, Santos RA, Lazar J, et al. Similarities and differences of X and Y chromosome homologous genes, SRY and SOX3, in regulating the renin-angiotensin system promoters. Physiol Genomics. 2015;47(5):177–86. doi: 10.1152/physiolgenomics.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava R, Zhang J, Go GW, Narayanan A, Nottoli TP, Mani A. Impaired LRP6-TCF7L2 Activity Enhances Smooth Muscle Cell Plasticity and Causes Coronary Artery Disease. Cell Rep. 2015;13(4):746–59. doi: 10.1016/j.celrep.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumida T, Naito AT, Nomura S, Nakagawa A, Higo T, Hashimoto A, et al. Complement C1q-induced activation of beta-catenin signalling causes hypertensive arterial remodelling. Nat Commun. 2015;6:6241. doi: 10.1038/ncomms7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102(6):720–8. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 54.McKernan AM, Calaresu FR. Insulin microinjection into the nucleus tractus solitarii of the rat attenuates the baroreceptor reflex. J Auton Nerv Syst. 1996;61(2):128–38. doi: 10.1016/s0165-1838(96)00074-4. [DOI] [PubMed] [Google Scholar]
- 55.Ruggeri P, Molinari C, Brunori A, Cogo CE, Mary DA, Picchio V, et al. The direct effect of insulin on barosensitive neurones in the nucleus tractus solitarii of rats. Neuroreport. 2001;12(17):3719–22. doi: 10.1097/00001756-200112040-00023. [DOI] [PubMed] [Google Scholar]
- 56.Huang HN, Lu PJ, Lo WC, Lin CH, Hsiao M, Tseng CJ. In situ Akt phosphorylation in the nucleus tractus solitarii is involved in central control of blood pressure and heart rate. Circulation. 2004;110(16):2476–83. doi: 10.1161/01.CIR.0000145116.75657.2D. [DOI] [PubMed] [Google Scholar]
- 57.Chiang HT, Cheng WH, Lu PJ, Huang HN, Lo WC, Tseng YC, et al. Neuronal nitric oxide synthase activation is involved in insulin-mediated cardiovascular effects in the nucleus tractus solitarii of rats. Neuroscience. 2009;159(2):727–34. doi: 10.1016/j.neuroscience.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 58.Tseng CJ, Liu HY, Lin HC, Ger LP, Tung CS, Yen MH. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27(1):36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- 59.Cheng PW, Chen YY, Cheng WH, Lu PJ, Chen HH, Chen BR, et al. Wnt Signaling Regulates Blood Pressure by Downregulating a GSK-3beta-Mediated Pathway to Enhance Insulin Signaling in the Central Nervous System. Diabetes. 2015;64(10):3413–24. doi: 10.2337/db14-1439. [DOI] [PubMed] [Google Scholar]
- 60.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3(7):683–6. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 61.Zelarayan L, Gehrke C, Bergmann MW. Role of beta-catenin in adult cardiac remodeling. Cell Cycle. 2007;6(17):2120–6. doi: 10.4161/cc.6.17.4632. [DOI] [PubMed] [Google Scholar]
- 62.van de Schans VA, van den Borne SW, Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC, et al. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49(3):473–80. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Q, Chen P, Xu Z, Li F, Yi XP. Expression and redistribution of beta-catenin in the cardiac myocytes of left ventricle of spontaneously hypertensive rat. J Mol Histol. 2013;44(5):565–73. doi: 10.1007/s10735-013-9507-6. [DOI] [PubMed] [Google Scholar]
- 64.Fujita A, Takahashi-Yanaga F, Morimoto S, Yoshihara T, Arioka M, Igawa K, et al. 2,5-Dimethylcelecoxib prevents pressure-induced left ventricular remodeling through GSK-3 activation. Hypertens Res. 2016 doi: 10.1038/hr.2016.122. [DOI] [PubMed] [Google Scholar]
- 65.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 66.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem. 2010;285(33):25221–31. doi: 10.1074/jbc.M110.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan M, Wu J, Cai Y. Suppression of Wnt signaling by the miR-29 family is mediated by demethylation of WIF-1 in non-small-cell lung cancer. Biochem Biophys Res Commun. 2013;438(4):673–9. doi: 10.1016/j.bbrc.2013.07.123. [DOI] [PubMed] [Google Scholar]
- 69.Han W, Han Y, Liu X, Shang X. Effect of miR-29a inhibition on ventricular hypertrophy induced by pressure overload. Cell Biochem Biophys. 2015;71(2):821–6. doi: 10.1007/s12013-014-0269-x. [DOI] [PubMed] [Google Scholar]
- 70.Roncarati R, Viviani Anselmi C, Losi MA, Papa L, Cavarretta E, Da Costa Martins P, et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2014;63(9):920–7. doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 71.Shao S, Cai W, Sheng J, Yin L. Role of SDF-1 and Wnt signaling pathway in the myocardial fibrosis of hypertensive rats. Am J Transl Res. 2015;7(8):1345–56. [PMC free article] [PubMed] [Google Scholar]
- 72.Marinou K, Christodoulides C, Antoniades C, Koutsilieris M. Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab. 2012;23(12):628–36. doi: 10.1016/j.tem.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 73.ter Horst P, Smits JF, Blankesteijn WM. The Wnt/Frizzled pathway as a therapeutic target for cardiac hypertrophy: where do we stand? Acta Physiol (Oxf) 2012;204(1):110–7. doi: 10.1111/j.1748-1716.2011.02309.x. [DOI] [PubMed] [Google Scholar]
- 74.Yu J. Wnt signaling and renal medulla formation. Pediatr Nephrol. 2011;26(9):1553–7. doi: 10.1007/s00467-011-1888-8. [DOI] [PubMed] [Google Scholar]
- 75.Halt K, Vainio S. Coordination of kidney organogenesis by Wnt signaling. Pediatr Nephrol. 2014;29(4):737–44. doi: 10.1007/s00467-013-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, et al. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem. 1998;273(18):10939–47. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 77.Binger KJ, Muller DN. Autophagy and the (Pro)renin Receptor. Front Endocrinol (Lausanne) 2013;4:155. doi: 10.3389/fendo.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327(5964):459–63. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 80.Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T, et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol. 2010;12(8):781–90. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- 81.Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20(14):1269–76. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 82.Hirose T, Hashimoto M, Totsune K, Metoki H, Asayama K, Kikuya M, et al. Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: the Ohasama study. Am J Hypertens. 2009;22(3):294–9. doi: 10.1038/ajh.2008.357. [DOI] [PubMed] [Google Scholar]
- 83.Ott C, Schneider MP, Delles C, Schlaich MP, Hilgers KF, Schmieder RE. Association of (pro)renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenet Genomics. 2011;21(6):347–9. doi: 10.1097/FPC.0b013e328344cdd2. [DOI] [PubMed] [Google Scholar]
- 84.Song R, Preston G, Kidd L, Bushnell D, Sims-Lucas S, Bates CM, et al. Prorenin receptor is critical for nephron progenitors. Dev Biol. 2015 doi: 10.1016/j.ydbio.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138(7):1247–57. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, et al. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23(3):637–51. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhandaru M, Kempe DS, Rotte A, Rexhepaj R, Kuhl D, Lang F. Hyperaldosteronism, hypervolemia, and increased blood pressure in mice expressing defective APC. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R571–5. doi: 10.1152/ajpregu.00070.2009. [DOI] [PubMed] [Google Scholar]
- 88.Just A. Going with the Wnt? Focus on “Hyperaldosteronism, hypervolemia, and increased blood pressure in mice expressing defective APC”. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R568–70. doi: 10.1152/ajpregu.00356.2009. [DOI] [PubMed] [Google Scholar]
- 89.Pauletto P, Sarzani R, Rappelli A, Chiavegato A, Pessina AC, Sartore S. Differentiation and growth of vascular smooth muscle cells in experimental hypertension. Am J Hypertens. 1994;7(7 Pt 1):661–74. doi: 10.1093/ajh/7.7.661. [DOI] [PubMed] [Google Scholar]
- 90.Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: novel targets in essential hypertension. J Hum Hypertens. 2014;28(8):510–6. doi: 10.1038/jhh.2013.117. [DOI] [PubMed] [Google Scholar]
- 91.Itaranta P, Chi L, Seppanen T, Niku M, Tuukkanen J, Peltoketo H, et al. Wnt-4 signaling is involved in the control of smooth muscle cell fate via Bmp-4 in the medullary stroma of the developing kidney. Dev Biol. 2006;293(2):473–83. doi: 10.1016/j.ydbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 92.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119(9):2538–49. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ Res. 2002;90(3):340–7. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- 94.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, et al. Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011;108(4):427–36. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 95.Mill C, George SJ. Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc Res. 2012;95(2):233–40. doi: 10.1093/cvr/cvs141. [DOI] [PubMed] [Google Scholar]
- 96.Keramati AR, Singh R, Lin A, Faramarzi S, Ye ZJ, Mane S, et al. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2011;108(5):1914–8. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, et al. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23(5):795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 98.Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282(6):L1296–304. doi: 10.1152/ajplung.00315.2001. [DOI] [PubMed] [Google Scholar]
- 99.Lin X, Wang Z, Gu L, Deuel TF. Functional analysis of the human platelet-derived growth factor A-chain promoter region. J Biol Chem. 1992;267(35):25614–9. [PubMed] [Google Scholar]
- 100.Park GH, Plummer HK, 3rd, Krystal GW. Selective Sp1 binding is critical for maximal activity of the human c-kit promoter. Blood. 1998;92(11):4138–49. [PubMed] [Google Scholar]
- 101.Zhang X, Diab IH, Zehner ZE. ZBP-89 represses vimentin gene transcription by interacting with the transcriptional activator, Sp1. Nucleic Acids Res. 2003;31(11):2900–14. doi: 10.1093/nar/gkg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR−/− mice by restraining noncanonical Wnt signals. Circ Res. 2015;117(2):142–56. doi: 10.1161/CIRCRESAHA.117.306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149(6):1298–313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–32. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Evans JG, Rose G. Hypertension. Br Med Bull. 1971;27(1):37–42. doi: 10.1093/oxfordjournals.bmb.a070812. [DOI] [PubMed] [Google Scholar]
- 106.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 107.Yusuf S, Lonn E, Pais P, Bosch J, Lopez-Jaramillo P, Zhu J, et al. Blood-Pressure and Cholesterol Lowering in Persons without Cardiovascular Disease. N Engl J Med. 2016;374(21):2032–43. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 108.Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, et al. Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374(21):2009–20. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]