Abstract
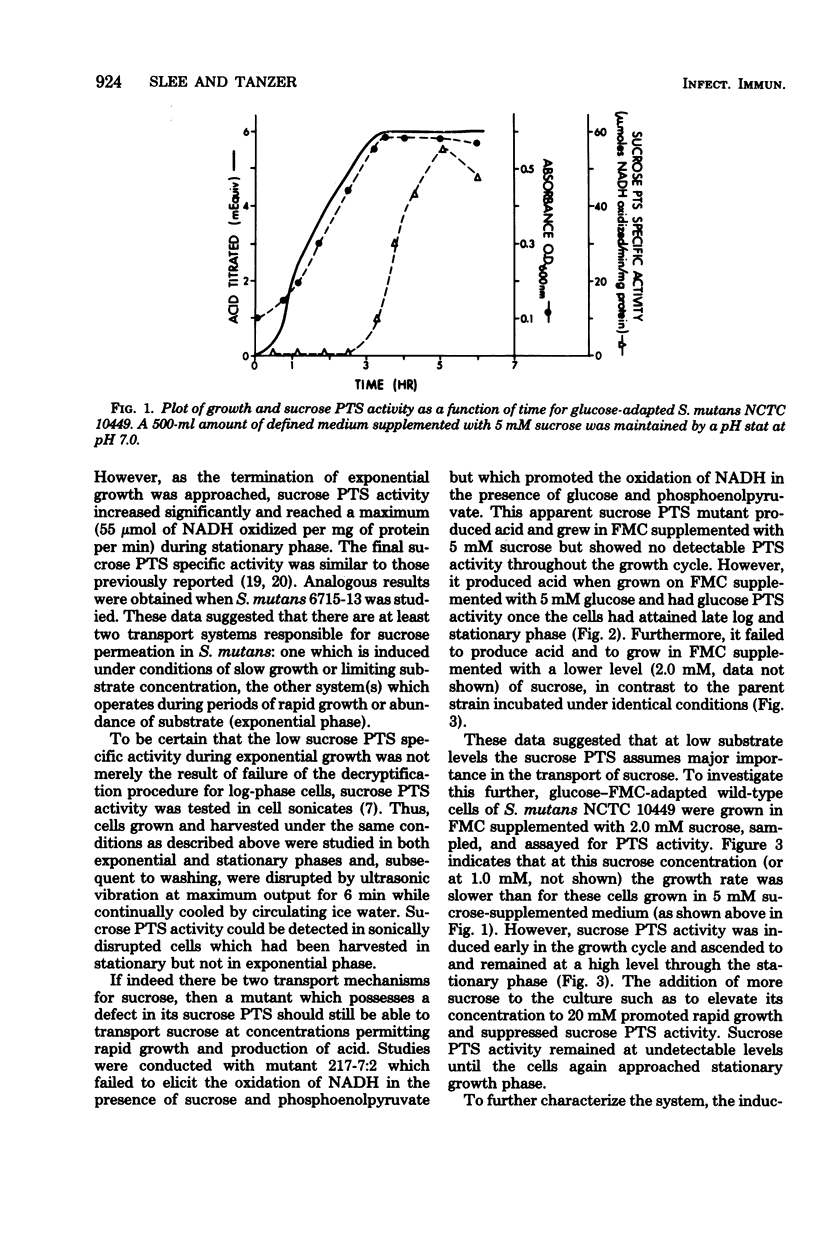
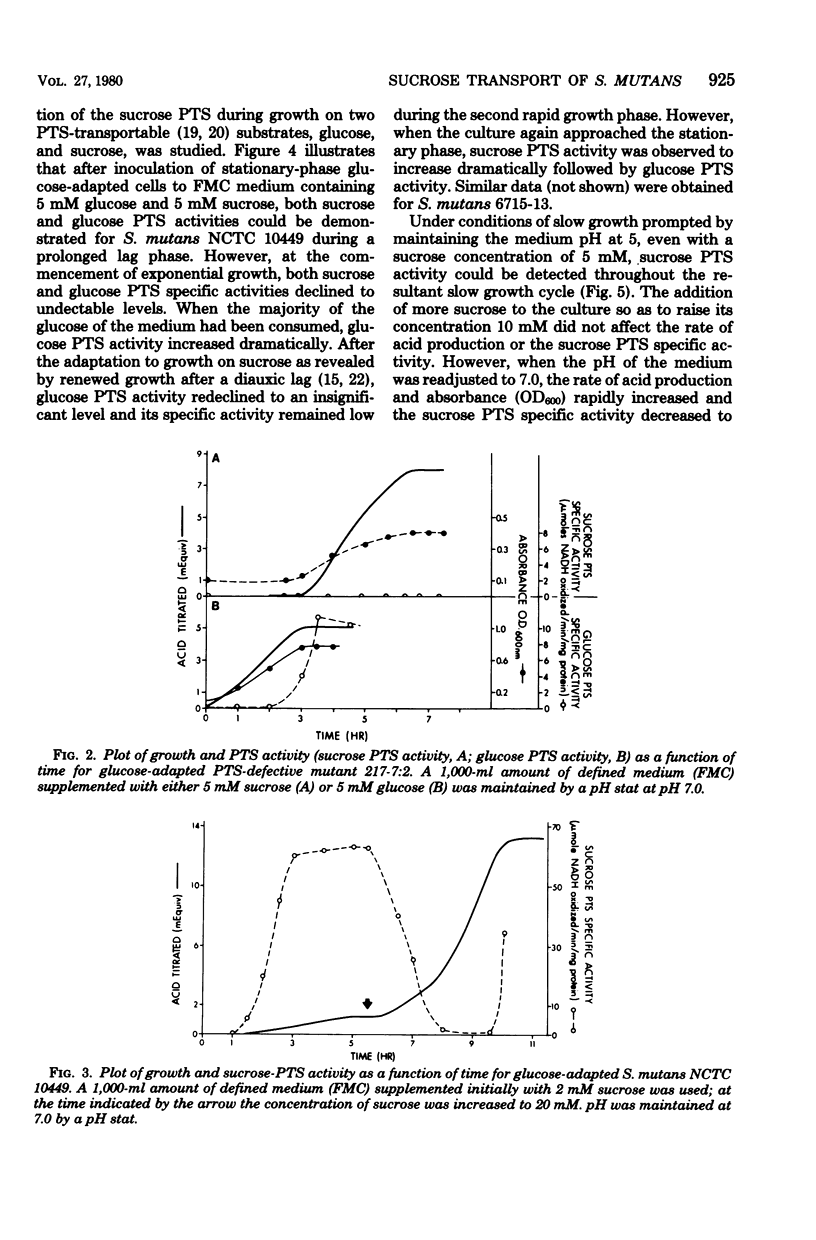
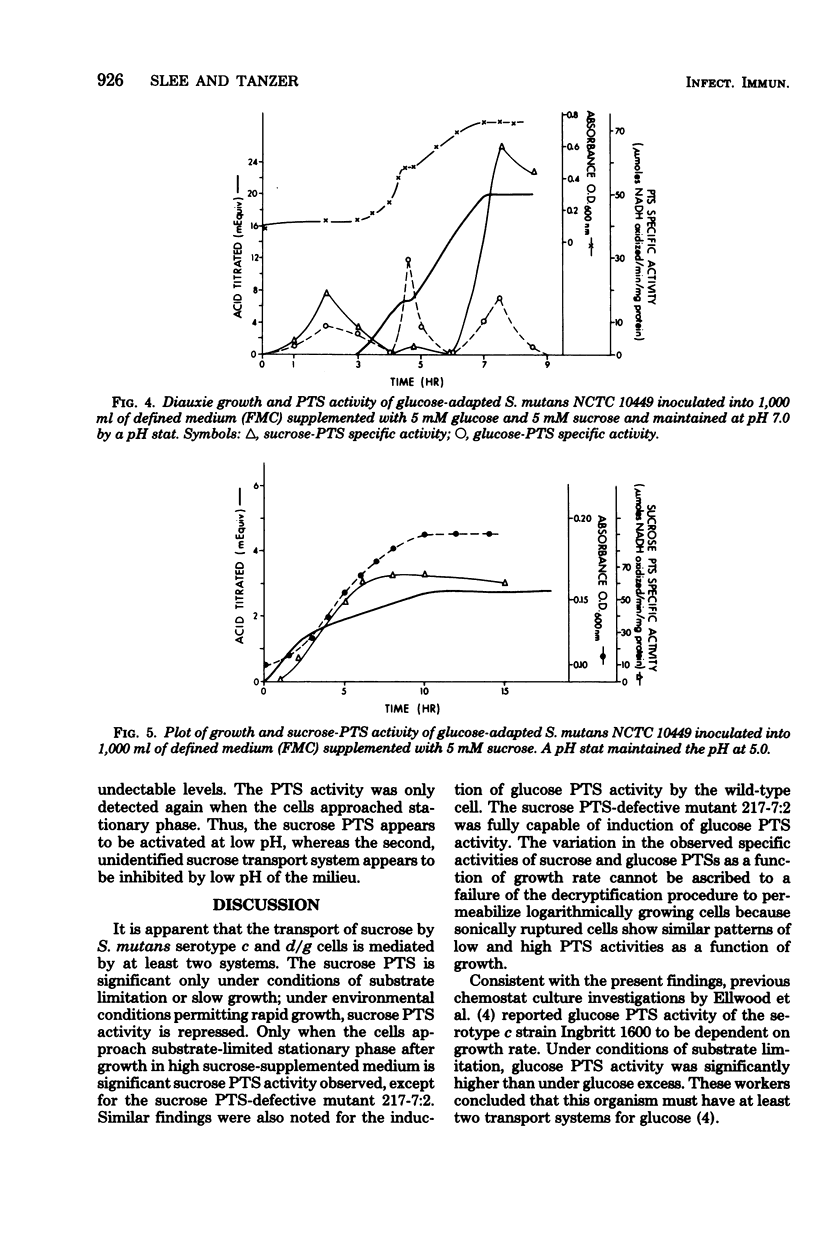
Sucrose and glucose phosphoenolpyruvate-dependent phosphotransferase (PTS) activities were studied in growing cultures of Streptococcus mutans serotype c and d/g cells adapted to either glucose or sucrose. Both acid production and optical absorbance were used to monitor growth in pH-controlled defined growth medium. The sucrose PTS activity appeared to be significant only under conditions of substrate limitation or slow growth as a result of low environmental pH. However, under environmental conditions which permitted rapid growth sucrose PTS activity appeared to be repressed, and only when the cells approached substrate-limited stationary phase after growth on high sucrose-supplemented medium was significant sucrose PTS activity again observed. A mutant apparently defective in sucrose PTS activity grew rapidly and produced acid under conditions of high environmental sucrose level but showed no sucrose PTS activity when the culture approached stationary phase. The mutant, however, after adaptation to glucose, demonstrated significant glucose PTS once the culture had attained the stationary growth phase. During diauxie growth in the presence of glucose and sucrose, there were sequential apparent inductions and repressions of glucose and sucrose PTS activities corresponding to decreases and increases of growth rate on the two substrates. Thus, S. mutans possesses at least two transport mechanisms for each substrate studied. One system (PTS) functions under conditions permitting slow growth and another functions under conditions permitting rapid growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlton G., Fitzgerald D. B., Keyes P. H. Hydrogen ion activity in dental plaques of hamsters during metabolism of sucrose, glucose and fructose. Arch Oral Biol. 1971 Jun;16(6):655–661. doi: 10.1016/0003-9969(71)90069-0. [DOI] [PubMed] [Google Scholar]
- Charlton G., Fitzgerald R. J., Keyes P. H. Determination of saliva and dental plaque pH in hamsters with glass micro-electrodes. Arch Oral Biol. 1971 Jun;16(6):649–654. doi: 10.1016/0003-9969(71)90068-9. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. A new assay of the phosphotransferase system in Escherichia coli. Biochem Biophys Res Commun. 1969 Feb 21;34(4):382–387. doi: 10.1016/0006-291x(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Kiel R. A., Tanzer J. M., Woodiel F. N. Identification, separation, and preliminary characterization of invertase and beta-galactosidase in Actinomyces viscosus. Infect Immun. 1977 Apr;16(1):81–87. doi: 10.1128/iai.16.1.81-87.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Freedman M. L. Chromosome replication and the division cycle of Escherichia coli B-r. J Bacteriol. 1971 Jul;107(1):95–99. doi: 10.1128/jb.107.1.95-99.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König K. G., Larson R. H., Guggenheim B. A strain-specific eating pattern as a factor limiting the transmissibility of caries activity in rats. Arch Oral Biol. 1969 Jan;14(1):91–103. doi: 10.1016/0003-9969(69)90024-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel I. D. Effects of dietary modifications on caries in humans. J Dent Res. 1970 Nov-Dec;49(6):1201–1211. doi: 10.1177/00220345700490060501. [DOI] [PubMed] [Google Scholar]
- Qureshi J. V., Goldner M., Riche W. H., Hargreaves J. A. Streptococcus mutans serotypes in young schoolchildren. Caries Res. 1977;11(3):141–152. doi: 10.1159/000260260. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J. A biochemical scheme for the separation of the five varieties of Streptococcus mutans. Arch Oral Biol. 1974 Nov;19(11):1079–1081. doi: 10.1016/0003-9969(74)90099-5. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in five serotypes of Streptococcus mutans. Infect Immun. 1979 Nov;26(2):783–786. doi: 10.1128/iai.26.2.783-786.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F. Identification, preliminary characterization, and evidence for regulation of invertase in Streptococcus mutans. J Bacteriol. 1973 Oct;116(1):192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L. Genetic alterations of Streptococcus mutans' virulence. Adv Exp Med Biol. 1978;107:661–672. doi: 10.1007/978-1-4684-3369-2_75. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Krichevsky M. I., Keyes P. H. The metabolic fate of glucose catabolized by a washed stationary phase caries-conducive streptococcus. Caries Res. 1969;3(2):167–177. doi: 10.1159/000259580. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



