Cardiac dysfunction and myocellular injury from cancer therapeutics are identified by reductions in left ventricular ejection fraction (LVEF) or greater than 15% deteriorations in myocardial strain.1 Myocardial strain may deteriorate due to increases in LV end-systolic volume (LVESV), reductions in LV end-diastolic volume (EDV), or both. Decreases in LVEDV due to hypovolemia from poor PO oral intake, emesis, or myocardial loss2 do occur during cancer treatment. We sought to determine the frequency by which decrements in myocardial strain were mediated by decreases in LVEDV versus increases in LVESV in patients receiving potentially cardiotoxic chemotherapy.
The study was approved by the local institutional review board and participants provided witnessed, written informed consent. Cardiovascular magnetic resonance (CMR) examinations were performed on a 1.5-T Siemens Avanto scanner <6 hours prior to chemotherapy administration both before and three months after initiation of cancer treatment. Left ventricular volumes, LVEF, LV mass, relative wall thickness, and mid-wall Eulerian circumferential strain (ECC) were calculated from a series of LV short axis white-blood cine stacks and a mid-cavity short axis grid tagged image.3 Additionally, global longitudinal strain (GLS) was assessed from high temporal resolution 2- and 4-chamber cine views in a subset of participants (n=34). Intra-observer variability was performed on 28 blinded, unpaired scans for LVEDV (Δ=1.8±1.4ml [p=0.21] with correlation of r=0.98 [p<0.001 for test against r=0.80]) and LVESV (Δ-0.47±1.3ml [p=0.73] with correlation of r=0.94 [p=0.003 for test against r=0.80]. Thresholds of change were defined a priori (ΔLVEDV ≥-10ml and ΔLVESV ≥5ml) and were >5× our analysis variability. Arterial Elastance index (EaI) was calculated as an assessment of afterload on ECC strain.4 All analyses were blinded to study visit and measures from other CMR examinations. Paired Student's t-tests compared changes in LV measures.
The study population included 101 participants (70% women, 82% white) that were aged 50±15 years and had hypertension (39%), diabetes (15%), hyperlipidemia (26%), coronary artery disease (8%), or a history of smoking (28%). Primary cancer diagnoses included breast cancer (50%), leukemia (12%), lymphoma (32%), or other soft tissue malignancy (7%). Chemotherapeutic agents included an admixture of anthracyclines (71%), alkylating agents (53%), antimicrotubule agents (37%), and tyrosine-kinase inhibitors (44%) with 12 participants receiving an anthracycline followed by trastuzumab.
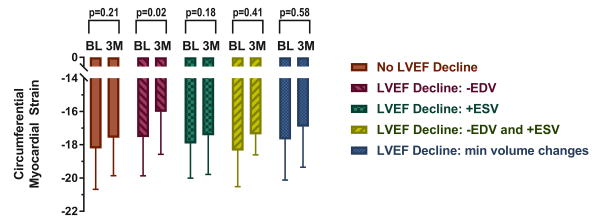
Overall, ECC strain declined from -17.99 to -17.23 (p=0.0052) concurrent with LVEF declines of 59.2% to 56.7% (p=0.0002). No LVEF change was observed in 39 participants. Sixty-two participants had an LVEF decline mediated by LVEDV declines (n=16), LVESV increases (n=35), combined LVEDV and LVESV changes (n=5), or LV volume changes below the thresholds (n=6). We observed an association between ECC strain deteriorations in those with decreases in LVEDV (p=0.02; Figure). In parallel with ECC changes, GLS was nominally associated with a 3-month decline (-15.44 to -14.79, p=0.069). GLS changes were associated with LVEDV changes (r=-0.36, p=0.03) and were nominally associated with LVEF changes (r=0.32, p=0.06) but not with LVESV changes (r=0.15, p=0.39). No changes were observed in LV mass index (p=0.23), relative wall thickness (p=0.65), or EaI (p=0.69). EaI was associated with changes in GLS (r=0.37, p=0.03) but not ECC (r=0.03, p=0.78).
Figure. Mid-wall circumferential myocardial strain measured with CMR in cancer patients prior to (BL) and 3 months (3M) after initiation of chemotherapy categorized by underlying cause for left ventricular ejection fraction (LVEF) decline.
Participants with a decline in LVEF due to a >5ml decrease in end diastolic volume exhibited subclinical deteriorations in myocardial strain. Myocardial strain changes were not observed in any other subgroup, including those with end systolic volume changes.
Three months after receipt of potentially cardiotoxic chemotherapy, up to 16% of individuals may experience a deterioration in myocardial strain mediated by a decline in LVEDV rather than an increase in LVESV without early changes in myocardial tissue volume. Our null findings of the effects of EaI on ECC strain suggest that afterload is not a mechanism responsible for ECC changes early after chemotherapy. Similar to those undergoing dialysis or with intravascular volume depletion due to blood donations,5 our data suggests LVEDV mediated relative strain changes of 9% may occur after chemotherapy causing a reduced preload. We have provided limited CMR-derived GLS data due to evolution of faster imaging sequences that occurred during the study. Although our subset was not powered to find differences, we were able to identify nominal associations of afterload-sensitive GLS change early after initiating potentially cardiotoxic chemotherapy. Continued work is needed to understand the role of CMR based strain measures in evaluating patients receiving potentially cardiotoxic chemotherapy.1
The data from this study underscore the importance of reviewing LV volume changes when interpreting LV strain deteriorations after receipt of cardiotoxic chemotherapy. One may not want to withhold potentially curative chemotherapy in an individual that may only need intravascular volume resuscitation or initiate cardioprotective medications such as beta-blockers or and angiotensin converting enzyme inhibitors (either of which could promote hypotension) in an individual with an already reduced LV preload and intravascular volume.
Acknowledgments
Sources of Funding: This research was supported in part by National Institutes of Health (NIH) grants R33CA12196, R01HL118740, R01CA167821 and the Susan G. Komen Foundation (BCTR07007769).
Footnotes
Conflict of Interest Disclosures: Marie-Pierre Jolly is an employee of Siemens Healthcare.
References
- 1.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Scully RE, Stevenson KE, Franco VI, Neuberg DS, Colan SD, Silverman LB, Moslehi JJ, Cheng S, Sallan SE. Hearts too small for body size after doxorubicin for childhood ALL: Grinch syndrome. J Clin Oncol. 2014;32:10021–10021. [Google Scholar]
- 3.Drafts BC, Twomley KM, D'Agostino R, Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int. 2013;2013:235194. doi: 10.1155/2013/235194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JO, Shin DH, Cho SW, Bin Song Y, Kim JH, Kim YG, Lee SC, Park SW. Effect of preload on left ventricular longitudinal strain by 2D speckle tracking. Echocardiography. 2008;25:873–879. doi: 10.1111/j.1540-8175.2008.00707.x. [DOI] [PubMed] [Google Scholar]