Introduction
Schistosomiasis is a neglected tropical disease (NTD) caused by infection with any of the five Schistosoma species: S. mansoni, S. japonicum, S. heaematobium. S. mekongi, or S. intercalatum [1]. It is endemic in 76 countries and territories and affects over 250 million people [2]. A quality-of-life assessment defines a significant 9.5–24% disability with the most aggressive schistosome species, S. japonicum [3, 4].
Schistosomiasis japonica is a major disease risk for more than 40 million people in China [5], and 7 million more in the Philippines [6]. It has more than 40 mammalian animals as reservoir hosts that play a role in maintaining the parasite and increasing the chances for human infection [7]. In China, there are several hundred thousand livestock, including over 200,000 buffalo currently infected [8]. Considering the zoonotic nature of schistosomiasis japonica and the important role buffalo and cattle play in environmental contamination and transmission to humans [9–15], a transmission blocking veterinary vaccine for domesticated bovines would provide an additional and unique approach to schistosomiasis japonica control.
Paramyosin is a 97-kDa myofibrillar protein with a coiled-coil structure found only in invertebrates. In addition to the muscles of all 3 developmental stages of Schistosoma japonicum, paramyosin is located on the tegumental surface of lung stage schistosomula and in the secretory glands of cercariae [16]. Immunization with paramyosin confers resistance to infection by S. mansoni in mice [17, 18], and an anti-paramyosin monoclonal antibody confers resistance to infection with S. japonicum in mice [19].
We have demonstrated the vaccine potential of S. japonicum paramyosin (Sj97) in mice immunized with protein biochemically purified from S. japonicum adult worms [20]. This work has also been extended to domestic sheep, pigs and water buffalo immunized with recombinant fragments of Sj97 [21–24]. In immuno-epidemiology studies conducted in an S. japonicum endemic region of the Philippines, we have demonstrated that individuals with a Th2 biased cellular response [25] or IgE biased humoral responses to Sj97 [26] are relatively resistant to reinfection following treatment compared to individuals without these responses.
Together, these data support paramyosin as a leading vaccine candidate for schistosomiasis, but the low yields of full-length recombinant protein have hampered its further development [27]. Our group overcame this scale-up challenge lately [28], which has enabled larger scale testing of both the safety and efficacy of recombinant full length Sj97. Here, we report the results of 3 vaccine-challenge experiments in buffalo conducted in 2008, 2013 and 2016 using full-length rSj97 produced at pilot scale.
Materials and Methods
1. Recombinant protein production and characterization
Pilot scale production of recombinant full-length paramyosin (rSj97) has been described previously [28]. Briefly, kanamycin-resistant recombinant pET30 plasmids were transformed into BL21(DE3) and expressed at the pilot scale using our published fermentation and chromatographic purification process (see figure S1 of reference[28]). Lyophilized, vialed rSj97 was resuspended in 1 ml of LPS-free water. LPS (endotoxin) levels were determined using an FDA-cleared LAL-assay (Lonza, USA). The protein concentration was assessed with a BCA-based protein assay containing bovine serum albumin as the standard (Pierce, Rockford, IL). Protein purity was assessed with 4–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and colloidal Coomassie staining (Gelcode Blue; Pierce, Rockford, IL). Proteins on stained SDS-PAGE gels were excised and subjected to trypsin digestion and nano-liquid chromatography-tandem mass spectrometry-based peptide sequencing (ProtTech, Norristown, PA). Residual SDS was measured using a quantitative colorimetric assay as described elsewhere [29]. Secondary and tertiary protein structure analyses were performed on 0.6 nM solutions of rSj97 in the presence and absence of 0.05% SDS by circular dichroism on a spectropolarimeter (J-185; Jasco Inc., Easton, MD) with a 0.2-mm-path-length cuvette with temperature control at 25°C. Raw data in millidegrees were converted to ellipticity and used to calculate helix fractions [30] and the presence of a coiled-coil tertiary structure [31]. The recombinant Sj97 was functionally characterized for IgG and collagen binding by enzyme-linked immunosorbent assay (ELISA)-based assays as described elsewhere [28].
2. Vaccination experiments
The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee at the Shanghai Veterinary Research Institute (ShVRI) in China, and the Animal Welfare Committee of the Office of Research Administration for the Lifespan IACUC.
Montanide™ ISA206 adjuvants were from Seppic, France. Adjuvant-protein formulation was prepared the day before vaccination following the manufacturer’s recommendation. In brief, LPS-free water reconstituted rSj97, or lyophilization buffer (50M sodium phosphate, 0.05% tween-20, 0.3% sucrose, pH7.4) as the control, was added to ISA206 at 50:50 (w:w) ratio. The mixtures were stirred at a low shear rate (300 rpm) for 10 min at 30°C in a glass beaker to form the water-in-oil-in-water emulsion [32].
Male and female water buffalo aged 10 to 17 months were purchased from non-schistosomiasis endemic area in Jiangsu, China. Animals were pre-screened for intestinal helminthes using 50g of stool with the modified saturated saline floatation method. An anti-soluble egg antigen (SEA) ELISA test was also carried out on all the buffalo using pre-immune serum in order to confirm their schistosomiasis-free status. All buffalo received 15mg/kg albendazole orally 4 weeks before the 1st immunization for presumptive treatment of potential geohelminth infections.
Vaccination protocols were based on those of Xu et al [33, 34]. Water buffalo were vaccinated at 0, 4 and 8 weeks by injecting emulsions subcutaneously at the lower third (near the shoulders) of a buffalo neck. At 12wk, all animals were challenged with 1,000 +/−3 cercariae shed from the infected snails percutaneously. Schistosoma japonica infected Oncomelania hupensis hupensis snails were kept in the lab of ShVRI for lifecycle maintenance.
The 2008 trial
Sixteen water buffalo were equally allocated into 2 groups based on their gender, age and weight. One group received 3 vaccinations of 250ug rSj97-ISA206 at 4-week intervals, and the unvaccinated control group was injected with ISA206 emulsified with the lyophilization buffer with the same schedule.
The 2013 trial
Thirty-two water buffalo were allocated into 2 vaccination groups with 11 buffalo each and 1 control group with 10 buffalo based on their gender, age and weight. One group received 3 doses of 250ug rSj97-ISA206 at 4-week intervals; the second group received 500 μg/dose for 3 injections at 4-week intervals. The controls were injected with ISA206 emulsified with the lyophilization buffer.
The 2016 trial
Sixteen water buffalo were allocated into 2 groups with 8 buffalo each based on their gender, age and weight. One group received 3 vaccinations of 500 μg/dose rSj97-ISA206 at 4-week intervals; the other group received injection of ISA206 emulsified with the lyophilization buffer.
3. Sample collection
Serum samples were collected from the animals pre- and 4 weeks post each vaccination and challenge infection, as well as at the time for perfusion. In the 2016 trial, we collected serum 2, 4 and 8 weeks post-challenge corresponding to weeks 0, 4, 8, 12, 14, 16, 18 and 20 of the experiment. Whole blood (K2EDTA-anticoagulated) was collected at 0, 4, 8 and 12 weeks for hemogram and chemistry analysis to monitor buffalo health status after vaccination.
4. Worm antigen preparation
SWAP (soluble worm antigen preparation) and SEA (soluble egg antigen) were prepared under endotoxin free conditions according to standard procedures [35]. In brief, 7–8 weeks after S. japonicum cercarial exposure, infected rabbits (~2500 cercariae/rabbit) were perfused and adult worms and rabbit livers were collected and rinsed with LPS-free PBS. The collected worms and purified eggs were re-suspended in PBS and sonicated for 4 times of 1 min on ice on full power (Fisher scientific model F60 sonic dismembrator, USA), followed by centrifuging at 30,000g for 30min at 4°C. The resulting supernatant was stored at −80°C.
5. Isotype-specific ELISA
An indirect-ELISA was used to detect SWAP, SEA, and rSj97 specific IgG1 and IgG2. One μg per well of each antigen were used to coat a 96-well Maxisorb Nunc immunoplate (Sigma-Aldrich,) overnight. The plates were blocked with 150 μL 0.5% gelatin-PBST for 1hr at 37°C. Antigen-specific IgG1 and IgG2 were detected using sheep anti-bovine IgG1-HRP and IgG2-HRP conjugates (Bio-rad, Raleigh, NC) at 1:6000 dilution. After a final wash, the plates were developed by adding 3, 3′, 5, 5′-Tetramethylbenzidine (TMB) liquid substrate (Sigma-Aldrich, Mannheim, Germany). The reaction was stopped with 1M H2SO4 and read on a micro-plate ELISA reader (Bio-rad, Raleigh, NC) at 450nm.
6. Vaccination safety assessments
On each vaccination day, the veterinary team weighed the animals, recorded rectal temperature, and evaluated the animals for health status using a standardized veterinary physical assessment tool, the Body Condition Score (BCS) [36, 37]. Whole blood samples were assayed for WBC (×109/L), RBC (×1012/L), HGB (hemoglobin, g/L), HCT (hematocrit, %), MCV (mean corpuscular volume, fL/cell), MCH (mean corpuscular hemoglobin, pg/cell), MCHC (mean corpuscular hemoglobin concentration, g/L) RDW (red blood cell distribution width, %), PLT (platelet count, ×109/L), MPV (mean platelet volume, fL), PDW (platelet distribution width, fL), PCT (plateletcrit, %), ALB (albumin, g/L), Cr (creatinine, μmol/L), GLU (glucose, mmol/L), ALT (alanine aminotransferase, u/L), AST (aspartate aminotransferase, u/L), ALP (alkaline phosphatase, u/L), UREA (blood urea concentration, mmol/L), LDH (lactate dehydrogenase, u/L), CK (creatine kinase, u/L), Ca (calcium, mmol/L), Mg (magnesium, mmol/L), and P (phosphate, mmol/L). Hemograms were measured on a BS-2800Vet automatic blood cell analyzer (MINDRAY Bio-medical electronics CO. LTD, Shenzhen, China). Chemistry assays were performed on a BS-200 Chemistry Analyzer (MINDRAY Bio-medical electronics CO. LTD, Shenzhen, China) following manufacture’s recommendation.
During the first 30 minutes following each immunization, animals were monitored for signs of anaphylactic shock, such as heavy breathing and restlessness. Rectal temperatures were taken for each buffalo 1hr after vaccination. Nose dryness and erythema at the injection site was closely observed for 4–5 hrs after each vaccination. Injection site erythema and nose dryness was also assessed and recorded again one day after each vaccination.
All animals were weighed and then euthanized at either 10 weeks (2008 trial) or 8 weeks (2013 and 2016 trials) post-challenge. Adult worms were obtained from the portal vein of euthanized water buffalo by perfusion of the descending thoracic aorta with saline and the intestinal mesentery and liver were examined after perfusion to collect any additional remaining worms.
All adult worms recovered from each water buffalo were counted, categorized and recorded as males, females and total worm number (worm burden). A five gram sample of the left lateral hepatic lobe from each water buffalo was digested in 5% KOH (w/v) for 18 hours at 37°C. The suspension was then agitated and a 1mL sample of the liver suspension was collected, and centrifuged at 3000rpm for 1 min. Then, the pellet was re-suspended in 200μL of PBS, and the total eggs were counted under a microscope. Using the average of three separate samples, the number of eggs per gram (EPG) of liver tissue was calculated for each animal.
7. Statistical analysis
Statistical analyses were performed with JMP v11 software (SAS Institute, Cary, NC) with exception of the Friedman test (SAS v9.4; SAS Institute, Cary, NC). Data were analyzed using the non-parametric Kruskal-Wallis test to compare continuous variables among three groups while the non-parametric Wilcoxon rank sum test was used for comparison of continuous variables between two groups. For comparisons among groups at each time point, the Friedman test was performed with multiple post-hoc comparisons. The Fisher’s exact test was applied to compare the ratio of gender among groups. Spearman’s correlation coefficient (rs) was used to determine linear relationships between liver egg number and worm burden. Efficacy is reported as the percent reduction in median total worm number between the treatment groups. A P-value < 0.05 was considered statistically significant.
Results
1. Recombinant protein production
The purified rSj97 was >95% pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis and was free of significant endotoxin contamination. We produced 2 lots of rSj97 for these buffalo experiments. Lot # 4530 was used for the trial in 2008. Lot # 7170 was used for the trials in 2013 and 2016. Each vial of lot # 4530 contains 0.07 EU per mg of protein and less than 0.01% SDS. Each vial of lot # 7170 contains 0.03 EU per mg of protein and 0.032% SDS. Both lots of rSj97 are stable when stored at 4°C for 45 days as assessed by a 4–15% SDS-PAGE separation under reducing conditions. An alpha-helical coiled-coil tertiary structure, immunoglobulin, and IgG and collagen binding characteristics were confirmed for both lots of the recombinant paramyosin (data not shown).
2. Vaccination safety (assessed for the 2013 and 2016 trials)
In both the 2013 and 2016 trials, we detected no significant differences between the treatment groups at baseline with regards to age, gender, weight, body condition score (BCS), hematologic parameters or chemistry analytes designed to assess hepatic and renal function at the baseline of the experiment (see Table 1 for 2013 data, and Supplement Table 1 for 2016 data). Laboratory values for all animals were within published reference intervals for this species [38, 39].
Table 1.
Baseline Characteristics in 2013 Trial.
| 250-rSj97 (n=11)
|
500-rSj97 (n=11)
|
ISA206 (n=10)
|
|||||
|---|---|---|---|---|---|---|---|
| Median or proportion | IQR | Median or proportion | IQR | Median or proportion | IQR | P* | |
| age (mon) | 14 | 13–15 | 14 | 13–15 | 14 | 13–15 | 0.960 |
| gender=M | 27.3 | 36.4 | 70 | 0.146 | |||
| BCS | 6 | 6–7 | 7 | 6–8 | 6 | 5–7 | 0.167 |
| Weight (g) | 250 | 200–305 | 255 | 205–300 | 257.5 | 210–295 | 0.995 |
| WBC (×109/L) | 9.7 | 9.1–11.2 | 10.9 | 8–13.2 | 11.8 | 9.3–13.2 | 0.366 |
| RBC (×1012/L) | 7.7 | 7.2–8.7 | 8 | 7.2–8.2 | 7.3 | 6.8–7.5 | 0.116 |
| HGB (g/L) | 138 | 128–147 | 137 | 131–145 | 129 | 124–136 | 0.135 |
| HCT (%) | 41.8 | 39–46.7 | 42.1 | 40.7–45.4 | 38.1 | 36.7–41.7 | 0.090 |
| MCV (fL/cell) | 53.8 | 49.3–57.2 | 53.8 | 52.6–56.5 | 54.3 | 50.9–57.2 | 0.860 |
| MCH (pg/cell) | 17.7 | 16.1–18 | 17.7 | 17–18 | 18.1 | 17–19.2 | 0.515 |
| MCHC (g/L) | 328 | 320–330 | 328 | 319–335 | 335.5 | 326–337 | 0.186 |
| RDW (%) | 17.3 | 16.3–18.2 | 17.3 | 16.7–17.6 | 17.5 | 16.9–18.1 | 0.686 |
| PLT (×109/L) | 338 | 294–353 | 296 | 275–367 | 316.5 | 309–358 | 0.963 |
| MPV (fL) | 7.2 | 6.9–7.9 | 7.5 | 7.1–8 | 7.1 | 6.6–8.1 | 0.464 |
| PDW (fL) | 15.1 | 14.6–15.3 | 15.2 | 14.9–15.5 | 15.1 | 15–15.5 | 0.403 |
| PCT (%) | 0.2 | 0.2–0.3 | 0.2 | 0.2–0.3 | 0.2 | 0.2–0.3 | 0.940 |
| ALB (g/L) | 39.1 | 38.1–39.2 | 37.8 | 36–38.4 | 37.5 | 35.7–40.4 | 0.311 |
| Cr (μmol/L) | 149.8 | 115.3–167.3 | 111.4 | 92.6–142.6 | 116.2 | 92.3–154.8 | 0.287 |
| ALT (u/L) | 55.5 | 47.3–73.8 | 60.5 | 49.1–64.8 | 57.5 | 38.7–65.3 | 0.848 |
| AST (u/L) | 191.7 | 171.5–213.6 | 189.4 | 164.8–215.3 | 196.7 | 177.8–232.4 | 0.717 |
| ALP (u/L) | 199.6 | 150.7–407.6 | 223.8 | 143–285.3 | 217.3 | 105–294.5 | 0.941 |
| UREA (mmol/L) | 6 | 5.8–7.5 | 6.6 | 5.7–7 | 6.6 | 5.9–7.4 | 0.765 |
| LDH (u/L) | 705.6 | 665.9–881.3 | 699.7 | 639.1–793.2 | 791.7 | 709–971.8 | 0.305 |
| CK (u/L) | 238.5 | 203.8–297.8 | 246.8 | 213.4–283.3 | 260.2 | 222.7–334.4 | 0.460 |
| Ca (mmol/L) | 2.3 | 2.2–2.4 | 2.2 | 2.1–2.3 | 2.2 | 2.2–2.3 | 0.277 |
| Mg (mmol/L) | 2.1 | 1.8–2.2 | 2 | 1.8–2.2 | 1.9 | 1.7–2 | 0.430 |
| P (mmol/L) | 2.6 | 2.2–2.9 | 2.5 | 2.4–2.8 | 2.4 | 2.2–2.5 | 0.434 |
Gender was analyzed by the Fisher’s exact test. Others were analyzed by Kruskal-Wallis test.
We did not detect any significant differences in rectal temperature, BCS or body weight between the treatment groups at any time point during the vaccination series (Figure S1, S2). We did not detect any significant differences in hemogram or chemistry analytes between the treatment groups at any time point during the vaccination series in the 2013 trial. In 2016 trial, LDH levels are significantly higher in vaccination group across all time points before challenge infection, while the average levels of AST, ALT and Cr differed at several time points sporadically between the control and vaccinated groups (Figure S3, S4).
In both the 2013 and 2016 trials, no severe adverse events attributable to the injection were observed. In the 2013 trial, at one day post-immunization, injection site erythema was noted in 5 animals (2 in control group, 2 in 250ug injection group, and 1 in 500 μg injection group) after the 1st injection; 3 (1 in control group and 2 in 250 μg injection group) after the 2nd injection; 3 (1 in control group and 2 in 250 μg injection group) after the 3rd injection. The diameter of the erythematous area was less than 1.0 cm in all cases. In the 2016 trial, buffalo skin reactions after vaccination were similar, with no erythematous area exceeding 1.0 cm in diameter in any animal at any time point.
In both the 2013 and 2016 trials, there was no more than a 0.3°C temperature change from baseline when measured 1hr after injection in all animals at all time points. At the time of perfusion, no significant gross anatomic lesions were observed in the abdominal organs of any animal in either trial.
3. Vaccination efficacy
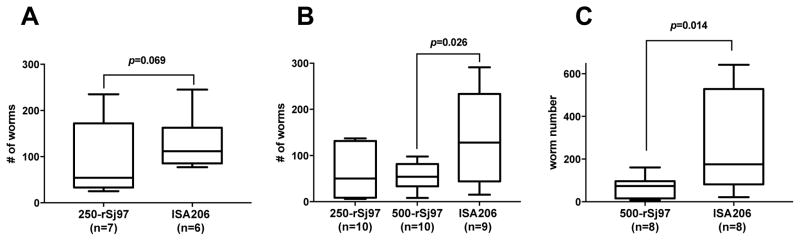
In the 2008 trial, buffalo vaccinated with 250 μg rSj97 per injection (n=7) had a 51.5% lower median worm burden compared to the control animals (n=6), but this difference did not achieve statistical significance (P = 0.069, Figure 1A). For the 2013 trial, we increased both the sample size and the dose of rSj97. Buffalo vaccinated with 250 μg rSj97 per injection (n=10) had a 60.9% lower median worm burden compared to the control animals (P = 0.069, n=9), while buffalo vaccinated with 500 μg rSj97 per injection (n=10) had a 57.8% lower median worm burden compared to the control animals (P = 0.026, Figure 1B).
Fig. 1.
Vaccination with rSj97 protects water buffalo from S. japonicum cercarial challenge. Median worm burden shown as the number of worms recovered at perfusion from the rSj97-ISA206 vaccinated and control groups. (A) the 2008 trial, (B) the 2013 trial, (C) the 2016 trial. Lines indicate the median, boxes indicate the 75th percentile and whiskers indicate the 90th percentile of the distribution.
In the 2016 trial, buffalo vaccinated with 500 μg rSj97 per injection (n=8) had a 57.8% lower median worm burden compared to the control animals (n=8, P = 0.014, Figure 1C).
In all three trials, worm burden was significantly correlated with the quantity of eggs trapped in liver tissue across all treatment groups (Spearman’s rs all >0.5, all P < 0.002). We did not detect a difference in the female to male worm ratio between any treatment groups for any trial, nor did we detect a difference in liver eggs per number of paired worms.
4. Buffalo mortality
Out of 64 buffalo under study, a total of 6 buffalo died during the trials. Autopsy of the carcasses revealed that two deaths were due to accidents, two deaths due to kidney stones, a relatively frequent problem among water buffalo in the region. There was no evidence implicating either the vaccine or adjuvant in the deaths of the animals.
In the 2008 trial, 1 buffalo in the 250 μg rSj97 injection group died 4 weeks after the challenge infection due to apparent weakness. Two animals died in the control group with 1 death occurring 2 days before the challenge infection and one occurring 2 days before perfusion. In this first animal, a carpentry nail was found embedded in the pericardium. In the second animal, the death 2 days post challenge infection may be related to the challenge inoculum.
In the 2013 trial, 1 buffalo in the 500 μg rSj97 injection group died 1 day before the 3rd immunization and this death was caused by urinary tract obstruction. One buffalo in the 250 μg group encountered an accident that caused a rear leg wound and died 4 days before the challenge infection due to weakness. One death in the control group occurred 12 days after challenge infection due to urinary obstruction.
No buffalo died during the 2016 trial.
These buffalo mortality results are consistent with the prior published data of other experienced schistosome vaccine research groups [22].
5. Antigen specific antibody response
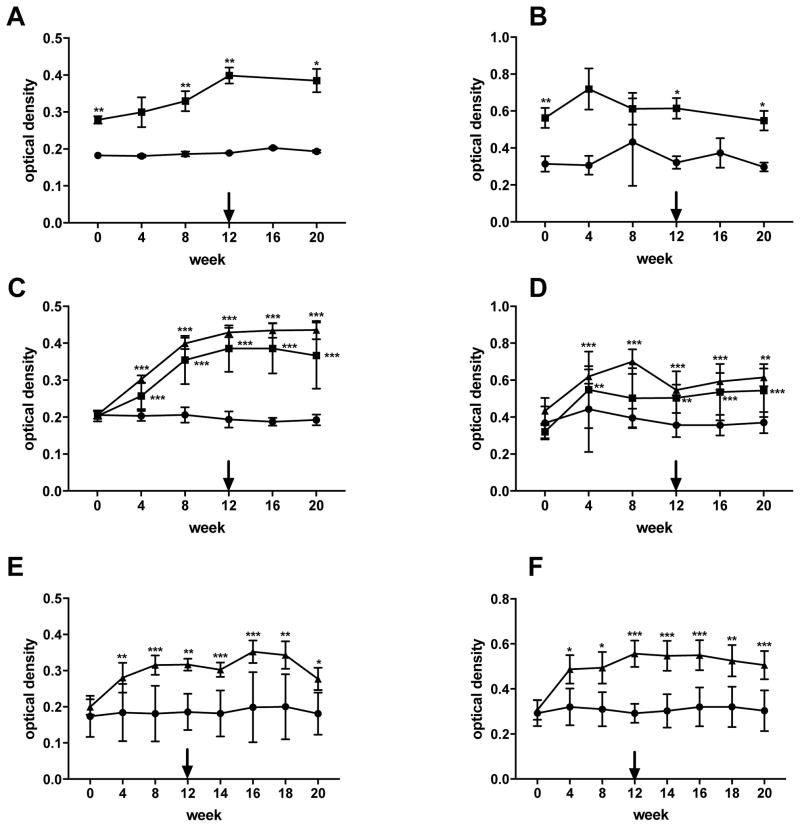
We measured rSj97 specific IgG1 and IgG2 in water buffalo at pre- and post-vaccination time points and after Schistosoma japonicum challenge. IgG2 responses developed more rapidly and achieved higher levels compare to IgG1 responses both after the vaccination series as well as after challenge infection. The levels of specific IgG1 and IgG2 remained low in the control group in all trials (Figure 2A–F). In the vaccination groups, rSj97-specific IgG1 levels reached their maxima prior to challenge infection and plateaued thereafter (Figure 2A, C and E), while the IgG2 levels peaked after either the 2nd or the 3rd vaccination (Figure 2B, D and F).
Fig. 2.
Vaccination with rSj97 generates both Th1 (IgG2) ad Th2 (IgG1) specific responses. Panels A, C and E, rSj97-specific IgG1. Panels B, D, and F, rSj97-specific IgG2. Panels A and B, the 2008 trial. Panels C and D, the 2013 trial. Panels E and F, the 2016 trial. Boxes depict the 250 μg rSj97/dose groups, triangles depict the 500 μg rSj97/dose groups, and dots depict the ISA206 alone control groups. Arrows indicate the time of challenge infection. P values reflect the result of the Friedman test comparing vaccinated versus control groups at a given time point. *P<0.05, **P<0.01, *** P<0.001
Neither anti-SWAP nor anti-SEA IgG1 or IgG2 levels in the vaccinated groups increased significantly before challenge infection compare to the controls. Moreover, SWAP- and SEA- specific IgG1 and IgG2 increased slightly after challenge infection, but no statistically significant differences were detected between the vaccination and control groups (data no shown).
Discussion
S. japonicum is a zoonosis with domesticated water buffalo, the key labor force for rice farming and one of the main sources of dairy products, suffering from the disease, as well as constituting a critical reservoir for continuing transmission to humans. This domesticated reservoir constitutes the target population for a veterinary anti-schistosome vaccine, which would improve bovine health and functional work capacity while reducing parasite transmission to humans.
In three independent vaccine trials, we show that full-length recombinant paramyosin produced at pilot scale, together with ISA206 as adjuvant, resulted in 51.5–60.9% median worm reduction in water buffalo following S. japonicum challenge infection. These levels of protection have not been previously obtained in large animal models with recombinant paramyosin either adjuvanted with BCG (43.2%–44.2%)[23], TiterMax (32.9–34.5%) [21] or QuailA (34%, not statistically significant) [22], nor any other S. japonicum vaccine candidate to date [15, 24], including a vaccination field trial with Sj28 GST and Sj23 DNA vaccine, which conferred 33–44% worm reduction in the vaccinated groups [40]. Because bovines play a major role in schistosomiasis japonica transmission in endemic areas [41, 42], a vaccine that generates more than 50% of protection in domestic cattle and water buffalo would constitute a key asset for disease control and elimination [43, 44].
In bovines, Th1 polarized responses drive the production of IgG2 isotype, while Th2 polarize responses drive IgG1 isotype production [45]. In our trials, immunization with rSj97 in ISA206 generated a mixed response with induction of both IgG1 and IgG2 anti-paramyosin antibodies. However, we were unable to show a conclusive correlation between protection and isotype distribution in our vaccinated animals.
In humans, protective antibody responses in general, and anti-paramyosin responses in particular, are correlated with type 2 cellular and IgE isotype specific responses [25, 26, 46, 47]. Although there is clear evidence of acquired protective immunity against S. japonicum in water buffalo [34], few studies have examined their humoral and cellular responses during schistosomiasis and, to our knowledge, none have explored antigen-specific IgG subclasses during vaccination [22, 48, 49]. Studies examining the regulation of immunoglobulin isotype expression in bovines indicate a role for IL-4 and IL-13 in the regulation of IgG1 and IgE expression in cattle [50, 51]. Unfortunately, we were unable to assay for rSj97 specific IgE or IL-13 levels in our experiments due to the unavailability of reagents for water buffalo. Future studies to better illustrate and understand bovine immune responses to schistosome infection and vaccination are warranted.
Montanide ISA206, which forms a stable water-in-oil-in-water emulsions, is a component of several licensed veterinary vaccines, including bovine vaccines for foot-and-mouth disease control [52]. In our trials, rSj97 adjuvanted with ISA206 showed an acceptable safety profile with no severe reactogenicity or body temperature oscillation among water buffalo in either vaccination or adjuvant only control groups after each injection. We detected no safety signals in the body conditions score, or in hematology parameters or serum tests of hepatic or renal function. Body weight showed the expected trend of increasing with age during the trial in both vaccine and adjuvant groups, but we did not have a non-adjuvant control group for comparison.
The present work demonstrated that rSj97 adjuvanted with ISA206 is safe, well tolerated and reliably generates greater than 50% reduction in median worm burdens when administered as 3 doses of 500 μg rSj97 per buffalo. Larger trials are necessary to optimize rSj97-adjuvant combinations and evaluate the optimal vaccine in the context of field exposure as opposed to the laboratory exposure employed in the present studies. We are currently conducting a buffalo trial with rSj97-ISA206 vaccination followed by 6 months of community based field exposure. In parallel with this bovine trial, we are conducting detailed assessment of the potential for Type 1 hypersensitivity reactions to rSj97 in S. japonicum exposed humans. Together, these data are necessary to support large scale (Phase II) trials of rSj97 in ISA206 in bovines and Phase I trials in humans.
Supplementary Material
Highlights.
We conducted 3 vaccine trials with recombinant full-length paramyosin (rSj97) in water buffalo.
The three-dose 500 μg/dose regimen was well tolerated in two trials of 18 buffalo.
Vaccinated buffalo had 51.5% to 60.9% lower worm burden post challenge compared to controls.
rSj97 is a safe and promising vaccine candidate for Schistosomiasis japonica.
Acknowledgments
Financial support: This work is supported by the US NIH/NIAID (Award No. 1R21AI097963-01A1 and 1R01AI101274-01A1 to J.D.K.), seed funds from the Department of Pathology and Laboratory Medicine, Rhode Island Hospital, the National Natural Science Foundation of China (Grant No. 30671581 to JJ. L. and 31472188 to ZQ. F.).
We thank all the staff from Shanghai Veterinary Research Institute involved in buffalo transportation, care and assistance during the perfusion. The authors would also like to thank Ms Youngeun Choi for carefully reviewing the manuscript with insightful questions.
Footnotes
All authors reported no conflicts of interest.
A subset of the data (abstract#1224) were orally presented at the 65th ASTMH annual meeting (Nov 13–17, 2016) in Atlanta, GA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sturrock RF. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem Inst Oswaldo Cruz. 2001;96(Suppl):17–27. doi: 10.1590/s0074-02762001000900003. [DOI] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 4.King CH. The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies. Washington (DC): The National Academies Press; 2011. Schistosomiasis: challenges and opportunities; pp. 323–42. [PubMed] [Google Scholar]
- 5.Jia TW, Zhou XN, Wang XH, Utzinger J, Steinmann P, Wu XH. Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull World Health Organ. 2007;85:458–65. doi: 10.2471/BLT.06.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blas BL, Rosales MI, Lipayon IL, Yasuraoka K, Matsuda H, Hayashi M. The schistosomiasis problem in the Philippines: a review. Parasitol Int. 2004;53:127–34. doi: 10.1016/j.parint.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Qian YJ, Li SZ, Xu J, et al. Potential schistosomiasis foci in China: a prospective study for schistosomiasis surveillance and response. Acta Trop. 2015;141:342–8. doi: 10.1016/j.actatropica.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Zhu C, Shi Y, et al. Surveillance of Schistosoma japonicum infection in domestic ruminants in the Dongting Lake region, Hunan province, China. PLoS One. 2012;7:e31876. doi: 10.1371/journal.pone.0031876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olveda DU, Li Y, Olveda RM, et al. Bilharzia in the Philippines: past, present, and future. Int J Infect Dis. 2014;18:52–6. doi: 10.1016/j.ijid.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Gray DJ, Williams GM, Li Y, McManus DP. Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS One. 2008;3:e4058. doi: 10.1371/journal.pone.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McManus DP, Bartley PB. A vaccine against Asian schistosomiasis. Parasitol Int. 2004;53:163–73. doi: 10.1016/j.parint.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Li YS, McManus DP, Lin DD, et al. The Schistosoma japonicum self-cure phenomenon in water buffaloes: potential impact on the control and elimination of schistosomiasis in China. Int J Parasitol. 2014;44:167–71. doi: 10.1016/j.ijpara.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Gray DJ, Li YS, Williams GM, et al. A multi-component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: design and baseline results of a 4-year cluster-randomised intervention trial. Int J Parasitol. 2014;44:659–68. doi: 10.1016/j.ijpara.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Inobaya MT, Olveda RM, Tallo V, et al. Schistosomiasis mass drug administration in the Philippines: lessons learnt and the global implications. Microbes Infect. 2015;17:6–15. doi: 10.1016/j.micinf.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 15.You H, McManus DP. Vaccines and diagnostics for zoonotic schistosomiasis japonica. Parasitology. 2015;142:271–89. doi: 10.1017/S0031182014001310. [DOI] [PubMed] [Google Scholar]
- 16.Gobert GN, Stenzel DJ, Jones MK, Allen DE, McManus DP. Schistosoma japonicum: immunolocalization of paramyosin during development. Parasitology. 1997;114(Pt 1):45–52. doi: 10.1017/s0031182096008001. [DOI] [PubMed] [Google Scholar]
- 17.Lanar DE, Pearce EJ, James SL, Sher A. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986;234:593–6. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- 18.Flanigan TP, King CH, Lett RR, Nanduri J, Mahmoud AA. Induction of resistance to Schistosoma mansoni infection in mice by purified parasite paramyosin. J Clin Invest. 1989;83:1010–4. doi: 10.1172/JCI113942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima S, Janecharut T, Hata H, Niimura M. Role of a mouse monoclonal IgE antibody in passive transfer of immunity to Schistosoma japonicum infection. Mem Inst Oswaldo Cruz. 1987;82(Suppl 4):237–41. doi: 10.1590/s0074-02761987000800045. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez BL, Kurtis JD, Wiest PM, et al. Paramyosin: a candidate vaccine antigen against Schistosoma japonicum. Parasite Immunol. 1996;18:49–52. doi: 10.1046/j.1365-3024.1996.d01-4.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Nara T, Zeng X, et al. Vaccination of domestic pig with recombinant paramyosin. against Schistosoma japonicum in China. Vaccine. 2000;18:2142–6. doi: 10.1016/s0264-410x(99)00541-1. [DOI] [PubMed] [Google Scholar]
- 22.McManus DP, Wong JY, Zhou J, et al. Recombinant paramyosin (rec-Sj-97) tested for immunogenicity and vaccine efficacy against Schistosoma japonicum in mice and water buffaloes. Vaccine. 2001;20:870–8. doi: 10.1016/s0264-410x(01)00405-4. [DOI] [PubMed] [Google Scholar]
- 23.Taylor MG, Huggins MC, Shi F, et al. Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine. 1998;16:1290–8. doi: 10.1016/s0264-410x(98)00055-3. [DOI] [PubMed] [Google Scholar]
- 24.Jiz MA, Wu H, Olveda R, Jarilla B, Kurtis JD. Development of Paramyosin as a Vaccine Candidate for Schistosomiasis. Front Immunol. 2015;6:347. doi: 10.3389/fimmu.2015.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenstra T, Acosta LP, Wu HW, et al. T-helper-2 cytokine responses to Sj97 predict resistance to reinfection with Schistosoma japonicum. Infect Immun. 2006;74:370–81. doi: 10.1128/IAI.74.1.370-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiz M, Friedman JF, Leenstra T, et al. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun. 2009;77:2051–8. doi: 10.1128/IAI.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McManus DP. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol. 2005;27:297–308. doi: 10.1111/j.1365-3024.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- 28.Jiz M, Wu HW, Meng R, et al. Pilot-scale production and characterization of paramyosin, a vaccine candidate for schistosomiasis japonica. Infect Immun. 2008;76:3164–9. doi: 10.1128/IAI.00409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusconi F, Valton E, Nguyen R, Dufourc E. Quantification of sodium dodecyl sulfate in microliter-volume biochemical samples by visible light spectroscopy. Anal Biochem. 2001;295:31–7. doi: 10.1006/abio.2001.5164. [DOI] [PubMed] [Google Scholar]
- 30.Rohl CA, Baldwin RL. Comparison of NH exchange and circular dichroism as techniques for measuring the parameters of the helix-coil transition in peptides. Biochemistry (Mosc) 1997;36:8435–42. doi: 10.1021/bi9706677. [DOI] [PubMed] [Google Scholar]
- 31.Zhou NE, Kay CM, Hodges RS. Synthetic model proteins. Positional effects of interchain hydrophobic interactions on stability of two-stranded alpha-helical coiled-coils. J Biol Chem. 1992;267:2664–70. [PubMed] [Google Scholar]
- 32.Dar P, Kalaivanan R, Sied N, et al. Montanide ISA 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle. Vaccine. 2013;31:3327–32. doi: 10.1016/j.vaccine.2013.05.078. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Shi F, Shen W, et al. Vaccination of sheep against Schistosoma japonicum with either glutathione S-transferase, keyhole limpet haemocyanin or the freeze/thaw schistosomula/BCG vaccine. Vet Parasitol. 1995;58:301–12. doi: 10.1016/0304-4017(94)00735-u. [DOI] [PubMed] [Google Scholar]
- 34.Xu S, Shi F, Shen W, et al. Vaccination of bovines against Schistosomiasis japonica with cryopreserved-irradiated and freeze-thaw schistosomula. Vet Parasitol. 1993;47:37–50. doi: 10.1016/0304-4017(93)90174-l. [DOI] [PubMed] [Google Scholar]
- 35.Tsang VC. New methods of purifying and identifying parasitic diagnostic antigens: a review of the systematic and quantitative approach with the Schistosoma model. Vet Parasitol. 1982;10:171–9. doi: 10.1016/0304-4017(82)90022-x. [DOI] [PubMed] [Google Scholar]
- 36.Alapati A, Kapa SR, Jeepalyam S, Rangappa SM, Yemireddy KR. Development of the body condition score system in Murrah buffaloes: validation through ultrasonic assessment of body fat reserves. J Vet Sci. 2010;11:1–8. doi: 10.4142/jvs.2010.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hady PJ, Domecq JJ, Kaneene JB. Frequency and precision of body condition scoring in dairy cattle. J Dairy Sci. 1994;77:1543–7. doi: 10.3168/jds.S0022-0302(94)77095-8. [DOI] [PubMed] [Google Scholar]
- 38.Xiong NN, Chen YN, Zhou J, Tang QL, Chen HL, Ning KJ. Determination of blood pyhsiological and biochemical indexes of Wandong cattle. Journal of Anhui Science and Technology University. 2014;28:19–22. [Google Scholar]
- 39.He BX, Su WW, Ma JJ, et al. Nanning suburban dairy buffalo and cattle liver funtion status survey. Chinese Journal of Veterinary Medicine. 2007;43:26–7. [Google Scholar]
- 40.Shi F, Zhang Y, Lin J, et al. Field testing of Schistosoma japonicum DNA vaccines in cattle in China. Vaccine. 2002;20:3629–31. doi: 10.1016/s0264-410x(02)00398-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang TP, Vang Johansen M, Zhang SQ, et al. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005;96:198–204. doi: 10.1016/j.actatropica.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Cao ZG, Zhao YE, Lee Willingham A, Wang TP. Towards the Elimination of Schistosomiasis japonica through Control of the Disease in Domestic Animals in The People’s Republic of China: A Tale of over 60 Years. Adv Parasitol. 2016;92:269–306. doi: 10.1016/bs.apar.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Dong GD, Liu JM, et al. Elimination of schistosomiasis japonica from formerly endemic areas in mountainous regions of southern China using a praziquantel regimen. Vet Parasitol. 2015;208:254–8. doi: 10.1016/j.vetpar.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Angeles JM, Leonardo LR, Goto Y, et al. Water buffalo as sentinel animals for schistosomiasis surveillance. Bull World Health Organ. 2015;93:511–2. doi: 10.2471/BLT.14.143065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson-Crispi KA, Mallard BA. Type 1 and type 2 immune response profiles of commercial dairy cows in 4 regions across Canada. Can J Vet Res. 2012;76:120–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Hagan P, Ndhlovu PD, Dunne DW. Schistosome immunology: more questions than answers. Parasitol Today. 1998;14:407–12. doi: 10.1016/s0169-4758(98)01325-8. [DOI] [PubMed] [Google Scholar]
- 47.Medhat A, Shehata M, Bucci K, et al. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobium adult worm antigens correlates with lack of reinfection after treatment. J Infect Dis. 1998;178:512–9. doi: 10.1086/515630. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Fu Z, Feng X, et al. Comparison of worm development and host immune responses in natural hosts of Schistosoma japonicum, yellow cattle and water buffalo. BMC Vet Res. 2012;8:25. doi: 10.1186/1746-6148-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McWilliam HE, Piedrafita D, Li Y, et al. Local immune responses of the Chinese water buffalo, Bubalus bubalis, against Schistosoma japonicum larvae: crucial insights for vaccine design. PLoS Negl Trop Dis. 2013;7:e2460. doi: 10.1371/journal.pntd.0002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trigona WL, Hirano A, Brown WC, Estes DM. Immunoregulatory roles of interleukin-13 in cattle. J Interferon Cytokine Res. 1999;19:1317–24. doi: 10.1089/107999099312993. [DOI] [PubMed] [Google Scholar]
- 51.Estes DM. Regulation of IgA responses in cattle, humans and mice. Vet Immunol Immunopathol. 2010;138:312–7. doi: 10.1016/j.vetimm.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Xiao Y, Chen HY, Wang Y, et al. Large-scale production of foot-and-mouth disease virus (serotype Asia1) VLP vaccine in Escherichia coli and protection potency evaluation in cattle. BMC Biotechnol. 2016;16:56. doi: 10.1186/s12896-016-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.