Abstract
Objectives
Previous studies suggest that colonization with non-toxigenic Clostridium difficile may protect against toxigenic C. difficile infection (CDI), yet most of the studies were conducted in men. Therefore, we conducted a study to examine this hypothesis in both genders.
Methods
Patients (n=1492) were classified by disease status at baseline and observed for 1 year. Cox proportional hazards regression was used to evaluate CDI rates within 8 weeks post-baseline (short-term) and from 8 weeks to 1 year (long-term follow-up).
Results
During short-term follow-up, CDI rates were 5 times greater in females with non-toxigenic Clostridium difficile compared to females without C. difficile (hazard ratio (HR) = 5.13; 95% CI: 1.47–17.83). The comparable HR in males was 0.44 (95% CI: 0.04–4.43). During long term follow-up, CDI rates were similar in those with non-toxigenic C. difficile and those without C. difficile at baseline, for both females and males. Mortality rates were significantly lower for patients colonized by non-toxigenic C. difficile than those with toxigenic C. difficile at baseline, for both genders combined (HR=0.51; 95% CI: 0.28–0.92) and were similar to those with no C. difficile at baseline (HR=0.78; 95% CI: 0.43–1.41).
Conclusions
There were gender differences in the short-term risk of CDI. Mortality was similar for patients colonized with non-toxigenic C. difficile and patients without C. difficile.
Keywords: Clostridium difficile, Infection, Gender, Non-toxigenic strains, Mortality
INTRODUCTION
Clostridium difficile infection (CDI) results from production of at least one of two toxins, TcdA and TcdB. A C. difficile isolate that does not produce at least one of these toxins is termed “non-toxigenic” and, in general, is not associated with disease [1]. Non-toxigenic C. difficile (NTCD) isolates have garnered attention for their capacity to colonize humans and potentially reduce the risk of CDI by competitively excluding toxigenic isolates in the gastrointestinal tract [2]. While several studies have investigated the protective effect of NTCD in hamsters [3–7], comparable evidence in human subjects is limited. A case report from 1987 found that administration of NTCD as a live culture resolved episodes of recurrent CDI in two patients [8]. In addition, a meta-analysis of four studies in which hospitalized patients were monitored with weekly rectal swabs demonstrated that asymptomatic colonization by C. difficile was associated with a lower rate of subsequent CDI (3.6% vs. 1.0%) [9]. Notably, 46% of the asymptomatically colonized patients carried NTCD, suggesting some of the benefit of colonization may have been related to NTCD. A more recent study also reported a protective effect of NTCD colonization against CDI among patients admitted to a Taiwanese hospital [10]. A phase I trial investigating NTCD as a therapeutic remedy for recurrent CDI revealed that NTCD was generally safe [11], and preliminary evidence from a phase II trial indicated a CDI recurrence rate of 11% in patients randomized to NTCD compared to 30% in the placebo group [12].
Most of the human studies regarding NTCD were conducted in male veteran populations [9] and information regarding possible outcomes in females is lacking. Moreover, there is recent evidence of horizontal gene transfer such that NTCD strains can be converted to toxin a producer [13], which has significant clinical implications regarding the feasibility of using NTCD as a preventive agent. With these findings in mind, we conducted a study to test the hypothesis that patients colonized with NTCD were protected against subsequent CDI compared to those patients without any C. difficile colonization.
MATERIALS AND METHODS
We conducted a longitudinal study within the University of Michigan Health System (UMHS), a large tertiary healthcare referral center. The study was approved by the University of Michigan Institutional Review Board. Study entry was from October 2010 to January 2013. Inpatients and outpatients who had C. difficile stool assays ordered by their physician for clinically indicated reasons (such as diarrhea) were potential subjects in the study. During the study entry period, all samples that tested positive in the clinical microbiology laboratory, and a random subset of samples that tested negative, were sent to our research laboratory. Positive samples were identified in the clinical laboratory by positive enzyme immunoassays for C. difficile glutamate dehydrogenase (GDH) and toxin or a positive GDH and PCR test for the presence of TcdB toxin gene. All stool samples were cultured anaerobically on taurocholate-cycloserine-cefoxitin-fructose agar (TCCFA) at 37°C. Toxin status of C. difficile isolates were confirmed by PCR as previously reported [14,15]. The patients were classified into three groups: those whose stool culture did not yield C. difficile, those whose stool sample yielded NTCD, and those whose stool culture yielded a toxigenic C. difficile strain. In instances in which a mixed culture (toxigenic C. difficile and NTCD) was obtained, the sample was excluded (n=1 sample). In instances in which duplicate stool samples existed from the same patient, only the first stool sample was included as the index sample. Our clinical microbiology laboratory also processes samples ordered by community physicians who do not use our electronic medical record; these samples were excluded from our study.
Patients were observed for 365 days from the initial date of stool collection by evaluating their medical records (both outpatient and inpatient visits). The primary outcome was toxigenic CDI determined by a positive C. difficile assay performed in the clinical microbiology laboratory as part of routine clinical care by treating healthcare providers (testing generally was performed for clinical suspicion for CDI). Since the transitory nature of NTCD colonization is not fully known, we considered outcomes during a shorter follow-up period (within 8 weeks post-baseline) as well as during a longer follow-up period (>56 days to 1 year post-baseline). The secondary outcome was all-cause mortality.
Patient characteristics at baseline were extracted from the electronic medical record within the UMHS through structured query or by two independent reviewers. These included age, gender, race, inpatient status at baseline, nursing home stay immediately prior to initial stool collection, Charlson-Deyo comorbidity score, number of hospital admissions during the previous 28 days, number of nights in the hospital during the previous 180 days, and number of prior positive C. difficile tests within the past year prior to baseline. Charlson-Deyo comorbidity scores were calculated as previously described [16,17]. These patient characteristics were selected as many of them have been shown to be predictive of CDI, severe CDI, or mortality in prior studies [18–23].
Patient characteristics were tabulated for the three clinical groups (no C. difficile, NTCD, and toxigenic C. difficile). One-way ANOVA was utilized to compare means across groups and Bartlett’s test was used to assess equality of variance. Chi-square testing was conducted to assess differences among categorical variables. Cox proportional hazards regression was used to model time-to-toxigenic C. difficile infection, with adjustment for patient characteristics (as listed above). Hazard ratios (HR) and 95% confidence intervals (CI) were calculated to compare hazards in patients who, at baseline, had NTCD, toxigenic C. difficile or no C. difficile. Censoring occurred at death or at 56 days (for the short-term analyses) or at 365 days post-baseline (for the longer-term analyses). Hazard models were stratified by inpatient status at baseline (yes/no) to allow for differing baseline hazard functions for inpatients versus outpatients. Post-diagnostic measures were used to assess underlying assumptions of proportionality (log-log plot, Kaplan-Meier and predicted survival plot), overall fit (Cox-Snell residuals), and outliers (deviance residuals). In secondary analyses, the risk of death during the 365 day follow-up period was examined by using Cox proportional hazards regression when adjusted for patient characteristics. Two-tailed alpha was set at 0.05. Analyses were conducted in Stata/MP 13.1.
RESULTS
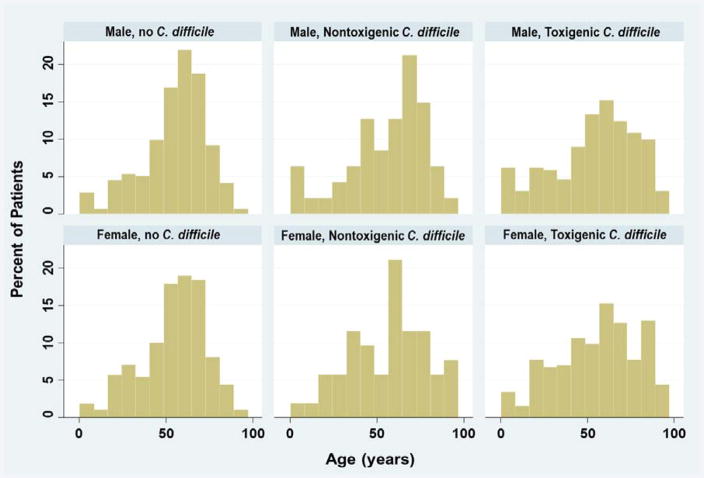
A total of 1492 patients met study eligibility. At baseline, 99 (7%) patients were colonized by NTCD, 710 (48%) patients had toxigenic C. difficile, and 683 (46%) patients were not colonized by C. difficile. Patients were similar in age, gender, race and number of comorbidities (Table 1). Pediatric patients constituted 5.9% of the participants (age range, 3 months to 17 years) and adult patients constituted 94.1% (age range, 18–97 years) (Figure 1). While the mean ages were similar across the three patient groups, the variance was greater in patients with toxigenic C. difficile at baseline (p<0.001). Use of antibiotics and proton pump inhibitors were common (Table 1). Patients with toxigenic C. difficile at baseline were more likely to have had a nursing home stay and less likely to have been hospitalized at the time of study entry. Patients with no C. difficile at baseline were less likely to have had prior history of positive C. difficile tests.
Table 1.
Baseline Patient Characteristics.
| Characteristics | No C. difficile (n=672) | NonToxigenic C. difficile (n=97) | Toxigenic C. difficile (n=651) | P-value |
|---|---|---|---|---|
| Age, years (mean, SD) | 54.7 (18.6) | 55.7 (22.3) | 54.9 (23.2) | 0.903 |
| Female (n, %) | 364 (54.2%) | 51 (52.6%) | 357 (54.8%) | 0.907 |
| Non-White (n, %) | 115 (17.1%) | 16 (16.5%) | 130 (20.0%) | 0.360 |
| Inpatient at baseline (n, %) | 555 (82.6%) | 72 (74.2%) | 400 (61.4%) | <0.001 |
| Nursing home (n, %) | 7 (1.0%) | 7 (7.2%) | 78 (12.0%) | <0.001 |
| Charlson Deyo Score (mean, SD) | 1.37 (1.60) | 1.32 (1.49) | 1.28 (1.53) | 0.581 |
| Antibiotics during past 2 weeks (n, %) | 425 (62.2%) | 54 (54.5%) | 359 (50.6%) | <0.001 |
| Proton pump inhibitors during past 2 weeks (n, %) | 323 (47.3%) | 47 (47.5%) | 228 (32.1%) | <0.001 |
| H2 receptor antagonists during past 2 weeks (n, %) | 132 (19.3%) | 19 (19.2%) | 115 (16.2%) | 0.292 |
| Number admissions during previous 28 days: | ||||
| 0 | 577 (85.9%) | 80 (82.5%) | 552 (84.8%) | |
| 1 | 86 (12.8%) | 14 (14.4%) | 91 (14.0%) | |
| 2+ | 9 (1.3%) | 3 (3.1%) | 8 (1.2%) | 0.614 |
| Number nights in hospital during previous 180 days (mean, SD) | 12.6 (16.2) | 13.6 (17.9) | 10.6 (16.7) | 0.042 |
| Number prior positive C. difficile tests: | ||||
| 0 | 636 (94.6%) | 80 (82.5%) | 531 (81.6%) | |
| 1 | 29 (4.3%) | 13 (13.4%) | 97 (14.9%) | |
| 2+ | 7 (1.0%) | 4 (4.1%) | 23 (3.5%) | <0.001 |
Figure 1.
Age Distributions of Patients by Status at Baseline and Gender.
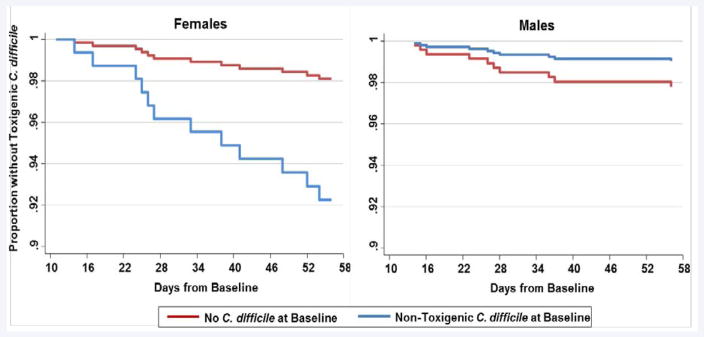
Within the first 8 weeks after baseline, the incidence of CDI was recorded for those patients who were colonized by NTCD and those without C. difficile at baseline. (Table 2) shows the hazard ratios for the incidence of CDI during this time period when stratified by gender and adjusted for patient characteristics. After adjustment, females with NTCD colonization developed CDI at 5 times the rate as females without C. difficile at baseline (HR=5.13; 95% CI 1.47, 17.83). This same pattern was not seen in males; the point estimate in males was below the null, suggesting a protective effect (HR=0.44; 95% CI 0.04, 4.43) although the results were not statistically significant. The survivor functions were plotted by gender, as shown in (Figure 2). The proportion of females without CDI decreased significantly over the 8 week period in those with NTCD at baseline. However, an opposite pattern was evident in males; the proportion of males without CDI appears highest in those with NTCD at baseline compared to those without any C. difficile at baseline.
Table 2.
Hazard Ratios for Clostridium difficile Infection within 8 Weeks after Baseline.
| Female | Male | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Baseline status: Nontoxigenic C. difficile compared to no C. difficile |
5.13 | 1.47, 17.83 | 0.010 | 0.44 | 0.04, 4.43 | 0.482 | 1.92 | 0.68, 5.46 | 0.221 |
| Covariates: | |||||||||
| Age | 1.00 | 0.97, 1.03 | 0.926 | 1.04 | 0.99, 1.08 | 0.100 | 1.02 | 0.99, 1.04 | 0.229 |
| Female | _ | _ | _ | _ | _ | _ | 1.10 | 0.47, 2.57 | 0.833 |
| Non-White | 0.89 | 0.19, 4.23 | 0.884 | 2.93 | 0.73, 11.77 | 0.130 | 1.63 | 0.59, 4.48 | 0.346 |
| Nursing home, previous | 11.42 | 1.08, 121.07 | 0.043 | 3.97 | 0.34, 46.57 | 0.273 | 3.89 | 0.78, 19.43 | 0.098 |
| Charlson Deyo score | 1.11 | 0.8, 1.54 | 0.512 | 1.06 | 0.73, 1.52 | 0.769 | 1.11 | 0.89, 1.40 | 0.357 |
| Number admissions during previous 28 days | 0.47 | 0.07, 3.06 | 0.429 | 1.43 | 0.3, 6.54 | 0.645 | 0.88 | 0.29, 2.68 | 0.816 |
| Number nights in hospital during previous 180 days | 0.99 | 0.95, 1.03 | 0.560 | 0.99 | 0.94, 1.04 | 0.633 | 0.99 | 0.95, 1.02 | 0.422 |
| Number prior positive C. difficile tests | 1.31 | 0.15, 11.58 | 0.809 | 1.48 | 0.65, 3.39 | 0.352 | 1.25 | 0.54, 2.91 | 0.602 |
Figure 2.
Cox Proportional Hazards Survival Curves for Toxigenic Clostridium difficile Infection within 8 Weeks by Gender and Baseline Status. The proportion of patients without toxigenic Clostridium difficile infection over time is shown.
Over the longer period of follow-up (8 weeks to 1 year), 104 individuals developed CDI (28 without C. difficile at baseline, 5 with NTCD at baseline and 71 with toxigenic C. difficile at baseline). The regression results for this time period are shown in (Table 3), adjusted for patient characteristics. Patients with NTCD at baseline developed CDI at a similar rate to those without C. difficile at baseline (HR=1.14; 95% CI: 0.44, 2.96) and at a similar rate to those with toxigenic C. difficile at baseline (HR=0.43; 95% CI: 0.17, 1.06). This held true for both females and males. However, patients with toxigenic C. difficile at baseline were significantly more likely to develop CDI (i.e., relapse) when compared to patients without C. difficile at baseline (HR=2.32 in females and HR=3.61 in males).
Table 3.
Hazard Ratios for Clostridium difficile Infection from 8 Weeks to 1 Year after Baseline.
| Female | Male | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value |
| Baseline status: Nontoxigenic C. difficile compared to no C. difficile |
1.24 | 0.4, 3.72 | 0.699 | 0.67 | 0.08, 5.30 | 0.703 | 1.14 | 0.44, 2.96 | 0.794 |
| Baseline status: Nontoxigenic C. difficile compared to toxigenic C. difficile |
0.53 | 0.19, 1.54 | 0.246 | 0.19 | 0.03, 1.37 | 0.098 | 0.43 | 0.17, 1.06 | 0.067 |
| Baseline status: Toxigenic C. difficile compared to no C. difficile |
2.32 | 1.30, 4.16 | 0.005 | 3.61 | 1.66, 7.86 | 0.001 | 2.67 | 1.68, 4.25 | <0.001 |
| Covariates: | |||||||||
| Age | 0.99 | 0.98, 1.00 | 0.019 | 1.00 | 0.98, 1.01 | 0.524 | 0.99 | 0.98, 1.00 | 0.022 |
| Female | _ | _ | _ | _ | _ | _ | 1.41 | 0.95, 2.10 | 0.089 |
| Non-White | 1.59 | 0.89, 2.84 | 0.114 | 0.59 | 0.24, 1.46 | 0.255 | 1.15 | 0.72, 1.85 | 0.566 |
| Nursing home, previous | 1.82 | 0.72, 4.62 | 0.204 | 1.92 | 0.72, 5.17 | 0.194 | 1.83 | 0.95, 3.54 | 0.071 |
| Charlson Deyo score | 1.01 | 0.85, 1.20 | 0.920 | 1.09 | 0.88, 1.36 | 0.441 | 1.04 | 0.9, 1.18 | 0.611 |
| Number admissions during previous 28 days | 1.20 | 0.74, 1.94 | 0.457 | 0.84 | 0.39, 1.83 | 0.667 | 1.06 | 0.72, 1.57 | 0.765 |
| Number nights in hospital during previous 180 days | 1.02 | 1.0, 1.03 | 0.004 | 1.02 | 1.00, 1.03 | 0.045 | 1.02 | 1.0, 1.03 | 0.001 |
| Number prior positive C. difficile tests | 1.31 | 0.95, 1.80 | 0.097 | 1.49 | 1.16, 1.92 | 0.002 | 1.42 | 1.16, 1.72 | 0.001 |
Antibiotic usage during the follow-up period was assessed for the 3 baseline groups (no C. difficile, NTCD, toxigenic C. difficile). The hypothesis was that antibiotic use would be more prevalent in the 2-week period prior to the development of CDI (compared to the 2-week period prior to the last follow-up date in those who did not develop CDI). The use of antibiotics was greater in those that developed CDI (17.8%) than in those who did not (4.2%). However, antibiotic usage was similar (p=0.515) across the 3 baseline groups for those who developed CDI and was similar (p=0.793) across the 3 groups for those who did not develop CDI.
Of the 1492 patients in the study, 265 (17.8%) died (from all causes) during the 365-day follow-up period; this included 133 (19%) of those with toxigenic C. difficile at baseline (71 females and 62 males), 12 (12%) with NTCD at baseline (7 females and 5 males), and 120 (18%) without C. difficile at baseline (57 females and 63 males). Patients with NTCD at baseline had similar death rates as those with no C. difficile at baseline, for both females (HR=0.98; 95% CI: 0.45, 2.15) and males (HR=0.60; 95% CI: 0.24, 1.50) (Table 4). However, patients with NTCD at baseline had lower death rates than those with toxigenic C. difficile at baseline. The hazard ratio (HR=0.51; 95% CI: 0.28, 0.92) was statistically significant when data from both females and males were pooled. In addition, females with toxigenic C. difficile at baseline had a greater mortality rate than females with no C. difficile at baseline (HR=1.67; 95% CI: 1.15, 2.43).
Table 4.
Hazard Ratios for Death by Baseline Status.
| Female | Male | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value |
| Baseline status: Nontoxigenic C. difficile compared to no C. difficile |
0.98 | 0.45, 2.15 | 0.958 | 0.60 | 0.24, 1.50 | 0.272 | 0.78 | 0.43, 1.41 | 0.406 |
| Baseline status: Nontoxigenic C. difficile compared to toxigenic C. difficile |
0.59 | 0.27, 1.28 | 0.181 | 0.45 | 0.18, 1.14 | 0.091 | 0.51 | 0.28, 0.92 | 0.026 |
| Baseline status: Toxigenic C. difficile compared to no C. difficile |
1.67 | 1.15, 2.43 | 0.007 | 1.32 | 0.90, 1.92 | 0.154 | 1.52 | 1.17, 1.99 | 0.002 |
| Covariates: | |||||||||
| Age | 1.02 | 1.0, 1.03 | <0.001 | 1.01 | 1.00, 1.02 | 0.016 | 1.02 | 1.0, 1.02 | <0.001 |
| Female | _ | _ | _ | _ | _ | _ | 0.92 | 0.72, 1.17 | 0.486 |
| Non-White | 0.42 | 0.22, 0.77 | 0.006 | 0.78 | 0.48, 1.26 | 0.308 | 0.60 | 0.4, 0.87 | 0.008 |
| Nursing home, previous | 0.95 | 0.5, 1.80 | 0.884 | 1.35 | 0.73, 2.52 | 0.341 | 1.04 | 0.67, 1.63 | 0.845 |
| Charlson Deyo score | 1.13 | 1.03, 1.24 | 0.013 | 1.15 | 1.03, 1.27 | 0.009 | 1.14 | 1.06, 1.22 | <0.001 |
| Number admissions during previous 28 days | 1.30 | 0.96, 1.76 | 0.090 | 0.76 | 0.48, 1.22 | 0.260 | 1.08 | 0.84, 1.38 | 0.561 |
| Number nights in hospital during previous 180 days | 1.01 | 1.0, 1.02 | 0.002 | 1.02 | 1.0, 1.03 | <0.001 | 1.02 | 1.0, 1.02 | <0.001 |
| Number prior positive C. difficile tests | 0.90 | 0.64, 1.27 | 0.550 | 0.68 | 0.43, 1.08 | 0.099 | 0.81 | 0.62, 1.06 | 0.129 |
DISCUSSION
This study suggests that there are different patient outcomes by gender after colonization with NTCD. Women tended to be at greater short-term risk of CDI when colonized by NTCD; this was not true in men. However, longer-term risk of CDI and death in those colonized by NTCD were similar in both genders. In addition, females with toxigenic C. difficile had significantly greater mortality rates compared to females with no C. difficile. The comparable hazard ratio in males was elevated but not statistically significant.
Population rates of CDI are higher in women than in men [24,25]. Sex-specific differences in the gut microbiome have been shown to be mediated by hormone levels, and transference of intestinal bacterial communities can alter sex hormone levels in animal studies [26,27]. Moreover, male castration attenuates these microbial differences suggesting that androgens may play a role [27]. Human studies have also reported sex differences in intestinal microbiota [28]. In a longitudinal study in humans, sex differences were present in three species of Clostridia, with higher abundance in males than females [29]. Clostridia species were also more abundant in males in another human study, with females showing significantly greater variability in gut microbial content [30]. However, relative abundance of toxigenic and non-toxigenic C. difficile was not reported.
In our study, females colonized by NTCD developed CDI at a greater rate than those without C. difficile. While the underlying reasons for this are not fully known, recent evidence indicates that not all NTCD strains are uniformly protective against specific toxigenic strains [31]. That is, NTCD strain T7 was shown to be less effective than M3 in preventing CDI caused by the B16 epidemic strain [31]. Therefore, the gender differences observed in our study may be a reflection of colonization and challenge by different C. difficile strains. There could also be other underlying factors that we were unable to capture within this study. Women are often caretakers (e.g., assistants in skilled nursing facilities and nurses in hospitals) and may have greater exposure to toxigenic C. difficile spores [32]. It is not known whether such activities were disproportionately experienced by women with NTCD versus those without C. difficile in our study.
Patients colonized with NTCD had similar mortality rates when compared to those without C. difficile at baseline, demonstrating that at minimum NTCD colonization is not associated with all-cause mortality. However, a larger investigation would be needed to substantiate these findings, given that there were 99 individuals colonized by NTCD in our study. In addition, toxigenic C. difficile at baseline was associated with increased mortality which agrees with prior literature [33]. Age and increased comorbidity were predictors of mortality as well, which is in agreement with prior work [23].
There is currently more information regarding the prevalence of NTCD in patients hospitalized or residing in long-term care facilities than there is among community dwellers [1]. In a study of 294 infants in France, NTCD colonization was common, with 27% of females and 26% of males affected [34]. An investigation of 1234 healthy adults in Japan (ages 18–65 years) indicated a NTCD prevalence of 3% [35]. In the United Kingdom, a study of 149 elderly individuals living at home (of whom 80% were women) reported the prevalence of NTCD (without toxigenic strains) as 2% and the prevalence of mixed cultures as 0.7% [36]. In our study, 6.6% of the subjects had NTCD at baseline of whom 25% were outpatients, ranging from ages 1 to 90 years in males and from ages 5 to 93 years in females.
This study has several limitations. Because subjects were included on the basis of C. difficile testing, it is likely that most or all of them had signs or symptoms suggestive of CDI to a healthcare provider. As such, we might have selected a group of NTCD colonized patients who had different host characteristics than the truly asymptomatic NTCD colonized population at large. In fact, prior work has shown that hospitalized patients with diarrhea, but without CDI, have a systemic inflammatory response that was not seen in asymptomatic outpatient controls [37]. Larger studies of asymptomatic individuals may be informative to evaluate whether NTCD colonization affects the incidence of CDI in the population as a whole. Another limitation is that, during the course of the follow-up period, it is possible that some patients received medical treatment outside of our health system. We estimate that the overall likelihood of this was low and, if this occurred, we assumed that these were evenly distributed across the three patient groups. Lastly, prospective studies with close monitoring of C. difficile status and exposures over time would be preferable to our retrospective approach.
In conclusion, our results do not support the premise that NTCD colonization is uniformly protective against subsequent CDI. Short-term risk of CDI was greater in females colonized by NTCD. Long-term risk of CDI was similar in those with NTCD and those without C. difficile, in both genders. Future studies that delve deeper and more specifically into NTCD epidemiology are needed to close current knowledge gaps.
Acknowledgments
We would like to thank the personnel of the University of Michigan Clinical Microbiology Laboratory for their expertise and support with the execution of this project. We would also like to thank Lynn Holevinski for database support as part of the University of Michigan Clinical Information Technology team.
Financial support: Support for this study was provided by the National Institutes of Health, grants K01AI097281 (S.T.W.) and 5U19AI090871 (D.M.A. and V.B.Y.), the Claude D. Pepper Older Americans Independence Center, grant AG-024824 (K.R.), and the Michigan Institute for Clinical and Health Research, grant 2UL1TR000433 (K.R.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Natarajan M, Walk ST, Young VB, Aronoff DM. A clinical and epidemiological review of non-toxigenic Clostridium difficile. Anaerobe. 2013;22:1–5. doi: 10.1016/j.anaerobe.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerding DN, Johnson S. Management of Clostridium difficile infection: thinking inside and outside the box. Clin Infect Dis. 2010;51:1306–1313. doi: 10.1086/657116. [DOI] [PubMed] [Google Scholar]
- 3.Wilson KH, Sheagren JN. Antagonism of toxigenic Clostridium difficile by nontoxigenic C. difficile. J Infect Dis. 1983;147:733–736. doi: 10.1093/infdis/147.4.733. [DOI] [PubMed] [Google Scholar]
- 4.Borriello SP, Barclay FE. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J Med Microbiol. 1985;19:339–350. doi: 10.1099/00222615-19-3-339. [DOI] [PubMed] [Google Scholar]
- 5.Sambol SP, Merrigan MM, Tang JK, Johnson S, Gerding DN. Colonization for the prevention of Clostridium difficile disease in hamsters. J Infect Dis. 2002;186:1781–1789. doi: 10.1086/345676. [DOI] [PubMed] [Google Scholar]
- 6.Merrigan MM, Sambol SP, Johnson S, Gerding DN. Prevention of fatal Clostridium difficile-associated disease during continuous administration of clindamycin in hamsters. J Infect Dis. 2003;188:1922–1927. doi: 10.1086/379836. [DOI] [PubMed] [Google Scholar]
- 7.Merrigan MM, Sambol SP, Johnson S, Gerding DN. New approach to the management of Clostridium difficile infection: colonisation with non-toxigenic C. difficile during daily ampicillin or ceftriaxone administration. Int J Antimicrob Agents. 2009;33:46–50. doi: 10.1016/S0924-8579(09)70017-2. [DOI] [PubMed] [Google Scholar]
- 8.Seal D, Borriello SP, Barclay F, Welch A, Piper M, Bonnycastle M. Treatment of relapsing Clostridium difficile diarrhoea by administration of a non-toxigenic strain. Eur J Clin Microbiol. 1987;6:51–53. doi: 10.1007/BF02097191. [DOI] [PubMed] [Google Scholar]
- 9.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351:633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 10.Hung YP, Lin HJ, Wu TC, Liu HC, Lee JC, Lee CI, Wu YH. Risk factors of fecal toxigenic or non-toxigenic Clostridium difficile colonization: impact of Toll-like receptor polymorphisms and prior antibiotic exposure. PLoS One. 2013;8:69577. doi: 10.1371/journal.pone.0069577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villano SA, Seiberling M, Tatarowicz W, Monnot-Chase E, Gerding DN. Evaluation of an Oral Suspension of VP2062, Spores of Nontoxigenic Clostridium difficile Strain M3, in Healthy Subjects. Antimicrob Agents Chemother. 2012;56:5224–5229. doi: 10.1128/AAC.00913-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safety and Efficacy Study of VP20621 for Prevention of Recurrent Clostridium difficile Infection. 2014 [Google Scholar]
- 13.Brouwer MS, Roberts AP, Hussain H, Williams RJ, Allan E, Mullany P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat Commun. 2013;4:2601. doi: 10.1038/ncomms3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson S, Torpdahl M, Olsen KE. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect. 2008;14:1057–1064. doi: 10.1111/j.1469-0691.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 15.Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW, et al. Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis. 2012;55:1661–168. doi: 10.1093/cid/cis786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Rao K, Micic D, Chenoweth E, Deng L, Galecki AT, Ring C, Young VB. Poor functional status as a risk factor for severe Clostridium difficile infection in hospitalized older adults. J Am Geriatr Soc. 2013;61:1738–1742. doi: 10.1111/jgs.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubberke ER, Yan Y, Reske KA, Butler AM, Doherty J, Pham V, Fraser VJ. Development and validation of a Clostridium difficile infection risk prediction model. Infect Control Hosp Epidemiol. 2011;32:360–366. doi: 10.1086/658944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welfare MR, Lalayiannis LC, Martin KE, Corbett S, Marshall B, Sarma JB. Co-morbidities as predictors of mortality in Clostridium difficile infection and derivation of the ARC predictive score. J Hosp Infect. 2011;79:359–363. doi: 10.1016/j.jhin.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653–659. doi: 10.1086/501989. [DOI] [PubMed] [Google Scholar]
- 23.Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with clostridium difficile-associated disease. Am J Gastroenterol. 2010;105:2040–2049. doi: 10.1038/ajg.2010.142. [DOI] [PubMed] [Google Scholar]
- 24.Rogers MA, Greene MT, Saint S, Chenoweth CE, Malani PN, Trivedi I, et al. Higher rates of Clostridium difficile infection among smokers. PLoS One. 2012;7:42091. doi: 10.1371/journal.pone.0042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers MA, Greene MT, Young VB, Saint S, Langa KM, Kao JY, et al. Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Med. 2013;11:121. doi: 10.1186/1741-7015-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 27.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre de Cárcer D, Cuív PO, Wang T, Kang S, Worthley D, Whitehall V, et al. Numerical ecology validates a biogeographical distribution and gender-based effect on mucosa-associated bacteria along the human colon. ISME J. 2011;5:801–809. doi: 10.1038/ismej.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaro KJ, Phillips ST, Cheknis AK, Sambol SP, Zukowski WE, Johnson S, et al. Nontoxigenic Clostridium difficile protects hamsters against challenge with historic and epidemic strains of toxigenic BI/NAP1/027 C. difficile. Antimicrob Agents Chemother. 2013;57:5266–5670. doi: 10.1128/AAC.00580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFarland LV, Beneda HW, Clarridge JE, Raugi GJ. Implications of the changing face of Clostridium difficile disease for health care practitioners. Am J Infect Control. 2007;35:237–253. doi: 10.1016/j.ajic.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Schmid D, Kuo HW, Simons E, Kanitz EE, Wenisch J, Allerberger F, et al. All-cause mortality in hospitalized patients with infectious diarrhea: Clostridium difficile versus other enteric pathogens in Austria from 2008 to 2010. J Infect Public Health. 2014;7:133–144. doi: 10.1016/j.jiph.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Rousseau C, Lemée L, Le Monnier A, Poilane I, Pons JL, Collignon A. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol. 2011;60:1112–1118. doi: 10.1099/jmm.0.029736-0. [DOI] [PubMed] [Google Scholar]
- 35.Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Takakuwa H, et al. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J Med Microbiol. 2001;50:720–727. doi: 10.1099/0022-1317-50-8-720. [DOI] [PubMed] [Google Scholar]
- 36.Miyajima F, Roberts P, Swale A, Price V, Jones M, Horan M, et al. Characterisation and carriage ratio of Clostridium difficile strains isolated from a community-dwelling elderly population in the United Kingdom. PLoS One. 2011;6:22804. doi: 10.1371/journal.pone.0022804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao K, Erb-Downward JR, Walk ST, Micic D, Falkowski N, Santhosh K, et al. The systemic inflammatory response to Clostridium difficile infection. PLoS One. 2014;9:92578. doi: 10.1371/journal.pone.0092578. [DOI] [PMC free article] [PubMed] [Google Scholar]