Abstract
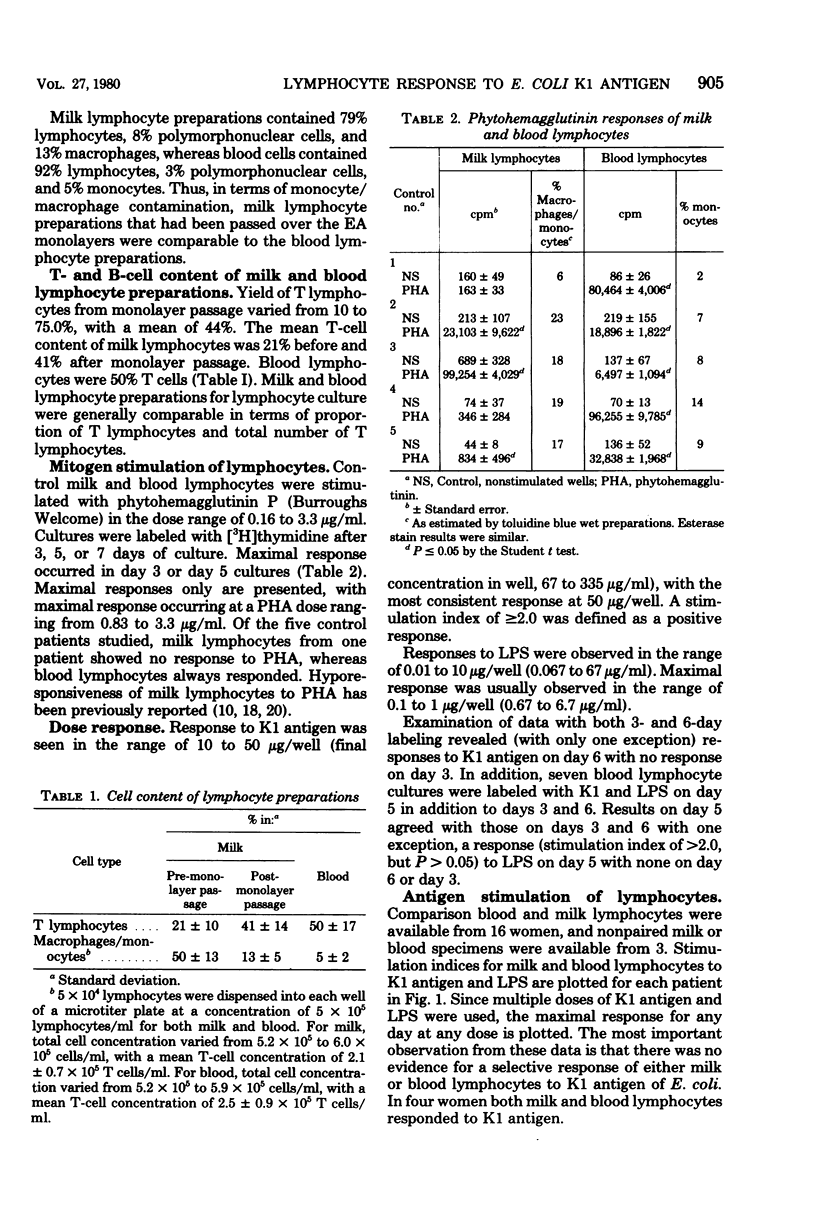
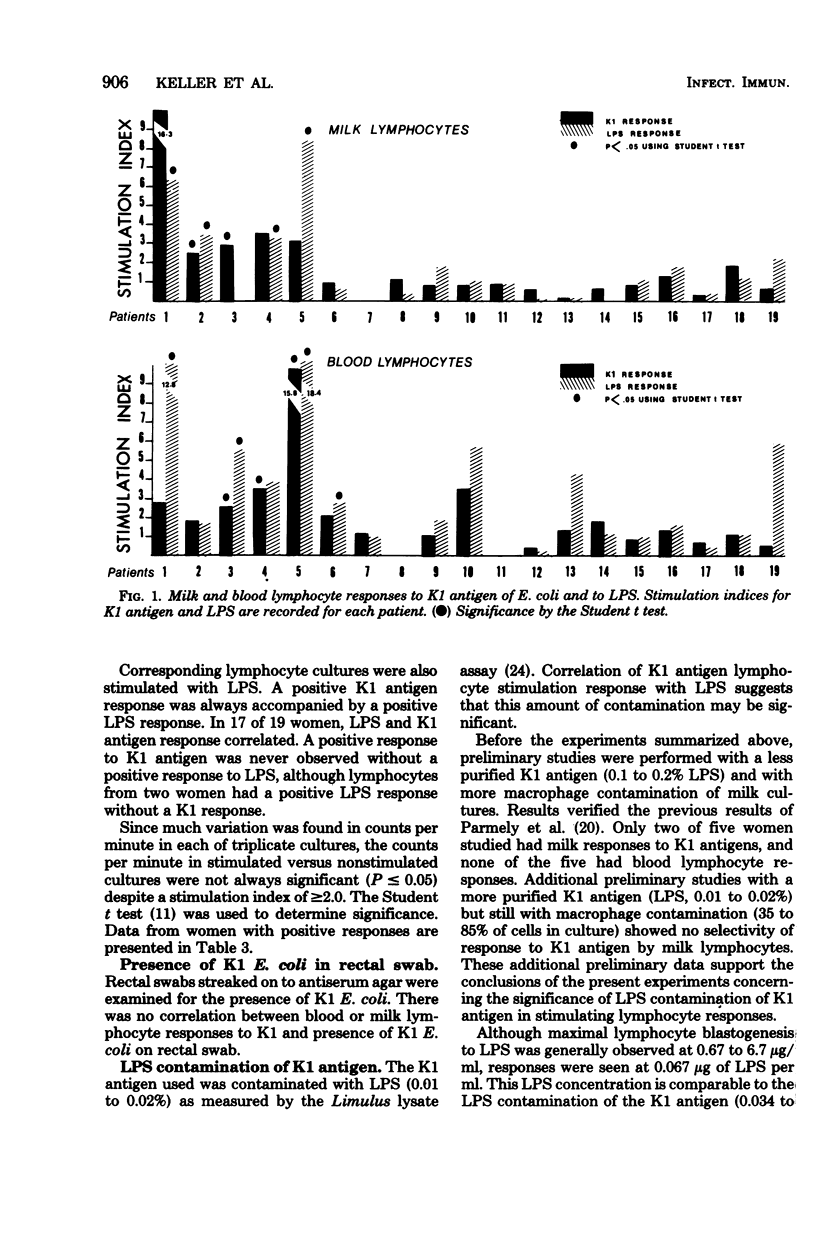
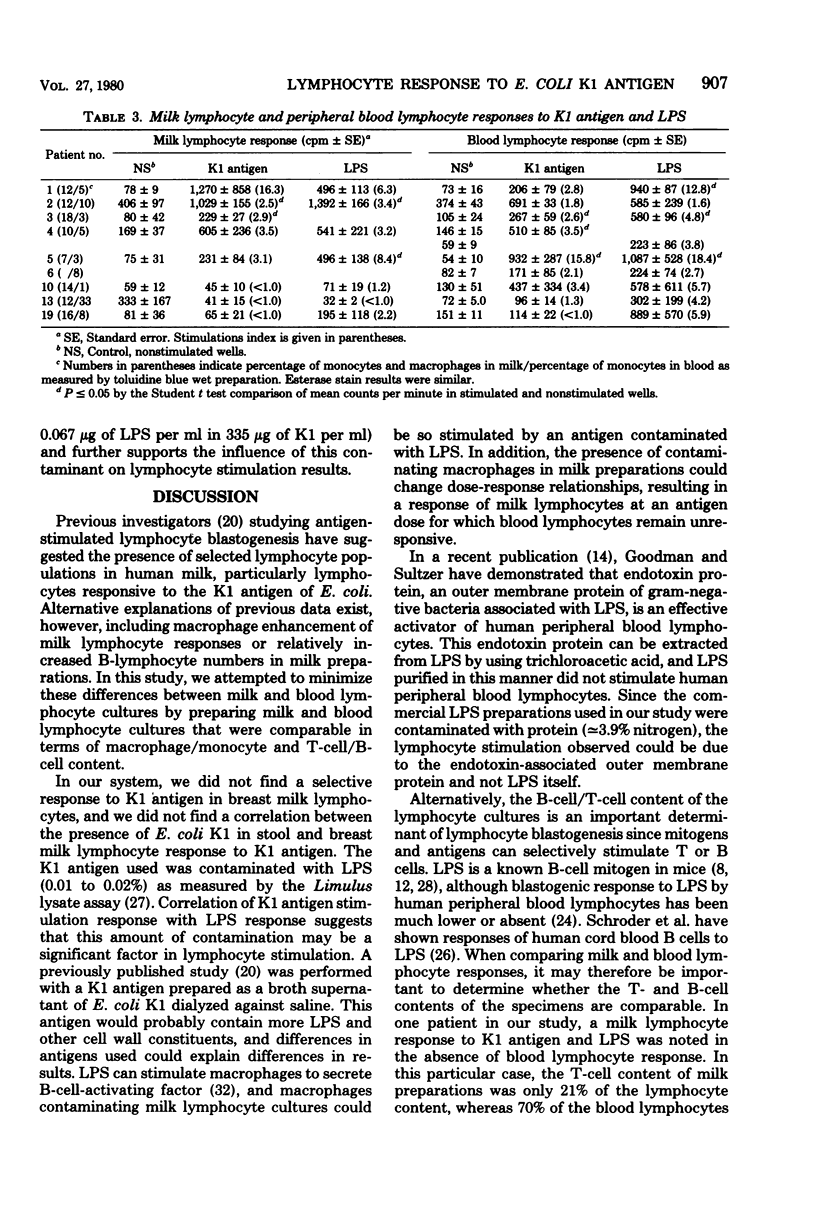
Comparison milk and blood lymphocyte blastogenic responses to the K1 antigen of Escherichia coli and lipopolysaccharide (LPS) from E. coli O127,B8 were examined in 16 postpartum women by [3H]thymidine uptake. Rabbit hemolysincoated sheep erythrocyte monolayers were used to deplete macrophages from milk lymphocyte preparations and to enrich for T lymphocytes in order to make milk preparations more comparable to blood preparations. Response was defined as a stimulation index of greater than or equal to 2.0. There was no evidence of selective response to K1 antigen by milk lymphocytes, since both blood and milk lymphocytes responded in four women and neither blood nor milk lymphocytes responded in nine. Milk lymphocytes alone responded to K1 in one woman, whereas blood lymphocytes alone responded in two women. Additional nonpaired milk or blood cultures were available from three women. None of these responded to K1 antigen. Corresponding lymphocyte cultures were stimulated with LPS. A positive K1 response was always accompanied by an LPS response, and the LPS response correlated with the K1 response in 17 of 19 women. Stool cultures examined with an antiserum agar showed no correlation between the presence of K1 E. coli in the stool and milk or blood lymphocyte response to K1 antigen. In the system used here, no selectivity of response of breast milk lymphocytes to K1 antigen was noted.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardyce R. A., Shearman D. J., McClelland D. B., Marwick K., Simpson A. J., Laidlaw R. B. Appearance of specific colostrum antibodies after clinical infection with Salmonella typhimurium. Br Med J. 1974 Aug 3;3(5926):307–309. doi: 10.1136/bmj.3.5926.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J. F., Dormont J. Further developments of the rosette inhibition test for the testing of antihuman lymphocyte serum. Transplantation. 1971 Jan;11(1):96–100. doi: 10.1097/00007890-197101000-00016. [DOI] [PubMed] [Google Scholar]
- Bakacs T., Gergely P., Klein E. Characterization of cytotoxic human lymphocyte subpopulations: the role of Fc-receptor-carrying cells. Cell Immunol. 1977 Aug;32(2):317–328. doi: 10.1016/0008-8749(77)90208-8. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Gupta R. K., Olquin M. V., Saif L. J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972 Sep;6(3):289–301. doi: 10.1128/iai.6.3.289-301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Jouanen E., Williams R. C., Jr T and B lymphocytes in human colostrum. Clin Immunol Immunopathol. 1974 Nov;3(2):248–255. doi: 10.1016/0090-1229(74)90011-7. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Further studies on the activation of lymphocytes by endotoxin protein. J Immunol. 1979 Apr;122(4):1329–1334. [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Kedar E., Ortiz de Landazuri M., Bonavida B. Cellular immunoadsorbents: a simplified technique for separation of lymphoid cell populations. J Immunol. 1974 Mar;112(3):1231–1243. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Ogra S. S., Ogra P. L. Immunologic aspects of human colostrum and milk. II. Characteristics of lymphocyte reactivity and distribution of E-rosette forming cells at different times after the onset of lactation. J Pediatr. 1978 Apr;92(4):550–555. doi: 10.1016/s0022-3476(78)80286-8. [DOI] [PubMed] [Google Scholar]
- Parmely M. J., Beer A. E., Billingham R. E. In vitro studies on the T-lymphocyte population of human milk. J Exp Med. 1976 Aug 1;144(2):358–370. doi: 10.1084/jem.144.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmely M. J., Beer A. E. Colostral cell-mediated immunity and the concept of a common secretory immune system. J Dairy Sci. 1977 Apr;60(4):655–665. doi: 10.3168/jds.S0022-0302(77)83915-5. [DOI] [PubMed] [Google Scholar]
- Parmely M. J., Reath D. B., Beer A. E., Billingham R. E. Cellular immune responses of human milk T lymphocytes to certain environmental antigens. Transplant Proc. 1977 Jun;9(2):1477–1483. [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- Pitt J. Breast milk leukocytes. Pediatrics. 1976 Nov;58(5):769–770. [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö B., Waterfield E. M., Möller E., Möller G. Polyclonal antibody secretion in human lymphocytes induced by killed staphylococcal bacteria and by lipopolysaccharide. Scand J Immunol. 1977;6(11):1159–1169. doi: 10.1111/j.1365-3083.1977.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Turunen O., Lundqvist C., de la Chapelle A. Cell surface markers in cord blood leucocytes after stimulation with lipopolysaccharide B. Acta Pathol Microbiol Scand C. 1978 Dec;86C(6):315–319. doi: 10.1111/j.1699-0463.1978.tb02596.x. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Winfield J. B., Lobo P. I., Hamilton M. E. Fc receptor heterogeneity: immunofluorescent studies of B, T, and "third population" lymphocytes in human blood with rabbit IgG b4/anti-b4 complexes. J Immunol. 1977 Nov;119(5):1778–1784. [PubMed] [Google Scholar]
- Wood D. D., Cameron P. M. The relationship between bacterial endotoxin and human B cell-activating factor. J Immunol. 1978 Jul;121(1):53–60. [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]


