Abstract
Background
Nevirapine (NVP) is a key component of antiretroviral prophylaxis and treatment for neonates. We evaluated current WHO weight-band NVP prophylactic dosing recommendations and investigated optimal therapeutic NVP dosing for neonates.
Methods
The PHPT-5 study in Thailand assessed the efficacy of ‘Perinatal Antiretroviral Intensification’ to prevent mother-to-child transmission of HIV in women with <8 weeks of antiretroviral treatment before delivery (NCT01511237). Infants received a 2-week course of zidovudine/lamivudine/NVP [NVP syrup/once daily: 2 mg/kg for 7 days; then 4 mg/kg for 7 days]. Infant samples were assessed during the first 2-weeks of life. NVP population PK parameters were estimated using non-linear mixed-effects models. Simulations were performed to estimate the probability of achieving target NVP trough concentrations for prophylaxis (>0.10 mg/L) and for therapeutic efficacy (>3.0 mg/L) using different infant dosing strategies.
Results
Sixty infants (55% male) were included. At birth, median (range) weight was 2.9 (2.3–3.6) kg. NVP concentrations were best described by a one compartment PK model. Infant weight and post-natal age influenced NVP PK parameters. Based on simulations for a 3-kg infant, ≥92% would have a NVP trough >0.1 mg/L after 48 hours through 2 weeks using the PHPT-5 and WHO-dosing regimens. For NVP-based therapy, a 6 mg/kg twice daily dose produced a trough >3.0 mg/L in 87% of infants at 48 hours and 80% at 2 weeks.
Conclusion
WHO weight-band prophylactic guidelines achieved target concentrations. Starting NVP 6 mg/kg twice daily from birth is expected to achieve therapeutic concentrations during the first 2 weeks of life.
Introduction
The rapid global scale up of antiretroviral therapy (ART) for all HIV-infected pregnant and breastfeeding women has helped to make major inroads towards ending the global pediatric HIV epidemic. Recent UNAIDS statistics have shown that new HIV infections among children have decreased by 50% since 2010, and globally 77% of HIV-infected pregnant women were accessing ART in 20151. These numbers are encouraging to meet the bold UNAIDS global target of less than 50,000 new HIV infections among children per year by 20202.
In the context of universal maternal ART, a remaining challenge is the identification and treatment of HIV-infected pregnant women who have no or late access to antenatal care (ANC) as these women are at the highest risk of transmitting HIV to their baby. Indeed, data from the PHPT-5 trial in Thailand indicated that less than 8 weeks of maternal ART and a baseline RNA viral load of ≥4 log10 copies/mL were independently associated with perinatal HIV transmission3. Potent antiretroviral prophylactic interventions are needed for infants who are at high risk of HIV acquisition during delivery and the postnatal period.
Nevirapine (NVP) is a key component of antiretroviral prophylaxis for infants. A combination of zidovudine (ZDV) plus a ‘3-dose’ infant NVP regimen (at birth, 48 and 144 hours of life) was significantly better than ZDV alone at preventing HIV transmission in women who received no ART during pregnancy4. Daily infant NVP starting at birth is also safe and effective at preventing mother-to-child transmission of HIV through breastfeeding5. The pharmacological goal of these prophylaxis regimens are to maintain NVP plasma concentrations in infants above the somewhat arbitrary value of 0.1 mg/L (10-fold the in vitro IC50) during the period of HIV exposure. Designed with the intent of a public health approach, the World Health Organization (WHO) recommends a simplified daily NVP infant prophylaxis weight-band dosing approached for infants born to mothers with HIV who are at high risk of acquiring HIV (WHO6), whether they are breastfed or formula fed, which does not include the standard ‘lead-in’ dose. These guidelines are based on relatively limited clinical data6.
Over the last few years there has also been an increasing demand for immediate/early ART for newborns identified as HIV-infected at birth (i.e. in utero infection). This strategy has been proposed to limit the development of cellular reservoirs containing replication-competent viruses in newborns, which in turn may facilitate drug-free remission in the future7. However, initiating ART within the first few days of life is challenging due to the limited number of child-friendly formulations available. Also, providing drugs to newborn babies poses unique pharmacokinetic challenges, such as the rapid maturation of the metabolism/excretion pathways after birth. Nevirapine has been one of the drugs favored within therapeutic antiretroviral treatment regimens starting at birth7, although insufficient PK data exist to recommend a neonatal dose8. Target therapeutic NVP concentrations are higher than those proposed for HIV prophylaxis with plasma NVP concentrations >3.0 mg/L associated with a reduced risk of virologic failure9, 10. There is currently a lack of pharmacokinetic data supporting the optimal therapeutic NVP dose in newborns.
Our aim was to develop a population pharmacokinetic model to describe NVP concentrations in newborns and to explore optimal NVP dosing for HIV prophylaxis (once daily) and treatment (twice daily) for neonates.
Materials and Methods
Study Design
The amended PHPT-5 trial was a multicenter, phase III, adaptive single-arm study in Thailand that assessed the efficacy of ‘Perinatal Antiretroviral Intensification’ to prevent mother-to-child transmission of HIV in pregnant women who received <8 weeks of ART before delivery (ClinicalTrials.gov NCT01511237). All women/infants enrolled received the standard of care for PMTCT in Thailand at the time the study was designed; mothers received zidovudine (ZDV)/lamivudine (3TC) plus lopinavir/ritonavir (LPV/r), twice a day, starting as soon as possible after 14 weeks of pregnancy, while newborns received ZDV monotherapy (4 mg/kg, every 12 hours) for 4 weeks. If any of the pregnant women received <8 weeks of ART before delivery ‘Perinatal ARV intensification’ was provided to both the mother and baby. Maternal ARV intensification involved a single maternal NVP dose (200 mg) during labor and continuation of ART for at least 4 weeks postpartum; while infant intensification involved a 2-week course of ZDV/3TC/NVP, followed by ZDV/3TC for 2 weeks. Nevirapine dosing was 2 mg/kg once daily (OD) for 7 days, then 4 mg/kg OD for 7 days. The initial doses of NVP were administered by the study team in the hospital. Mothers were instructed how to administer the infant’s prophylaxis treatment before hospital discharge and adherence to the prescribed prophylaxis was assessed at the week 2 visit. All infants were formula-fed and tested for HIV at birth, 1, 2, 4, 6 months. This study was approved by the Ethics Committees at Harvard T. H Chan School of Public Health, Boston, USA, the Ministry of Public Health, Thailand, the Faculty of Associated Medical Sciences, Chiang Mai University and local hospital ECs.
The safety and efficacy results of the main trial have been presented and they indicate that the posterior probability of intrapartum HIV transmission, with no transmissions observed, was 0.39% (95%CrI 0.12–1.4) with intensification compared to 2.0% (0.3–5.2) without11. This current analysis focuses on the assessment of NVP plasma concentrations in infants who received ARV intensification.
Maternal and Infant Blood Sampling
Stored plasma samples collected within the routine follow-up of women and infants enrolled were used for NVP measurement. Blood samples were centrifuged and plasma frozen at −20°C until analysis. Cord blood samples and random blood samples drawn from infants on the first day and at 14 days of life were assessed.
Quantification of Nevirapine Plasma Concentrations
Nevirapine plasma concentrations were determined using a validated reversed-phase high-performance liquid chromatography method with ultraviolet detection12. The method was internally validated over the concentration range of 0.05–15 mg/L. The average accuracy was 98% to 101% and precision (interassay and intra-assay) was <3% (coefficient of variation). The laboratory participates in two international external quality control (QC) programs for quantification of antiretroviral drugs: (i) the HIV/AIDS Clinical Pharmacology Quality Assurance program from the University at Buffalo, NY, which performs standardized interlaboratory testing twice a year13, and (ii) ASQUALAB Quality Control program, France (http://www.asqualab.com/).
Population Pharmacokinetic Analysis
Non-linear mixed effects regression models was used to estimate the population means and variances of NVP pharmacokinetic parameters. The software program NONMEM (Version VII, ICON Development Solutions, MD, USA), with a Fortran Compiler was used to fit concentration-time data using the first-order conditional estimation method (FOCE) with interaction. The software Wings for NONMEM was utilized to run the individual models (V.741: http://wfn.sourceforge.net/wfndown.htm) and diagnostic graphs were generated using RfN using the R program.
Pharmacokinetic structural and residual models were assessed using both statistical and graphical methods. Nested models were compared using the minimal value of the objective function (OFV) provided by NONMEM (equivalent to minus twice the maximum logarithm of the likelihood (-2LL) of the data). Exponential error models used to describe inter-individual variability (IIV) in pharmacokinetic parameters. Inter-occasion variability (IOV) in the pharmacokinetic parameters was also assessed.
For the PK model, it was assumed that NVP cord blood concentrations represented systemic infant concentrations at birth. Using the raw NVP cord blood concentration as “Time Zero” the residual NVP concentration in the infant received through the placenta (i.e. via maternal transfer after sd-NVP) was estimated using a mono-exponential decay of cord blood samples concentrations: e.g. Actual Cord Blood Conc. × e(−K*TABS); [TABS refers to absolute time since cord blood sample; K = NVP elimination rate constant]. This residual concentration of NVP was added to the estimate of the infant NVP concentration following infant NVP dosing.
Covariate Analysis
Infant demographic data included: body weight, sex, body surface area, post-natal age (PNA), post-menstrual age (PMA) and laboratory measures of liver (alanine aminotransferase, ALT) and kidney function (plasma creatinine). The investigator chose the best estimate of gestational age based on either last menstrual period (LMP), fundal height or sonogram. Additive, exponential and power models were assessed for continuous covariates. Linear, exponential and sigmoidal models were tested to describe the maturation of NVP clearance as a function of age14, 15. Both PMA and PNA were assessed to describe the time course of maturation of drug clearance14. Covariates were tested using a standard stepwise forward inclusion (and backward elimination model building procedure16.
Autoinduction of NVP metabolism by 1.5 to 2-fold occurs following during the first 2 to 4 weeks of treatment17. An exponential model was chosen to describe the autoinduction process whereby the fraction of induction at NVP treatment initiation was fixed to 0.66 (e.g. 1.5-fold below the maximum) and the TM50 (time to reach 50% of induction) was fixed to 134 hours (i.e. max. induction after 4 weeks of NVP): Induction = 1 − (1 − 0.66) × exp(−Time*0.693/134).
Model Validation
The final model was evaluated using a visual predictive check (VPC) and bootstrap re-sampling technique. The 5th, 50th, and 95th percentiles of the simulated concentrations were plotted and a VPC was performed by overlying the observed NVP concentrations. The bootstrap re-sampling technique was performed using Wings for NONMEM (400 replicate bootstrap datasets generated using the original dataset with replacement).
Simulations of Prophylactic and Therapeutic doses of Nevirapine
Using the final model, the probability to achieve NVP trough concentrations above the prophylactic and therapeutic target concentrations following different doses were assessed using 1,000 Monte Carlo simulations. A NVP trough target of >0.1 mg/L was used for prophylaxis doses and a trough target of >3.0 mg/L for therapeutic doses. The probability of achieving a target was calculated by dividing the number of simulated concentrations above target by the total number of simulated data.
Results
Sixty infants (55% male) had at least one sample with NVP concentration data available during the first 2 weeks of life. Median (range) gestational age at birth was 38.6 (35.7–41.7) weeks and infant weight was 2.9 (2.3–3.6) kg. Maternal sd-NVP was not administered to 8 of the mothers during labour due to various reasons (e.g. arrived at the hospital in active labour). Median (range) NVP cord blood concentration was 0.88 (<0.05–2.02) mg/L, at a median of 4.1 (0.2–32) hours after maternal sd-NVP intake. Eighty-one infant plasma samples were available with a median NVP concentration of 1.75 (0.25–6.2) mg/L within 48 hours of life and 2.18 (0.71–4.5) mg/L at 2 weeks of life. No major protocol deviations with regards to adherence to the prescribed infant prophylaxis during the first 2 weeks of life were reported.
Nevirapine Population Pharmacokinetic Model
A 1-compartment model with first order absorption and elimination best described the NVP concentration data. It was not possible to accurately estimate the absorption rate constant (Ka) due to a limited number of samples during the absorption phase so Ka it was fixed to 0.39 hr−1 (based on previous data18). No major covariance between the parameters were observed and a proportional residual error model was selected.
The inclusion of body weight as a covariate on CL/F and/or Vd/F significantly reduced the OFV for all models tested. The largest decreases in OFV were observed with the power models, either on CL/F (↓165.62) or Vd/F (↓53.37). Allometrically scaled models significantly reduced the OFV; however, when estimating the exponents the inter-subject variability increased for both CL/F and Vd/F. Body weight allometrically scaled on both CL/F and Vd/F with the exponents fixed to 0.75 and 1.0, respectively, and centered on a 3 kg infant, was selected for inclusion in the model.
Significant reductions in OFV were found for the linear, exponential and sigmoid maturation models using post-natal age. Nevertheless, the estimation of the age component in the linear model was not well estimated (RSE% >70%); while for the exponential and sigmoid models extrapolating the CL/F and Vd/F to estimate adults values yielded higher values than those reported. Maturation models including post-menstrual age did not significantly reduce the OFV. The inclusion of post-natal age using a power model led to a significant reduction in OFV and this was retained in the model. The influence of sex, ALT and creatinine were tested but found not to improve the model fit.
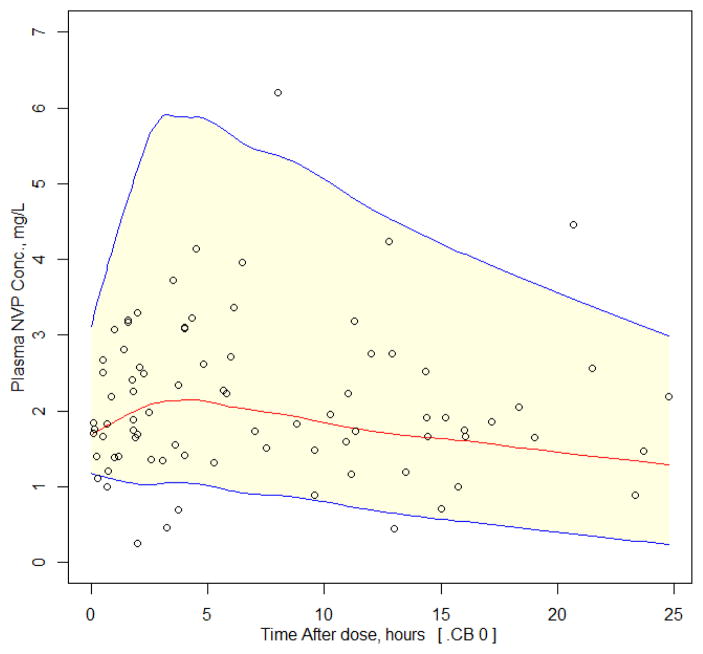
The final population pharmacokinetic parameter estimates for NVP along with the results of the bootstrap analysis are shown in Table 1. The VPC is shown in Figure 1 and there was good agreement between the observed and simulated data. Approximately 9% of the observed data were outside the 90%CI [6% below the 5th percentile and 4% above the 95th percentile] demonstrating a good model fit to the data.
Table 1.
Final nevirapine population pharmacokinetic parameter estimates
| Final Model | Bootstrap^ | |||
|---|---|---|---|---|
|
| ||||
| NVP PK Parameters | Estimate | RSE (%) | Median | 5th – 95th percentile |
|
| ||||
| CL/F (L/h/3 kg) | 0.058 | 17.3 | 0.062 | 0.02–0.14 |
|
| ||||
| θPNA | 0.53 | 11.5 | 0.53 | 0.2–1.8 |
|
| ||||
| Vd/F (L/3 kg) | 5.92 | 16.6 | 5.72 | 3.4–11.8 |
|
| ||||
| Ka (h−1) | 0.39 (fix) | - | ||
| TIND (hours) | 134 (fix) | - | ||
| INDX | 0.66 (fix) | - | ||
|
| ||||
| Inter-individual variability (IIV) | ||||
| IIV (CL/F) | 0.45 | 10.8 | 0.44 | 0.27–1.9 |
| IIV (Vd/F) | 1.17 | 16.8 | 1.18 | 0.58–2.0 |
|
| ||||
| Residual Variability | ||||
|
| ||||
| σ (Proportional) | 0.049 | 38.2 | 0.044 | 0.007 – 0.09 |
Note: CL/F oral clearance; V/F; apparent volume of distribution; Ka absorption rate constant; TIND induction half-time; INDX Fraction of steady state CL/F at time of NVP initiation; RSE%: relative standard error (standard error of estimate/estimate*100);
Statistics from 1,000 bootstrap analyses; CL/F = 0.058 × (WT/3)0.75 × (PNA/24) 0.53 × [1 − (1 − INDX)*exp(−Time*0.693/TIND)]; Vd/F = 5.92 × (WT/3)1
Figure 1.
Visual predictive check for the NVP population PK model. Lines represent the predicted population 5th, 50th and 95th percentiles and shaded area is the 90% CIs. Observed concentrations from the infants are overlaid (black circles).
Simulations of Prophylactic and Therapeutic NVP doses in infants
Prophylactic infant NVP doses
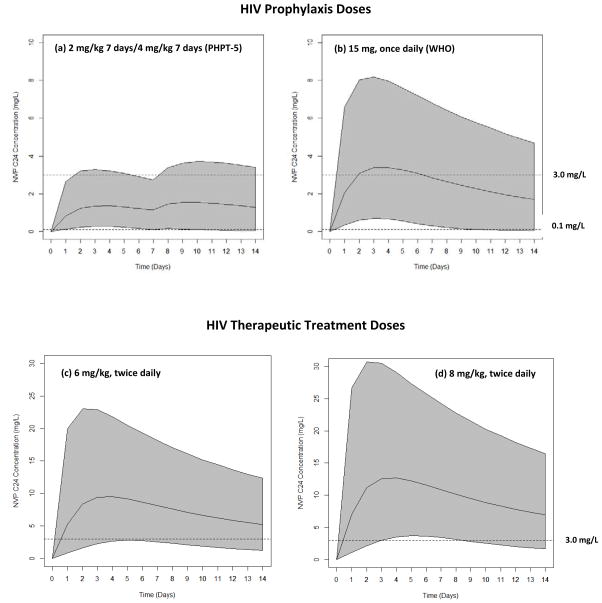
Model simulations for a 3 kg infant at birth, when the mother did not receive sd-NVP, were performed for infants receiving 2 mg/kg NVP syrup once daily for 7 days and then 4 mg/kg once daily for 7 days (as in PHTP-5) and for infants receiving 15 mg once daily per WHO-simplified NVP prophylaxis guidelines. The simulations for NVP C24 over the first 2 weeks of life for both doses are shown in Figure 2. It was predicted that >99% of infants would have a NVP C24 >0.1 mg/L after 2 days with the PHPT-5 and WHO dosing schedules; but this would decrease for both regimens to approximately 93% at 14 days of life.
Figure 2.
Simulated NVP C24 in newborns following (a) 2 mg/kg OD at birth, then 4 mg/kg OD at day 7 to 14 (PHPT-5 dosing) for HIV prophylaxis.; (b) 15 mg once daily from birth until days 14 of life (WHO guidelines); for HIV prophylaxis; (c) 6 mg/kg twice daily and (d) 8 mg/kg twice daily from birth until days 14 of life for HIV treatment. Note: middle line is the 50th percentile, lower/upper solid lines represent 5th & 95th percentiles of simulated data. Trough targets of 0.1 mg/L for prophylaxis and 3.0 mg/L for therapeutic treatment are shown with dotted lines.
Therapeutic infant NVP doses
Simulations for a 3 kg infant at birth, when the mother did not receive sd-NVP, were performed for infants receiving 6 mg/kg NVP twice daily and infants receiving 8 mg/kg twice daily. The simulations for NVP C12 over the first 2 weeks of life for both doses are shown in Figure 2. It was predicted that 87% of infants would have a NVP C12 >3.0 mg/L at 2 days of life with the 6 mg/kg dose [median NVP conc. 8.4 (1.6–23.0) mg/L] compared to 91% with the 8 mg/kg dose [NVP 11.2 (2.2–31.0) mg/L]. At 14 days of life, the NVP C12 >3.0 mg/L decreases to 80% for the 6 mg/kg dose and 88% for the 8 mg/kg dose.
Discussion
A population pharmacokinetic model to describe NVP plasma concentrations in infants initiating NVP syrup once daily at birth through 2 weeks of life for HIV prophylaxis was developed. The final model predicted that the WHO simplified HIV prophylaxis dosing guidelines rapidly achieves and maintains target NVP trough concentrations during the first 2 weeks of life. Therapeutic NVP doses of 6 or 8 mg/kg twice daily from birth were predicted to rapidly achieve target trough concentrations; however, the percentage of children with concentrations below target would start to rise during the 2nd week of life.
Several population pharmacokinetic models for NVP in children and adults have been reported but models including neonates with repeated dosing have not been published. In the present study the Ka to 0.39 hr−1 based on data from babies who received sd-NVP data18 and this value was similar to that reported in HIV-infected children receiving therapeutic doses of NVP19. Higher values of Ka between 1.2 and 1.6 hr−1 have been reported in adults20–22 demonstrating that considerable variability exists regarding the absorption kinetics of NVP.
Multiple infant covariates were found to improve the model fit. The inclusion of infant body weight on CL/F and Vd/F was not unexpected as previous pharmacokinetic models for NVP have identified weight as a significant covariate on these parameters21, 23, 24, including a study in HIV-infected Thai adults25. Both exponential23 and sigmoid24 maturation models for NVP CL/F as a function of age have been reported to describe NVP concentration data in children but these models did not provide acceptable parameter estimates in the current study. It is likely that the limited age range of the current study population explained the lack of fit of these models. Including post-natal age as a covariate on NVP CL/F provided a significant improvement in OFV and the goodness of fit plots. The main limitation of using a power model rather than a maturation model was that it was not possible to extrapolate the parameter estimates outside of age of the study population; thus any model simulations were limited to infants from birth through 2 weeks of life.
Perhaps a controversial choice was the inclusion of an autoinduction component on CL/F in the final model with the parameters fixed to published data40. A weakness of this approach is the assumption that the autoinduction parameters observed in adults also applies to infants. Given the complex nature of the autoinduction process during the first few weeks of life, particularly the transcriptional regulation and induction pathways of metabolic enzymes (e.g. CYP3A4 by pregnane X receptor (PXR) and constitutive androstane receptor (CAR)26,27), it is likely that the rate and extent of autoinduction differs in infants. However, given the lead-in dose period of NVP is the same for adults and children down to 15 days of age provides some reassurance that the effect may not be too dissimilar.
The prophylactic target of 0.1 mg/mL (approximately 10-fold the in vitro IC50) was arbitrary chosen based on the safety data at that time of the first studies assessing NVP to prevent perinatal transmission28. NVP-based prophylaxis regimens designed to meet this target have been shown to be efficacious and safe to prevent perinatal HIV transmission29 and HIV transmission during breastfeeding5. The model predicted that the NVP prophylaxis regimen used in the PHPT-5 study (2 mg/kg OD for 7 days; then 4 mg/kg OD for 7 days) would ensure >99% of infants achieve target prophylactic trough concentration at 48 hours of life. Breast-feeding infants in the HIVNET-023 study received NVP prophylaxis daily from birth for 24 weeks (2 mg/kg OD for 14 days; then 4 mg/kg OD) and all infants had concentrations >0.1 mg/L30. Despite the NVP dose increase after 7 days in PHPT-5, it was predicted that 8% of infants would have a trough below target at 14 days of life. A similar percentage of infants below target at 14 days of life was predicted for infants administered the WHO NVP prophylactic dosing regimen (15 mg once daily at birth; ≥2500g). It would be expected that this percentage would continue to increase until the recommended dose increase to 20 mg once daily at 6 weeks per WHO guidelines. Unfortunately, due to the limitations of the model it was not possible to predict NVP concentrations at 6 weeks of life so the percentage of infants with levels below target at this time remains unknown.
A population pharmacokinetic model of NVP during the first month of life using concentration data pooled from 5 clinical trials (4 assessing infant NVP prophylaxis, and 1 assessing early NVP-based ART) was recently presented31. This model estimated an average NVP CL/F of 0.0439 L/hr/kg0.75 and Vd/F 2.54 L/kg at birth for full-term infants. In our study the estimate of infant CL/F during the first day of life was lower, 0.015 L/hr/kg, but the population estimate of Vd/F was similar. By 2 weeks of age the estimates of NVP CL/F using both models were approximately 0.08 L/hr/kg. The different approaches relating age and CL/F (i.e. power vs. exponential maturation) may explain the difference in CL/F during the first few days of life.
Clinical case reports are emerging regarding initiating therapeutic doses of NVP in newborns (6 mg/kg, twice daily) and the NVP concentrations reported over the first 2 weeks of life are consistent with those predicted with our population model32. Early data presented from the IMPAACT P1115 study showed that among infants receiving 6 mg/kg twice daily the median NVP trough concentration at 1 week of age was 8.7 mg/L; which is comparable to our model predicted trough concentration of 8.14 mg/L.
Several limitations of our model are apparent, including the limited sampling per subject and narrow age range. The inability to assess host genetic polymorphisms is also a weakness. Several population pharmacokinetic models of NVP in adults and children have included the assessment of host genetic polymorphisms as part of the covariate analysis, especially the CYP2B6 516G>T genotype, which is associated with slower clearance22, 33. Unfortunately, the CYP2B6 516G>T genotype information was not available in our study population so the genotype could not be included in the model.
Overall, we have developed of a robust population pharmacokinetic model to describe NVP concentrations in newborns during the first 2 weeks of life. The value of our model is important to support NVP dosing for HIV-exposed infants and HIV-infected infants. These results provide reassurances regarding the current NVP prophylaxis recommendations. Also, in terms of early infant antiretroviral treatment, the data generated using a population approach supports assessing the safety and efficacy of therapeutic NVP doses in the range of 6 to 8 mg/kg twice daily in infants initiating treatment within the first few days of life.
Acknowledgments
We would like to thank the subjects that participated in the PHPT-5 trial and the study staff conducting the protocol at the sites. Health Promotion Center Region 10 Suraphan Sangsawang, Kanokwan Jittayanun; Chiangrai Prachanukroh Hospital Jullapong Achalapong, Kanchana Preedisripipat; Chiang Kham Hospital Chaiwat Putiyanun, Vanichaya Wanchaitanawong; Prapokklao Hospital Prapap Yuthavisuthi, Chaiwat Ngampiyaskul; Banglamung Hospital Prateep Kanjanavikai, Siriluk Phanomcheong; Chonburi Hospital Nantasak Chotivanich, Suchat Hongsiriwon; Rayong Hospital Weerapong Suwankornsakul, Phantip Sreshthatat; Bhuddasothorn Hospital Annop Kanjanasing, Ratchanee Kwanchaipanich; Nopparat Rajathanee Hospital Boonsong Rawangban, Sadhit Santadusit; Hat Yai Hospital Tapnarong Jarupanich, Boonyarat Warachit; Khon Kaen Hospital Thitiporn Siriwachirachai, Pornpimon Rojanakarin; Regional Health Promotion Centre 7, Khon Kaen Kraisorn Vivatpatanakul, Sansanee Hanpinitsak; Kalasin Hospital Phaiboon Wanasiri, Sakulrat Srirojana; Nakhonpathom Hospital Rucha Kongpanichkul, Suthunya Bunjongpak; Samutprakarn Hospital Prapan Sabsanong, Achara Puangsombat; Lampang Hospital Prateung Liampongsabuddhi, Kultida Pongdetudom; Sanpatong Hospital Prayoon Khamja, Noppadon Akarathum; Songkhla Hospital Supha-arth Phon-in, Wannee Limpitikul; Fang Hospital Jantana Jungpipun, Ratikorn Petprakorp; Maharaj Nakhon Si Thammarat Hospital Sukit Mahattanan, Somsri Kotchawet; Wiangpapao Hospital Toranong Pilalai.
This study was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, USA. Grants numbers: R01HD052461, R01HD056953.
Footnotes
Meetings at which parts of the data were presented: these data were presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, February 22–25, 2016
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest to disclose. The study was funded by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, USA. Grants numbers: R01HD052461, R01HD056953.
References
- 1.UNAIDS. [Accessed 14 January 2017];Global AIDS UP DATE 2016. 2016 http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf.
- 2.UNAIDS. [Accessed 14 January 2017];AIDS by the Numbers. 2016 http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf.
- 3.Lallemant M, Le Coeur S, Sirirungsi W, et al. Randomized noninferiority trial of two maternal single-dose nevirapine-sparing regimens to prevent perinatal HIV in Thailand. AIDS. 2015;29(18):2497–507. doi: 10.1097/QAD.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen-Saines K, Watts DH, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366(25):2368–79. doi: 10.1056/NEJMoa1108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362(24):2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection 2016. [Accessed 14 January 2017];Recommendations for a public health approach. 2016 http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed]
- 7.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Accessed (January 31, 2017)];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 9.de Vries-Sluijs TE, Dieleman JP, Arts D, et al. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet. 2003;42(6):599–605. doi: 10.2165/00003088-200342060-00009. [DOI] [PubMed] [Google Scholar]
- 10.Veldkamp AI, Weverling GJ, Lange JM, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. Aids. 2001;15(9):1089–95. doi: 10.1097/00002030-200106150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lallemant M, Amzal B, Urien S, et al. Antiretroviral Intensification to Prevent Intrapartum HIV Transmission in Late Comers. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Vancouver, Canada. July, 19–22; 2015.2015. [Google Scholar]
- 12.Hollanders RM, van Ewijk-Beneken Kolmer EW, Burger DM, Wuis EW, Koopmans PP, Hekster YA. Determination of nevirapine, an HIV-1 non-nucleoside reverse transcriptase inhibitor, in human plasma by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;744(1):65–71. doi: 10.1016/s0378-4347(00)00231-0. [DOI] [PubMed] [Google Scholar]
- 13.DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93(6):479–82. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 15.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug metabolism and pharmacokinetics. 2009;24(1):25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 16.Cressey TR, Urien S, Hirt D, et al. Influence of body weight on achieving indinavir concentrations within its therapeutic window in HIV-infected Thai patients receiving indinavir boosted with ritonavir. Ther Drug Monit. 2011;33(1):25–31. doi: 10.1097/FTD.0b013e3182057f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viramune . Viramune Tablets, Oral Suspension, Package Insert Labeling. Boehringer-Ingelheim Pharmaceuticals Inc; Ridgefield: Conn FDA Approved 1996 Revised: 01/2014. [Google Scholar]
- 18.Benaboud S, Ekouevi DK, Urien S, et al. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2011;55(1):331–7. doi: 10.1128/AAC.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foissac F, Blanche S, Dollfus C, et al. Population pharmacokinetics of atazanavir/ritonavir in HIV-1-infected children and adolescents. Br J Clin Pharmacol. 2011;72(6):940–7. doi: 10.1111/j.1365-2125.2011.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappelhoff BS, van Leth F, MacGregor TR, Lange J, Beijnen JH, Huitema AD. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir Ther. 2005;10(1):145–55. [PubMed] [Google Scholar]
- 21.Molto J, Valle M, Miranda C, et al. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. J Antimicrob Chemother. 2008;62(4):784–92. doi: 10.1093/jac/dkn268. [DOI] [PubMed] [Google Scholar]
- 22.Schipani A, Wyen C, Mahungu T, et al. Integration of population pharmacokinetics and pharmacogenetics: an aid to optimal nevirapine dose selection in HIV-infected individuals. J Antimicrob Chemother. 2011;66(6):1332–9. doi: 10.1093/jac/dkr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikanjam M, Kabamba D, Cressey TR, et al. Nevirapine exposure with WHO pediatric weight band dosing: enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience. Antimicrob Agents Chemother. 2012;56(10):5374–80. doi: 10.1128/AAC.00842-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foissac F, Bouazza N, Frange P, et al. Evaluation of nevirapine dosing recommendations in HIV-infected children. Br J Clin Pharmacol. 2013;76(1):137–44. doi: 10.1111/bcp.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wattanagoon Y, Na Bangchang K, Hoggard PG, et al. Pharmacokinetics of zidovudine phosphorylation in human immunodeficiency virus-positive thai patients and healthy volunteers. Antimicrob Agents Chemother. 2000;44(7):1986–9. doi: 10.1128/aac.44.7.1986-1989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci. 2005;94(6):1169–86. doi: 10.1002/jps.20324. [DOI] [PubMed] [Google Scholar]
- 27.Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther. 2006;317(3):1200–9. doi: 10.1124/jpet.105.098160. [DOI] [PubMed] [Google Scholar]
- 28.Mirochnick M, Fenton T, Gagnier P, et al. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Pediatric AIDS Clinical Trials Group Protocol 250 Team. J Infect Dis. 1998;178(2):368–74. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 29.Lallemant M, Jourdain G, Le Coeur S, et al. A Randomized, Double-blind Trial Assessing the Efficacy of Single-dose Perinatal Nevirapine Added to a Standard Zidovudine Regimen for the Prevention of Mother-to-child Transmission of HIV-1 In Thailand. 11th Conference on Retroviruses and Opportunistic Infections Abstract 40LB; 2004; San Francisco, USA. 2004. [Google Scholar]
- 30.Shetty AK, Coovadia HM, Mirochnick MM, et al. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast-feeding infants from birth to 6 months. J Acquir Immune Defic Syndr. 2003;34(5):482–90. doi: 10.1097/00126334-200312150-00006. [DOI] [PubMed] [Google Scholar]
- 31.Mirochnick M, Capparelli E, Nielsen K, et al. Nevirapine dosing for treatment in the first month of life. Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MA, USA. February 22–25, 2016; 2016.2016. [Google Scholar]
- 32.Bolaris MA, Keller MA, Robbins BL, Podany AT, Fletcher CV. Nevirapine Plasma Concentrations in Human Immunodeficiency Virus-Exposed Neonates Receiving High-Dose Nevirapine Prophylaxis as Part of 3-Drug Regimen. Journal of the Pediatric Infectious Diseases Society. 2016 doi: 10.1093/jpids/piv084. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. Aids. 2007;21(16):2191–9. doi: 10.1097/QAD.0b013e3282ef9695. [DOI] [PubMed] [Google Scholar]