Abstract
Background and Purpose
Targeting the prostacyclin/IP receptor to reduce stroke injury has been hindered by the lack of selective drugs. MRE-269 is the active metabolite of selexipag showing a very high selectivity toward the IP receptor. Selexipag has been recently approved for clinical use in pulmonary hypertension. We hypothesized that post-ischemic treatment with MRE-269 provides long-lasting neuroprotection with improved neurological outcomes in a clinically relevant rat stroke model.
Methods
Aged male Sprague-Dawley rats underwent transient middle cerebral artery occlusion (MCAO) and were randomly selected to receive either vehicle or MRE-269 (0.25 mg/kg) intravenously starting at 4.5h post-ischemia. Accelerating rotarod and adhesive removal tests were conducted before and at 3, 7, 14 and 21 days after stroke. Infarct volume was quantified by MRI at 48h and 21 days post-MCAO. In parallel experiments, cerebral cortex samples from stroke and non-stroke sides from vehicle- and MRE-269-treated groups were collected at 18h post-MCAO for molecular biology analyses.
Results
Quantitative MRI data showed that post-ischemic MRE-269 treatment significantly reduced infarct volume compared with vehicle-treated rats at both 48 h and three weeks after stroke. MRE-269 treatment resulted in a significant long-term recovery in both locomotor and somatosensory functions following MCAO, which was associated with a reduced weight loss in animals receiving the IP receptor agonist. Post-ischemic MRE-269 treatment reduced pro-inflammatory cytokines/chemokines and oxidative stress. Damage to the blood-brain barrier, as assessed by extravasation of immunoglobulin G to the ischemic brain, was significantly reduced by MRE-269, which was associated with a reduction in matrix metalloproteinase-9 (MMP-9) activity in the brain of stroked aged rats given the IP agonist at 4.5h after ischemia onset.
Conclusions
Our data suggest that targeting the IP receptor with MRE-269 is a novel strategy to reduce cerebral ischemia injury and promote long-term neurological recovery in ischemic stroke.
Keywords: IP receptor, middle cerebral artery occlusion, MRE-269, neuroinflammation, oxidative stress, blood-brain barrier, matrix metalloproteinase-9
Introduction
Prostacyclin, also known as prostaglandin I2 (PGI2), is an arachidonic acid-derived eicosanoid, which is predominantly produced by the endothelium1. PGI2 induces vasodilation, is a potent inhibitor of platelet aggregation, and reduces microvascular permeability1. PGI2 IP receptor deficient (IP−/−) mice displayed exacerbated neuronal death following brain ischemia2, 3. In addition, PGI2 analogs significantly reduced ischemic brain injury2, 4–6. However, the use of PGI2 and known analogs have limited therapeutic value due to chemical instability, rapid metabolism (t½~ 4 min)1, as well as lack of selectivity for the IP receptor resulting in unsafe side effects7. Kuwano et al. discovered a novel, brain-permeable, and highly selective IP agonist coded as NS-304 (selexipag), a pro-drug of the active form termed MRE-269 that is most active at a low nM range7, 8. Pharmacokinetics in rats, dogs, and humans showed a significantly improved t½ of 6–8h7, 8. After successful clinical trials9, 10, selexipag/MRE-269 has FDA approval for pulmonary hypertension.
In an animal model of excitotoxic brain injury, MRE-269 was found to exert neuroprotective effects11, which is a significant finding since excitotoxicity contributes to neuronal death in stroke. There is very limited information regarding the protective mechanisms and the potential to target the IP receptor pathway to reduce stroke injury and improve long-term neurological recovery. Here we hypothesized that post-ischemic treatment with MRE-269 will provide long-lasting neuroprotection with improved neurological outcomes after ischemic stroke. Utilizing an aged rat ischemic stroke model, we found that MRE-269 significantly reduced infarct volume and dramatically improved long-term recovery of both locomotor and somatosensory functions following MCAO. Neuroprotection of MRE-269 in ischemic stroke was through a reduction in pro-inflammatory cytokines/chemokines and oxidative stress. Moreover, post-ischemic treatment with MRE-269 significantly reduced brain MMP-9 activity and protected against stroke-induced BBB disruption. Collectively, these data suggest that targeting the IP receptor with MRE-269 is a novel strategy to reduce cerebral ischemia injury and promote long-term neurological recovery in ischemic stroke.
Materials and Methods
A detailed description of all the experiments is provided as an online-only Data Supplement. A brief description of the main methodology is provided below.
Animals and middle cerebral artery occlusion (MCAO) model
All experimental procedures were in accordance with the National Institutes of Health guidelines and protocols approved by the University of Florida Institutional Animal Care and Use Committee. An a priori sample size calculation was performed using the G*Power v.3.1.3 software12 and detailed in Supplemental Methods. A total of 57 aged male Sprague Dawley rats (18–20 months, Hilltop Laboratories, Scottdale, PA, USA) were used in this study and randomly assigned to treatment groups. Transient MCAO for 90 min was induced using an intraluminal silicone-coated filament method as previously described by our group13.
Experimental design and drug administration
For the intravenous administration of vehicle or MRE-269, a catheter was inserted into the right femoral vein of ischemic rats at the time of MCAO surgery. MRE-269 ([4-[(5,6-diphenylpyrazinyl)(1-methylethyl)amino]butoxy]-acetic acid; Cat. No. 10010412; Cayman Chemical, Ann Arbor, MI, USA) was dissolved in dimethyl sulfoxide (DMSO) and then diluted in sterile saline. For the infarct size and neurobehavioral tests experiments, twenty-six rats underwent transient MCAO and were randomly assigned to vehicle or treatment group with administration of 1% DMSO in saline (n=13) or MRE-269 (0.25 mg/kg, n=13) starting at 4.5 h post-MCAO. Additional doses were given every 12 h for the first 48 h, and then one injection daily for 7 days post-MCAO. The MRE-269 dose and treatment schedule was based on our preliminary findings in young rats (3–4 months) showing that MRE-269 (0.1–0.5 mg/kg, i.v.) given at 1.5 h after stroke onset produced a dose-dependent reduction in infarct volume as measured by TTC staining at 48 h (data not shown). The optimal dose of 0.25 mg/kg was therefore used in the present study in aged rats.
For the biochemical experiments, 31 rats were randomly assigned to the following groups: sham-operation (n=5), vehicle (1% DMSO, n=13) or MRE-269 (0.25 mg/kg, n=13). Drug or vehicle were given intravenously starting at 4.5 h after stroke and an additional dose was given at 12 h after stroke onset. Animals were sacrificed at 18 h post-MCAO and perfused with ice-cold saline. Samples from the ipsilateral and contralateral cerebral cortices were obtained for RNA isolation, immunoblotting, and lipid peroxidation analyses.
MRI, image analysis, and behavioral tests
At 48 h and 21 days after MCAO, rats treated with vehicle or MRE-269 (0.25 mg/kg) were imaged in a Bruker 4.7T MRI scanner. Behavioral tests were performed pre-MCAO and at 3, 7, 14 and 21 days post-MCAO by an independent investigator blinded to the experimental groups. Somatosensory deficits were assessed using the adhesive removal test as described previously14. Locomotor impairments were assessed with the accelerating rotarod as described previously15. Five rats in the vehicle and 4 rats in the MRE-269 groups died during the 21 days post-MCAO period. Further details are described in the Supplemental Methods.
RNA extraction and real-time PCR
Total RNA isolation from cortical tissue, cDNA synthesis, and real-time PCR were performed as described previously by our group13. Results are presented as normalized expression relative to sham-operated group. Additional details are described in the Supplemental Methods.
Immunoblotting
Details of the immunoblotting technique, antibody incubations, and signal detection are provided in the Supplemental Methods.
Determination of 8-iso-prostaglandin F2α
Levels of 8-iso-prostaglandin F2α (8-iso-PGF2α), a highly sensitive biomarker of oxidative stress, were measured using an ELISA kit (Cat. No. 516351, Cayman Chemical, Ann Arbor, MI) following the manufacturer’s protocol.
Immunoglobulin G (IgG) extravasation and MMP-9 activity assay
The permeability of the BBB was examined by the extravasation of IgG from the blood into the brain parenchyma using an IgG ELISA kit (Cat. No. E-25G; Portland, OR) according to the manufacturer’s instructions. The MMP-9 activity in cortical tissue was measured using a fluorescence resonance energy transfer (FRET) peptide immunoassay as described in our previous studies13, 16.
Statistical Analysis
All values were expressed as mean ± SEM. Statistical analysis was performed by unpaired Student’s t-test for comparisons between two groups, one-way or two-way ANOVA followed by Bonferroni posttests for comparisons of multiple groups. GraphPad Prism 6 was used to conduct data analysis, and P < 0.05 was considered statistically significant.
Results
Long-lasting neuroprotective efficacy of delayed MRE-269 administration in aged rats following transient MCAO
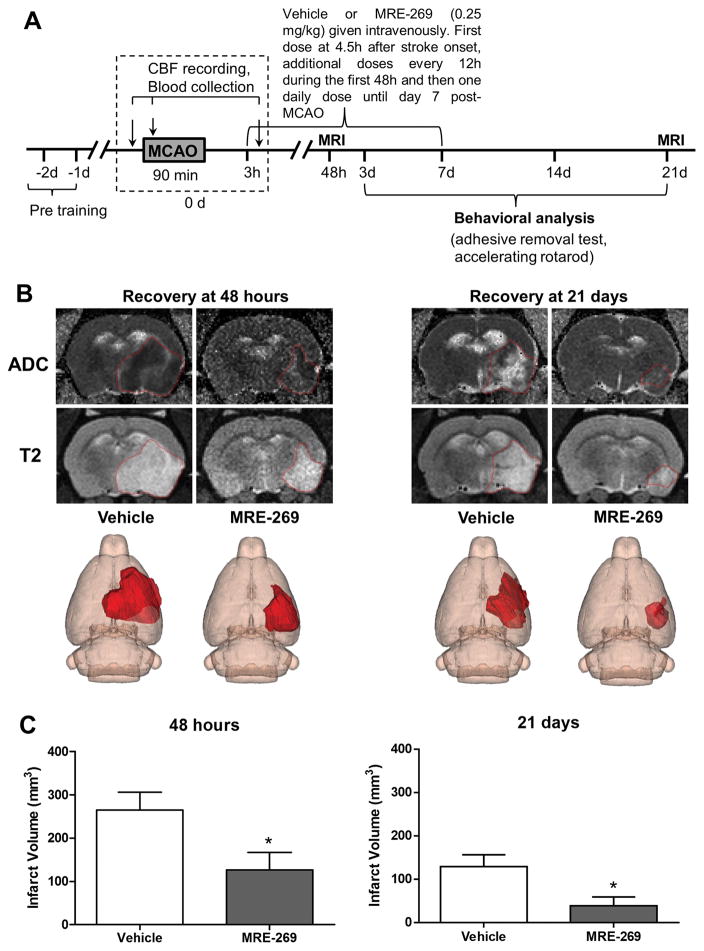
Since age is the single most important risk factor in stroke17, it is important to know whether post-ischemic treatment with MRE-269 is able to confer long-lasting neuroprotection in aged rats subjected to ischemic stroke. MRI-based infarct size analysis was performed at 48 h (acute phase) and at 21 days following stroke. As shown in Figure 1, delayed treatment with MRE-269 at 0.25 mg/kg resulted in a remarkable reduction in infarct volume, which was sustained for three weeks after stroke.
Figure 1. Delayed treatment with the highly selective IP receptor agonist, MRE-269, significantly reduced infarct volume in aged rats subjected to ischemic stroke.
A) Timeline of MRE-269 administration, MRI scanning and behavioral testing. B) Representative ADC, T2-weighted MRI scans and 3D reconstruction images of the ischemic lesion in vehicle- and MRE-269-treated rats are shown in panel B. The ischemic area was marked with a discontinuous red line in ADC and T2 images and depicted as a dark red area in the right hemisphere in 3D maps. Quantification of infarct volume at 48 h and 21 days (C) showed a marked neuroprotective effect of delayed MRE-269 administration. Data were expressed as mean ± SEM, *P<0.05 versus vehicle. At 48 h, n=11 for vehicle and n=10 for MRE-269; At 21 days, n=8 for vehicle and n=9 for MRE-269, respectively.
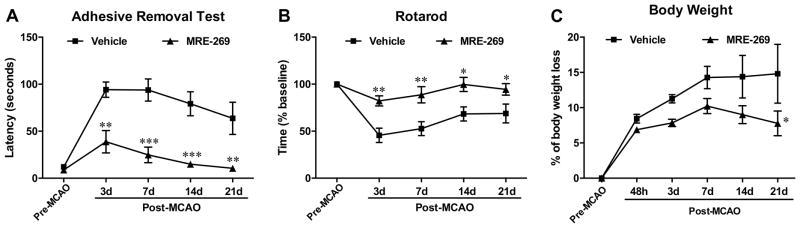
MRE-269 treatment resulted in a significant long-term recovery in both somatosensory (Figure 2A) and locomotor functions (Figure 2B) following MCAO compared to the vehicle group, which was associated with less body weight loss (Figure 2C) in animals receiving the IP receptor agonist. To determine if there was any association between major physiological parameters and the neuroprotective effect of MRE-269 in ischemic stroke, physiological variables including blood pH, blood oxygen (PO2), carbon dioxide saturation (PCO2), ion concentrations, blood glucose, hematocrit, hemoglobin, rectal temperature, and cerebral blood flow (CBF) were measured 15 min before and after MCAO respectively, as well as at 15 min after initial dose of vehicle (1% DMSO) or MRE-269 (0.25 mg/kg) injection. As shown in the Supplemental Table I and Supplemental Figure I, we did not observe a significant difference in these physiological parameters, including CBF, at any time point between the vehicle- and MRE-269-treated groups. These data suggest that changes in major physiological variables are unlikely to explain the neuroprotective effects of MRE-269 in ischemic stroke.
Figure 2. Effect of MRE-269 on neurological function and body weight loss in aged rats subjected to transient MCAO.
The drug was given as detailed in the legend of Fig. 1, and neurobehavioral analyses and body weight measurement were performed at pre- and post-MCAO. The same animals were MRI-scanned at 48 h and at 21 days (data presented in Fig. 1). Delayed MRE-269 treatment significantly improved long-term neurological outcomes assessed by adhesive removal test (A) and rotarod (B) compared to the vehicle group from day 3 to day 21 after MCAO. C) Bar graphs showing body weight loss was continuously increased from 48 h to day 21 after MCAO in the vehicle group, but MRE-269 treatment significantly attenuated body weight loss after one week following MCAO. Data in panels A to C were expressed as mean ± SEM, *P<0.05, **P<0.01 and ***P<0.001 versus vehicle. Vehicle, n=8; MRE-269, n=9.
Effect of MRE-269 on cortical gene expression of cytokines and chemokines in aged rats following transient MCAO
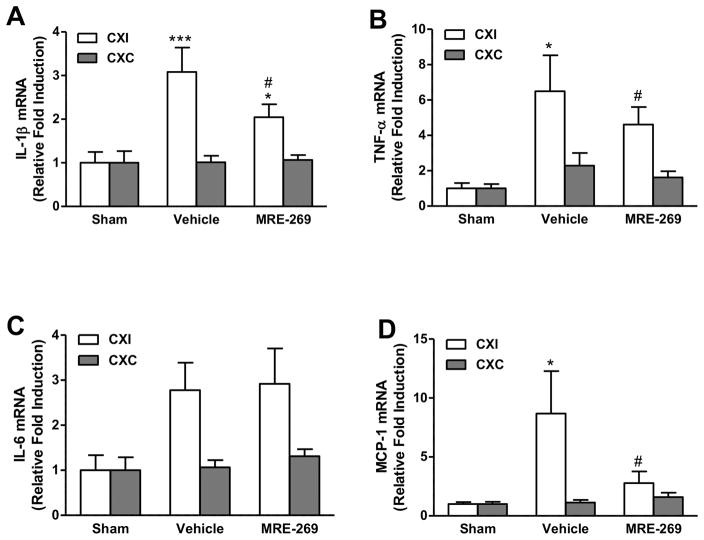
To examine whether the neuroprotective effect of MRE-269 in ischemic brain was through regulation of neuroinflammatory mediators, cortical gene expression of pro-inflammatory cytokines interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6 and chemokine monocyte chemoattractant protein-1 (MCP-1) were measured by quantitative RT-PCR in sham-operated, vehicle- and MRE-269-treated aged rats sacrificed at 18 h post-MCAO. Transient MCAO significantly increased mRNA expression of IL-1β (Figure 3A), TNF-α (Figure 3B), MCP-1 (Figure 3D), and trends toward a considerable increase in IL-6 (Figure 3C) in the ipsilateral cerebral cortex compared to the sham group. Post-ischemic treatment with MRE-269 significantly reduced the mRNA levels of IL-1β (Figure 3A), TNF-α (Figure 3B) and MCP-1 (Figure 3D) in the ipsilateral cerebral cortex compared to vehicle-treated rats. Taken together, these data suggest that delayed MRE-269 treatment can suppress transient MCAO-induced expression of pro-inflammatory cytokines IL-1β, TNF-α, and chemokine MCP-1 in the aged rat brain subjected to ischemic stroke.
Figure 3. Effect of MRE-269 on cortical gene expression of cytokines and chemokines in aged rats following transient MCAO.
Aged male rats underwent 90 min of MCAO and were randomly selected to receive either vehicle or MRE-269 (0.25 mg/kg; i.v.) given at 4.5 h and an additional dose at 12 h after stroke onset. Animals were sacrificed 18 h post-MCAO and perfused with ice-cold saline, the ipsilateral and contralateral cerebral cortex were saved for RNA extraction and western blot studies. Transcript levels of IL-1β (A), TNF-α (B), IL-6 (C), and MCP-1 (D) were determined by RT-qPCR. Data were expressed as mean ± SEM, *P<0.05, ***P<0.001 versus sham and #P<0.05 versus vehicle. Sham, n=5; Vehicle, n=12; MRE-269, n=13. CXI = cortex ipsilateral to stroke; CXC = cortex contralateral to stroke.
IP receptor activation with MRE-269 significantly reduced oxidative stress in aged rats following transient MCAO
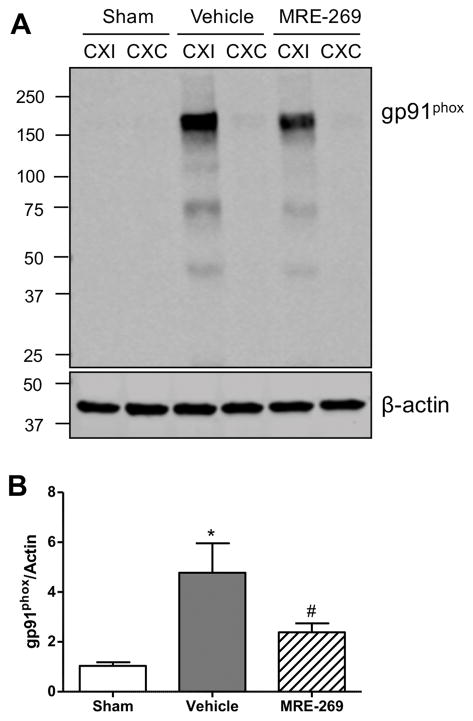
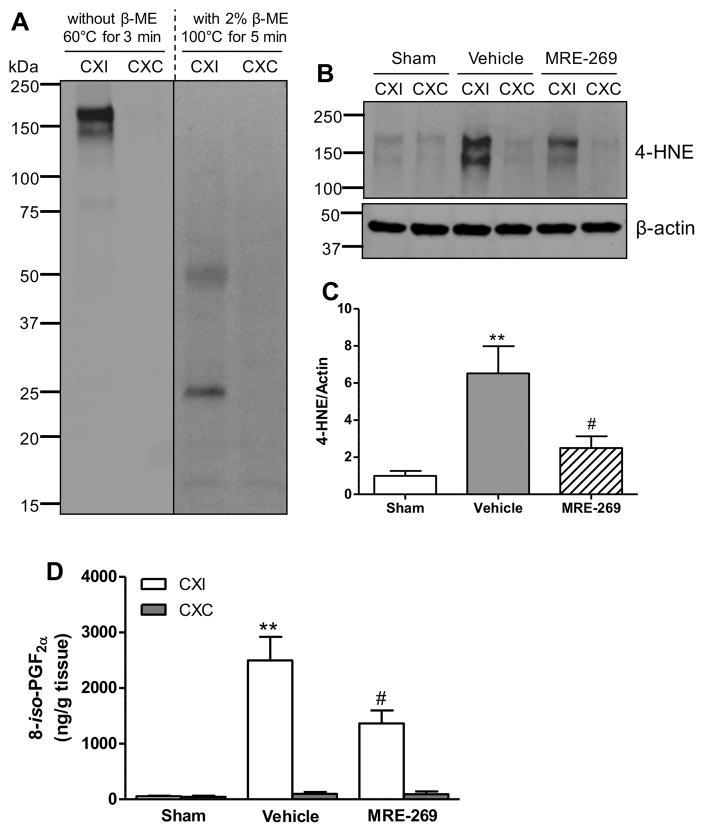
Next we examined the effects of MRE-269 on measures of oxidative damage to the brain tissue. We first determined the protein level of gp91phox (NOX2), a glycosylated subunit of NADPH oxidase (NOX), which is a major source of superoxide generation18. Immunoblot analyses showed a dramatic increase of the gp91phox subunit in the ipsilateral cortex of MCAO-treated aged rats at 18h post-MCAO compared to the sham group, and MRE-269 treatment significantly reduced the gp91phox levels induced by transient MCAO (Figure 4A and 4B). Also, we investigated the effects of delayed treatment with MRE-269 on lipid peroxidation, as assessed by quantification of 4-hydroxynonenal (4-HNE) and 8-iso-prostaglandin F2α (8-iso-PGF2α), two sensitive biomarkers of oxidative stress in ischemic brain damage19. As shown in Figures 5A–D, transient MCAO resulted in dramatic increases in both 4-HNE and 8-iso-PGF2α levels in the ischemic cerebral cortex. A 4.5–h delayed treatment with MRE-269 significantly reduced oxidative damage induced by ischemic stroke in aged rats.
Figure 4. MRE-269 reduced gp91phox protein level induced by transient MCAO in aged rats.
A) A representative immunoblot for gp91phox subunit in the cortex of sham-, vehicle- and MRE-269- treated groups. B) Densitometric analysis showed that transient MCAO significantly increased gp91phox protein level in the ischemic cortex compared to the sham group, and the gp91phox level was dramatically decreased in the MRE-269-treated rats compared to the vehicle group. Data were expressed as mean ± SEM, *P<0.05 versus sham and #P<0.05 versus vehicle. Sham, n=5; Vehicle, n=12; MRE-269, n=13. CXI = cortex ipsilateral to stroke; CXC = cortex contralateral to stroke.
Figure 5. MRE-269 reduced oxidative damage as assessed by levels of 4-HNE and 8-iso-PGF2α in aged rats following transient MCAO.
A) Immunoblot for 4-HNE under non-reduced and reduced conditions in the cortex of aged rat brain subjected to stroke, which shows better signal of 4-HNE under the non-reduced conditions. B) Immunoblot for 4-HNE in the cortex of sham-, vehicle- and MRE-269- treated rats. C) Transient MCAO significantly increased 4-HNE protein level in the ischemic cortex compared to the sham rats, and the 4-HNE level was dramatically decreased in the MRE-269-treated rats compared to the vehicle group. D) Delayed treatment with MRE-269 (0.25 mg/kg) significantly reduced 8-iso-PGF2α content in the ischemic cerebral cortex at 18 h following transient MCAO. Data were expressed as mean ± SEM, **P<0.01 versus sham and #P<0.05 versus vehicle. Sham, n=5; Vehicle, n=12; MRE-269, n=13. CXI = cortex ipsilateral to stroke; CXC = cortex contralateral to stroke.
Reduced BBB damage and MMP-9 activity in stroked aged rats given MRE-269
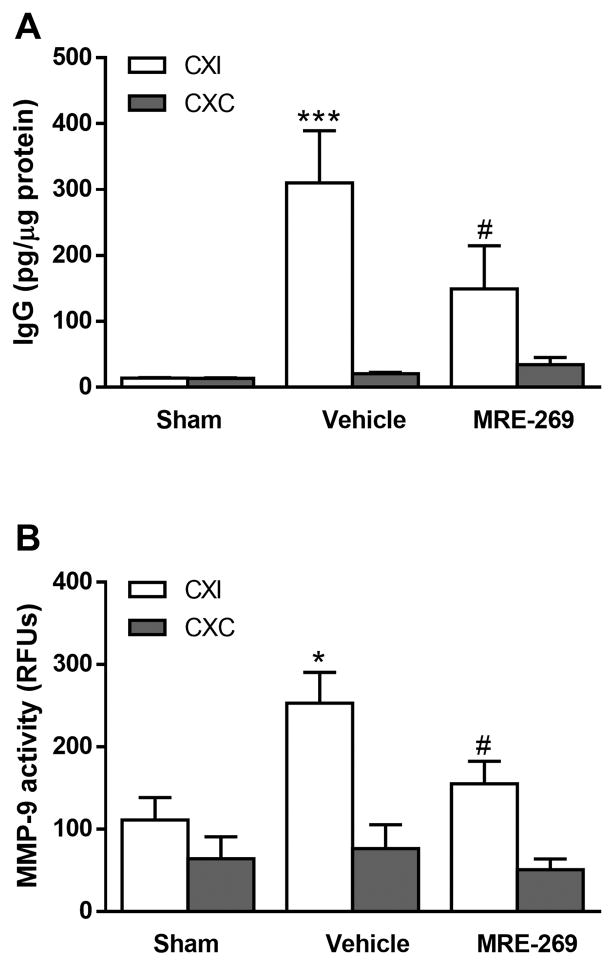
Since increased oxidative stress and pro-inflammatory cytokines contribute to BBB breakdown after stroke18, 20, 21, we next investigated the effects of MRE-269 on ischemia-induced BBB opening. At 18 h after ischemia, we observed a dramatic increase in IgG extravasation in the ipsilateral cerebral cortex of aged rats given the vehicle. Rats receiving MRE-269 (0.25 mg/kg; i.v.) at 4.5h after stroke onset had significantly less BBB damage compared with the vehicle group (Fig. 6A). Because increased MMP-9 activation after stroke is a key mediator of BBB disruption21–24, we next measured MMP-9 activity in the ipsilateral and contralateral cortices in both treatment groups. Ipsilateral MMP-9 activity was significantly increased in vehicle and MRE-269 groups when compared to either sham or their respective contralateral values (Fig. 6B). Post-ischemic treatment with MRE-269 significantly reduced MMP-9 activity in the ischemic brain as shown in Fig 6B.
Figure 6. MRE-269 reduced BBB damage and MMP-9 activity in aged rats following transient MCAO.
A) Ischemic stroke significantly increased BBB permeability as assessed by IgG extravasation, and treatment with MRE-269 (0.25 mg/kg; i.v.) at 4.5h after stroke onset significantly reduced the IgG extravasation into the ipsilateral cerebral cortex compared to vehicle rats. B) Activation of matrix metalloproteinase (MMP)-9 is a key pathological mechanism in stroke leading to BBB breakdown. Administration of MRE-269 (0.25 mg/kg; i.v.) significantly reduced stroke-induced MMP-9 activity in the ipsilateral cerebral cortex compared to vehicle group. Data were expressed as mean ± SEM, *P<0.05, ***P<0.001 versus sham ipsilateral and #P<0.05 versus vehicle ipsilateral. Sham, n=5; Vehicle, n=12; MRE-269, n=13. CXI = cortex ipsilateral to stroke; CXC = cortex contralateral to stroke.
Discussion
The present study demonstrated for the first time that a 4.5-h delayed administration of MRE-269, a highly specific and clinically used IP receptor agonist, provided sustained neuroprotection and improved long-term neurological recovery in aged rats subjected to ischemic stroke. Molecular studies indicated that MRE-269 reduced pro-inflammatory mediators such as IL-1β, TNF-α, and MCP-1, and reduced oxidative stress and BBB damage, which likely contributed to the observed neuroprotective effect. MRE-269 is safe in the clinical setting. Repurposing its use as a potential treatment for stroke is highly significant. The marked and long-lasting neuroprotection seen with a 4.5-h delayed administration of MRE-269 in aged rats add a significant translational value to our findings.
This is the first study to demonstrate the sustained neuroprotective effect of an IP receptor agonist given post-stroke in a clinically relevant model in aged rats. Moreover, the effects of MRE-269, a highly selective IP agonist used in the clinic, has never been studied in any animal model of brain injury. Another novel aspect of this study is our finding that a delayed administration of MRE-269 significantly reduces inflammatory mediators, oxidative stress, BBB damage, and MMP-9 activity in the post-ischemic brain.
Prostacyclin/IP receptor activation has potent vasodilatory effects1, which raises the possibility that increased brain perfusion could contribute to neuroprotection by IP agonists. However, low doses of PGI2 do not alter CBF in humans25. Consistent with these previous reports, we did not observe significant changes in CBF between vehicle and MRE-269 groups, which suggests that the neuroprotection by MRE-269 at a dose of 0.25 mg/kg is not due to improvement in microcirculatory reperfusion. At low doses, MRE-269 has no effect on mean arterial blood pressure or heart rate7.
It has been reported that IP receptor gene deletion exacerbated neurological deficits and infarct size in both transient MCAO and permanent MCAO model in mice2. Conversely, treatment with beraprost, an IP receptor agonist, significantly improved negative stroke outcomes in wild-type (WT) mice2 and reduced CA1 hippocampal neuronal death after global ischemia in aged mice26. MRE-269 has a much higher selectivity at low nM range and a longer half-life (6–8 h) compared to the classical IP receptor agonists such as beraprost and iloprost. More importantly, beraprost and iloprost also bind to other prostanoid receptors especially the EP3 receptor7, 8, potentially causing unsafe side effects such as marked hypotension, increased heart rate, impaired gastrointestinal function, and lung arterial contraction7, 27, 28, which limits their clinical use. As a highly specific and clinically available IP receptor agonist used in the treatment of pulmonary hypertension9, 10, our data for the first time demonstrated that delayed treatment with MRE-269 reduced infarct volume in aged rats subjected to transient MCAO, suggesting a potentially valuable application in the treatment of ischemic stroke.
Because age is the single most important risk factor in stroke17, it is of great clinical significance to know whether post-ischemic treatment with MRE-269 is able to confer long-lasting neuroprotection in aged rats subjected to ischemic stroke. Consistent with our findings in young rats, delayed treatment with MRE-269 (0.25 mg/kg) also exerted neuroprotective effects demonstrated by reduced infarct volume in aged rats subjected to transient MCAO. Importantly, sufficiently delayed drug administration is lacking in most experimental stroke studies which reduces their translational relevancy and may contribute to the discrepancy between the efficacy of pharmaceutical stroke treatment in animal studies and in the clinic. Also, considerable stroke research has been limited to the acute phase of focal ischemia injury in young animals rather than aged, which is less clinically relevant to most stroke populations in human. In this study, we tried to mimic a clinical setting by using aged rats tested for 3 weeks after MCAO to evaluate the efficacy of MRE-269 with an initial administration at 4.5 h post-occlusion. Delayed MRE-269 treatment resulted in a significant improvement of long-term recovery in both locomotor and somatosensory functions after MCAO. Collectively, our data provide a solid indication that post-ischemic intervention with MRE-269 is a promising neuroprotective therapy for ischemic stroke.
Ischemia/reperfusion triggers immune responses, excessive oxidative stress, adhesion molecule upregulation, and peripheral leukocyte recruitment, which ultimately cause inflammatory cell activation and infiltration. The activated inflammatory cells release many neuroinflammatory mediators including cytokines, chemokines, and matrix metalloproteinases (MMPs), thus, resulting in BBB disruption and neuronal cell death in ischemic stroke20. In this study, we found that stroke-induced neurobehavioral deficits and infarction were associated with upregulation of pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 as well as MCP-1 in the ipsilateral cerebral cortex of aged rats. As reported previously in a rat distal transient MCAO model, both IL-1β and TNF-α mRNA are induced in the ischemic cortex as early as 1 h after reperfusion, peaking between 3 to 6 h post-stroke, and remains elevated for up to 48 h after MCAO29. Administration of IL-1β to rats increases brain injury30, and IL-1β-deficient mice showed smaller infarct volume compared with WT mice31. Intracerebroventricular administration of recombinant TNF-α to spontaneously hypertensive rats 24 h before 80 or 160 min transient MCAO exacerbated infarct size and neurological deficit, and inhibition of TNF-α with a monoclonal antibody significantly reduced brain damage after ischemic stroke32. IL-6 is another pro-inflammatory cytokine that was detected as early as 3 h after stroke onset in rats with peak concentrations after 24 h and remained detectable for up to 14 days33. However, IL-6 deficient mice did not show improved neurological function and infarct volume after stroke compared with WT mice34. MCP-1 is one of the most commonly expressed chemokines that regulate migration and infiltration of monocytes/macrophages during neuroinflammation. Increased MCP-1 in the brain markedly exacerbated ischemic damage, which was correlated with inflammatory cell recruitment35. Mice deficient in CCR2 (CCR2-/-), the receptor for MCP-1, had a dramatic reduction in infarct size, BBB permeability, and edema following focal transient MCAO compared with WT mice. The protection seen in CCR2−/− mice was associated with a marked reduction in immune cell infiltration as well as reduced expression/production of pro-inflammatory cytokines such as IL-1β and TNF-α36. In line with these reports, our data also found that transient MCAO significantly increased mRNA expression of IL-1β, TNF-α, MCP-1, and trends toward a considerable increase of IL-6 in the ipsilateral cerebral cortex at 18 h post-stroke. Further findings indicated that delayed treatment with MRE-269 significantly reduced the mRNA levels of IL-1β, TNF-α and MCP-1, but not IL-6 induced by MCAO, which suggests that suppression of IL-1β, TNF-α and MCP-1 expression by MRE-269 may be one of the mechanisms underlying the neuroprotective effects of MRE-269 in ischemic stroke.
In addition to inflammatory cytokines/chemokines, oxidative stress also plays a critical role in the pathogenesis of ischemic stroke. In the CNS, there are several sources generating free radicals, including the mitochondria, xanthine oxidase, uncoupled nitric oxide synthase, and cyclooxygenases37. However, the NOX family of enzymes seems to be a major contributor to oxidative stress following ischemia. NOX2 (namely gp91phox) is a critical contributor to worse stroke outcomes since it is a major source of superoxide generation and a key contributor to ischemic injury38, 39. In this study, we found for the first time that neurological impairment and brain infarction were associated with a significant increase of gp91phox protein after stroke in the aged rat brain, and delayed MRE-269 treatment dramatically attenuated its increase. Previous studies have shown that activation of the IP receptor reduces gp91phox levels in peripheral endothelial cells40, 41. This is the first study to show reduced gp91phox levels by a PGI2 analog in stroke. Additionally, a dramatic increase in 8-iso-PGF2α and 4-HNE-modified proteins were observed in the ischemic cerebral cortex after stroke. MRE-269 significantly reduced both 8-iso-PGF2α and 4-HNE levels in the aged ischemic brain. Collectively, these findings suggest that the neuroprotective effect of MRE-269 in stroke may be partly through the reduction of oxidative stress induced by transient MCAO.
Stroke-induced pro-inflammatory cytokines and free radicals have been shown to exacerbate brain injury, in part, by increasing BBB permeability18, 20. Treatment with MRE-269 resulted in less BBB opening, which was associated with a significant reduction in ischemia-induced MMP-9 activation. While activation of the IP receptor has been shown to reduce ischemia-induced brain microvascular permeability42, 43, this is the first study showing that MRE-269 attenuates BBB damage after ischemic stroke likely by reducing MMP-9 activation.
In conclusion, we show that the delayed treatment with the highly selective IP receptor agonist, MRE-269, provided sustained protection, which correlated with a marked improvement in neurological function, reduction in infarct size, and reduced body weight loss in a stroke model in aged rats. Mechanistically, the downregulation of pro-inflammatory cytokines IL-1β, TNF-α and chemokine MCP-1, as well as the reduction of oxidative damage and BBB disruption by MRE-269 likely contributed to the observed neuroprotective effect. These data strongly suggest that targeting the IP receptor with MRE-269 is a novel strategy to reduce cerebral ischemia injury and promote long-term neurological recovery in ischemic stroke. Repurposing the use of selexipag/MRE-269 for ischemic stroke is of great translational relevance since this clinically approved drug has therapeutic potential to ameliorate ischemic brain injury.
Supplementary Material
Acknowledgments
We thank Dr. Kimberly E. Hawkins for her critical reading of the manuscript and Dr. Huadong Zeng for his assistance in using the MRI system.
Funding
This research was supported by a Grant-in-Aid from the American Heart Association (AHA; grant number 16GRNT31020048), the NIH (R01 NS065849 to ECJ), the McKnight Brain Institute, University of Florida, and the Thomas Maren Junior Investigator Award to ECJ.
Footnotes
Disclosures
None.
References
- 1.Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11. [PubMed] [Google Scholar]
- 2.Saleem S, Shah ZA, Maruyama T, Narumiya S, Dore S. Neuroprotective properties of prostaglandin i2 ip receptor in focal cerebral ischemia. Neuroscience. 2010;170:317–323. doi: 10.1016/j.neuroscience.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei G, Kibler KK, Koehler RC, Maruyama T, Narumiya S, Dore S. Prostacyclin receptor deletion aggravates hippocampal neuronal loss after bilateral common carotid artery occlusion in mouse. Neuroscience. 2008;156:1111–1117. doi: 10.1016/j.neuroscience.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karasawa Y, Komiyama H, Yoshida S, Hino N, Katsuura Y, Nakaike S, et al. Effect of ttc-909 on cerebral infarction following permanent occlusion of the middle cerebral artery in stroke prone spontaneously hypertensive rats. Journal of pharmacological sciences. 2003;91:305–312. doi: 10.1254/jphs.91.305. [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Takamatsu H, Kakiuchi T, Ohba H, Kataoka Y, Yokoyama C, et al. Neuroprotection by a central nervous system-type prostacyclin receptor ligand demonstrated in monkeys subjected to middle cerebral artery occlusion and reperfusion: A positron emission tomography study. Stroke. 2006;37:2830–2836. doi: 10.1161/01.STR.0000245088.60282.22. [DOI] [PubMed] [Google Scholar]
- 6.Takamatsu H, Tsukada H, Watanabe Y, Cui Y, Kataoka Y, Hosoya T, et al. Specific ligand for a central type prostacyclin receptor attenuates neuronal damage in a rat model of focal cerebral ischemia. Brain Res. 2002;925:176–182. doi: 10.1016/s0006-8993(01)03280-2. [DOI] [PubMed] [Google Scholar]
- 7.Kuwano K, Hashino A, Asaki T, Hamamoto T, Yamada T, Okubo K, et al. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-n-(methylsulfonyl )acetamide (ns-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther. 2007;322:1181–1188. doi: 10.1124/jpet.107.124248. [DOI] [PubMed] [Google Scholar]
- 8.Kuwano K, Hashino A, Noda K, Kosugi K, Kuwabara K. A long-acting and highly selective prostacyclin receptor agonist prodrug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-n-(methylsulfonyl )acetamide (ns-304), ameliorates rat pulmonary hypertension with unique relaxant responses of its active form, {4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}acetic acid (mre-269), on rat pulmonary artery. J Pharmacol Exp Ther. 2008;326:691–699. doi: 10.1124/jpet.108.138305. [DOI] [PubMed] [Google Scholar]
- 9.Skoro-Sajer N, Lang IM. Selexipag for the treatment of pulmonary arterial hypertension. Expert opinion on pharmacotherapy. 2014;15:429–436. doi: 10.1517/14656566.2014.876007. [DOI] [PubMed] [Google Scholar]
- 10.Asaki T, Kuwano K, Morrison K, Gatfield J, Hamamoto T, Clozel M. Selexipag: An oral and selective ip prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Journal of medicinal chemistry. 2015;58:7128–7137. doi: 10.1021/acs.jmedchem.5b00698. [DOI] [PubMed] [Google Scholar]
- 11.An Y, Belevych N, Wang Y, Zhang H, Nasse JS, Herschman H, et al. Prostacyclin mediates endothelial cox-2-dependent neuroprotective effects during excitotoxic brain injury. Journal of inflammation research. 2014;7:57–67. doi: 10.2147/JIR.S63205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 13.Frankowski JC, DeMars KM, Ahmad AS, Hawkins KE, Yang C, Leclerc JL, et al. Detrimental role of the ep1 prostanoid receptor in blood-brain barrier damage following experimental ischemic stroke. Sci Rep. 2015;5:17956. doi: 10.1038/srep17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 15.Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, et al. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins KE, DeMars KM, Yang C, Rosenberg GA, Candelario-Jalil E. Fluorometric immunocapture assay for the specific measurement of matrix metalloproteinase-9 activity in biological samples: Application to brain and plasma from rats with ischemic stroke. Molecular brain. 2013;6:14. doi: 10.1186/1756-6606-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American heart association prevention conference. Iv. Prevention and rehabilitation of stroke. Risk factors. Stroke. 1997;28:1507–1517. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 18.Tang XN, Cairns B, Kim JY, Yenari MA. Nadph oxidase in stroke and cerebrovascular disease. Neurol Res. 2012;34:338–345. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brault S, Martinez-Bermudez AK, Marrache AM, Gobeil F, Jr, Hou X, Beauchamp M, et al. Selective neuromicrovascular endothelial cell death by 8-iso-prostaglandin f2alpha: Possible role in ischemic brain injury. Stroke. 2003;34:776–782. doi: 10.1161/01.STR.0000055763.76479.E6. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 22.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type iv collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 24.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook PJ, Maidment CG, Dandona P, Hutton RA, James IM. The effect of intravenous epoprostenol (prostacyclin, pgi2) on cerebral blood flow and cardiac output in man. Br J Clin Pharmacol. 1983;16:707–711. doi: 10.1111/j.1365-2125.1983.tb02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakil H, Saleem S. Prostaglandin i2 ip receptor agonist, beraprost, prevents transient global cerebral ischemia induced hippocampal ca1 injury in aging mice. J Neurol Disord. 2014;2:1000174. doi: 10.4172/2329-6895.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian YM, Jones RL, Chan KM, Stock AI, Ho JK. Potent contractile actions of prostanoid ep3-receptor agonists on human isolated pulmonary artery. Br J Pharmacol. 1994;113:369–374. doi: 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison K, Ernst R, Hess P, Studer R, Clozel M. Selexipag: A selective prostacyclin receptor agonist that does not affect rat gastric function. J Pharmacol Exp Ther. 2010;335:249–255. doi: 10.1124/jpet.110.169748. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of tnf-alpha and il-1 beta mrnas follows early response gene expression in transient focal ischemia. Molecular and chemical neuropathology/sponsored by the International Society for Neurochemistry and the World Federation of Neurology and research groups on neurochemistry and cerebrospinal fluid. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- 31.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of il-1alpha and il-1beta in ischemic brain damage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke; a journal of cerebral circulation. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 33.Block F, Peters M, Nolden-Koch M. Expression of il-6 in the ischemic penumbra. Neuroreport. 2000;11:963–967. doi: 10.1097/00001756-200004070-00013. [DOI] [PubMed] [Google Scholar]
- 34.Clark WM, Rinker LG, Lessov NS, Hazel K, Hill JK, Stenzel-Poore M, et al. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke; a journal of cerebral circulation. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, et al. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 36.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor ccr2 protects against cerebral ischemia/reperfusion injury in mice. Stroke; a journal of cerebral circulation. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 37.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 38.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, et al. Ischemic stroke injury is reduced in mice lacking a functional nadph oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 39.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, et al. Nadph oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke; a journal of cerebral circulation. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 40.Chang TC, Huang CJ, Tam K, Chen SF, Tan KT, Tsai MS, et al. Stabilization of hypoxia-inducible factor-1{alpha} by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells. J Biol Chem. 2005;280:36567–36574. doi: 10.1074/jbc.M504280200. [DOI] [PubMed] [Google Scholar]
- 41.Muzaffar S, Shukla N, Lobo C, Angelini GD, Jeremy JY. Iloprost inhibits superoxide formation and gp91phox expression induced by the thromboxane a2 analogue u46619, 8-isoprostane f2alpha, prostaglandin f2alpha, cytokines and endotoxin in the pig pulmonary artery. Br J Pharmacol. 2004;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pluta R, Salinska E, Lazarewicz JW. Prostacyclin reduces early ischemic changes in central nervous system. Acta neurobiologiae experimentalis. 1990;50:295–302. [PubMed] [Google Scholar]
- 43.Pluta R, Salinska E, Lazarewicz JW. Prostacyclin attenuates in the rabbit hippocampus early consequences of transient complete cerebral ischemia. Acta Neurol Scand. 1991;83:370–377. doi: 10.1111/j.1600-0404.1991.tb03966.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.