Abstract
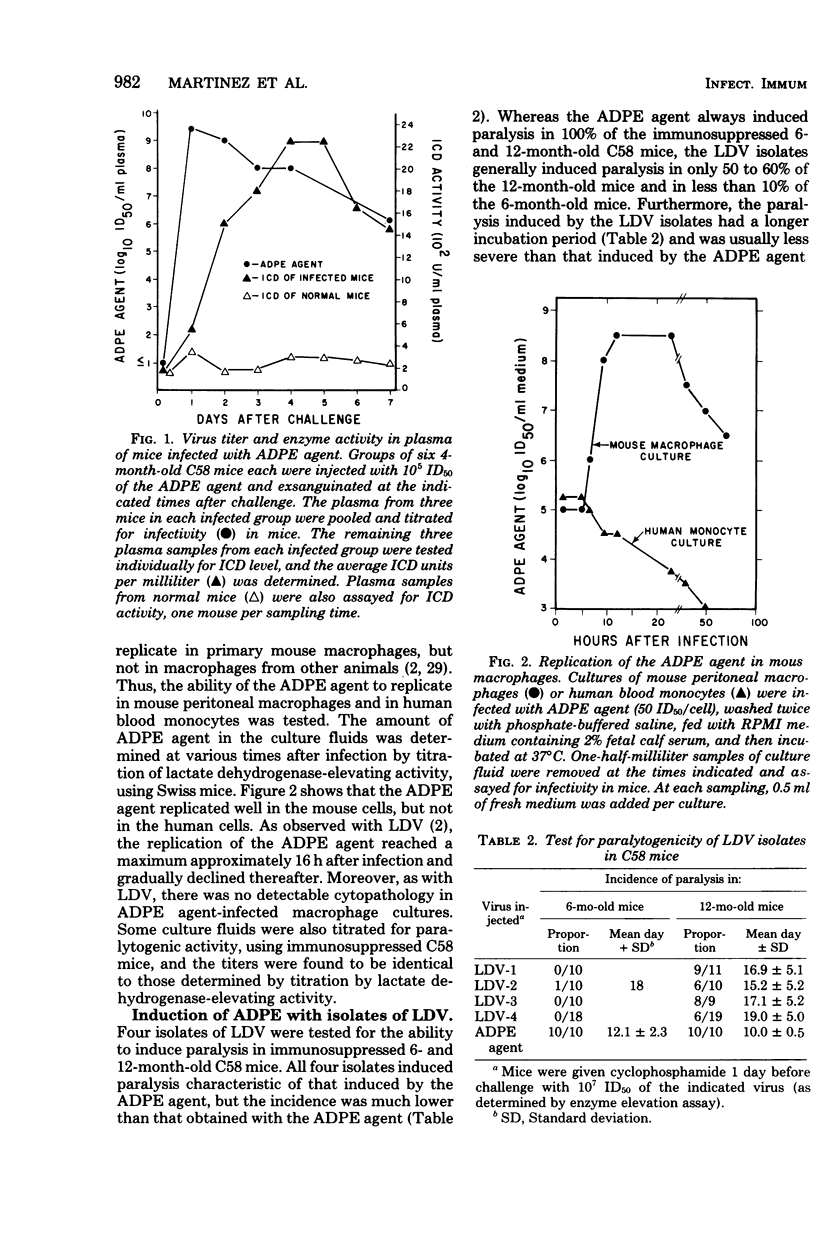
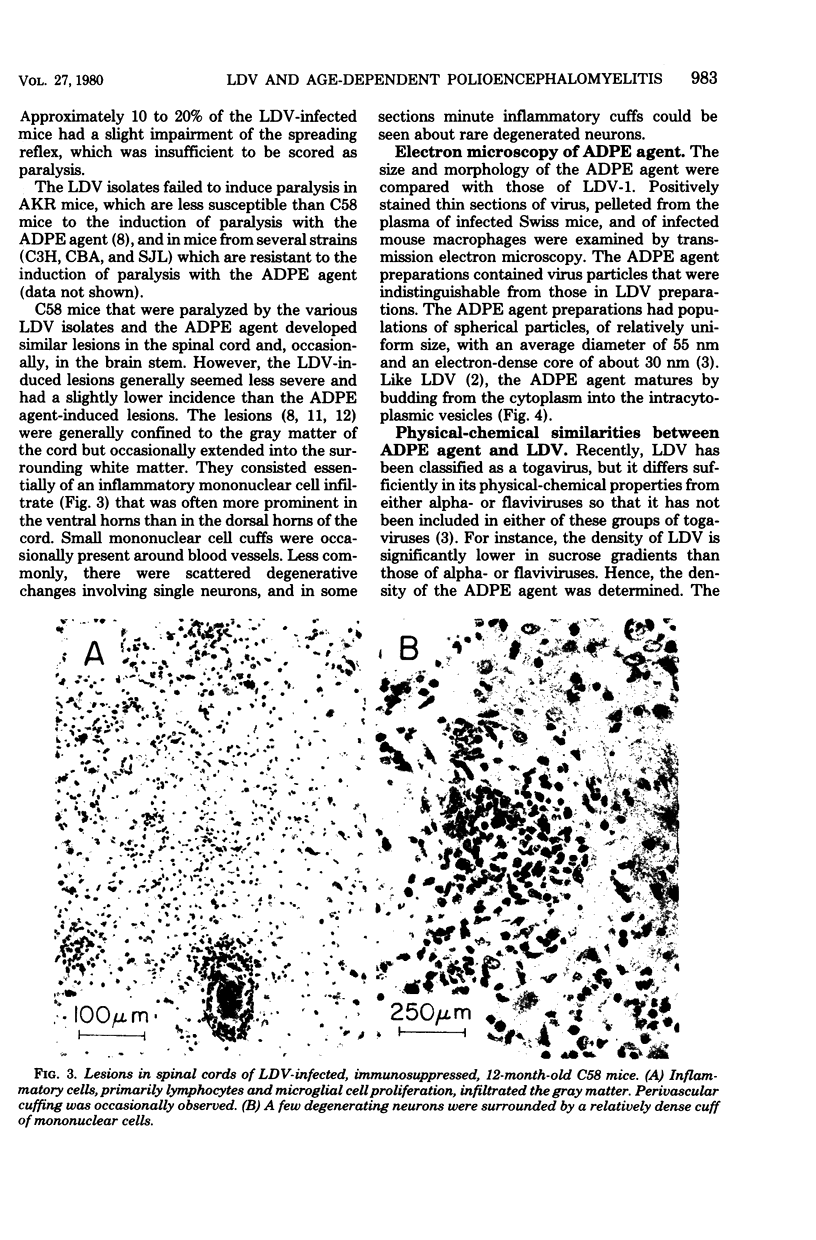
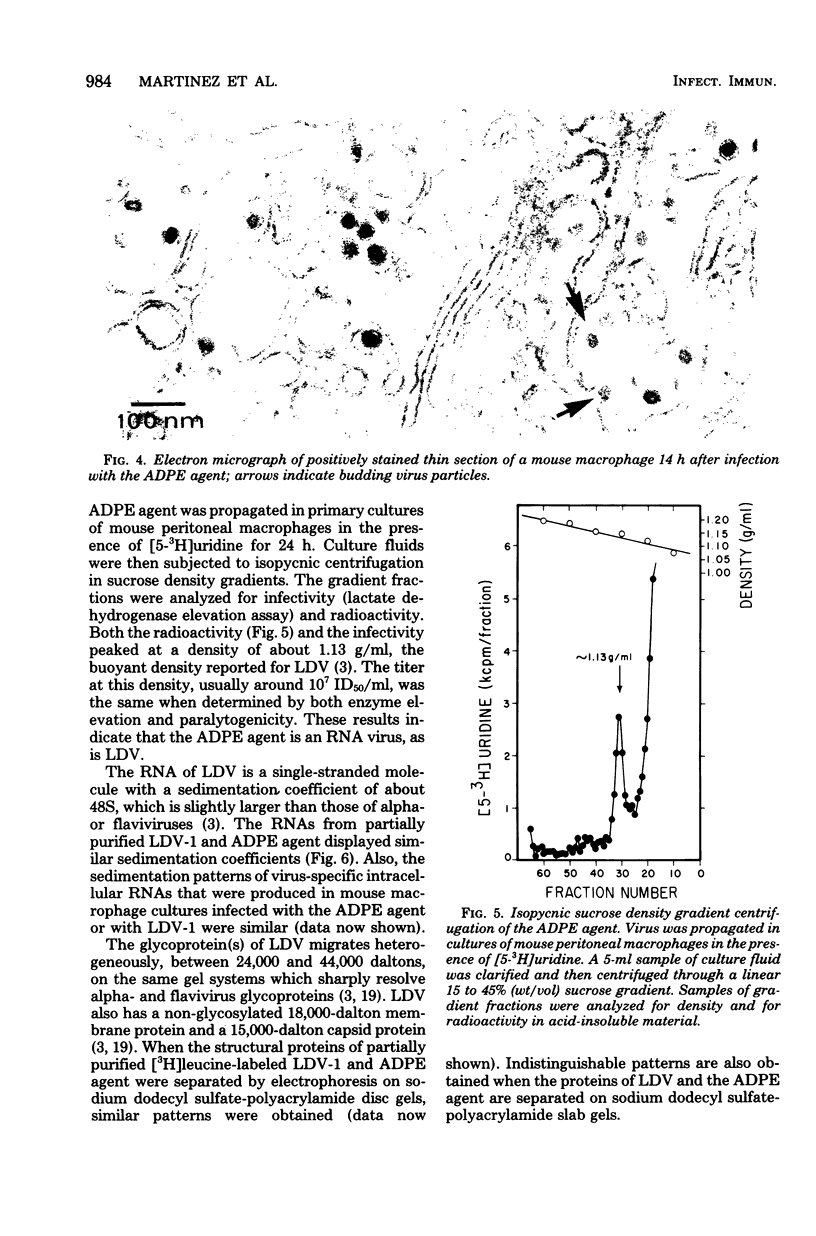
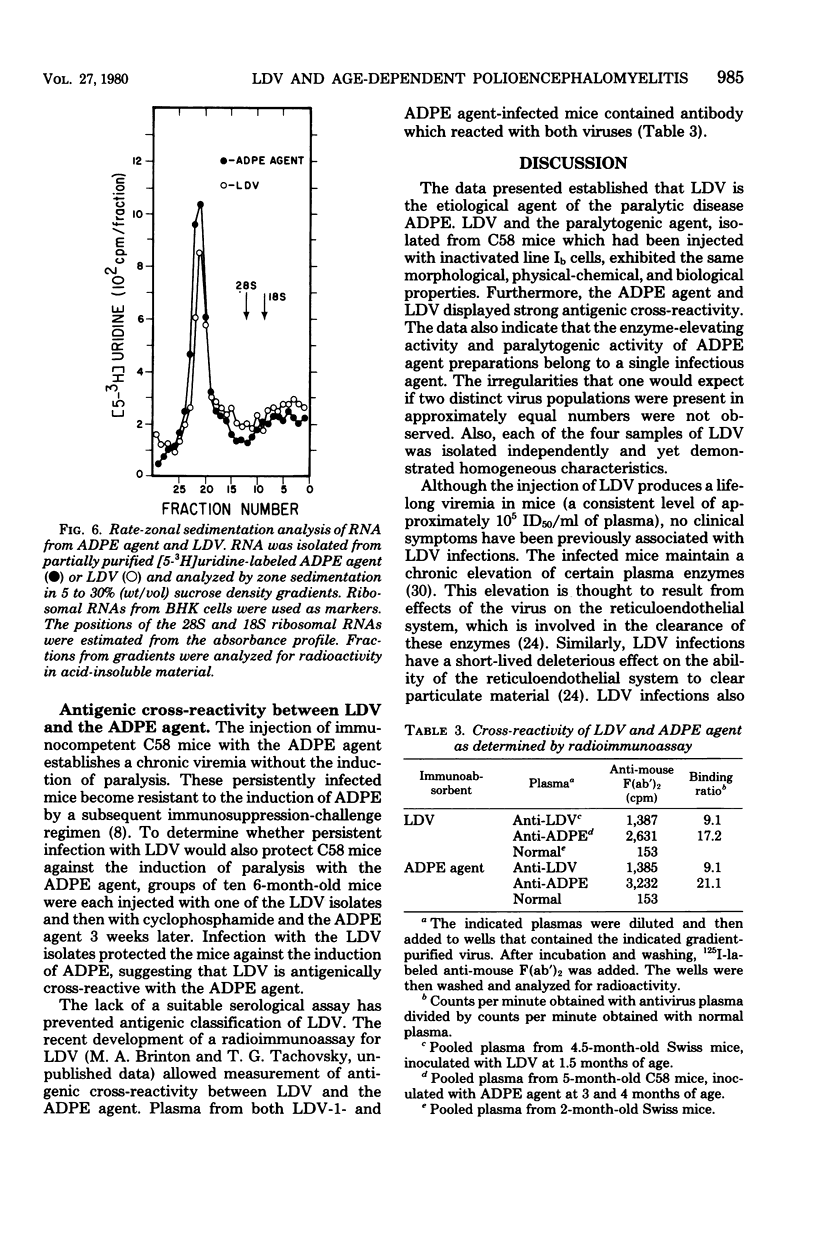
The etiological agent of genetically restricted, age-dependent polioencephalomyelitis of mice (the ADPE agent) and several isolates of lactate dehydrogenase-elevating virus (LDV) were compared by biological, physical-chemical and antigenic criteria. The data indicate that the ADPE agent is a strain of LDV. Like LDV, the ADPE agent induced a selective elevation of plasma enzymes and splenomegaly in mice. The enzyme-elevating activity and the paralytogenic activity of the ADPE agent preparations were shown to belong to the same virus. The ADPE agent demonstrated LDV-like replication kinetics in vivo and in vitro. Moreover, the ADPE agent required primary mouse macrophages for in vitro replication, as does LDV. In turn, the LDV isolates induced a paralytic disease with ADPE-like lesions in the spinal cords of immunosuppressed C58 mice. However, the LDV isolates showed a stronger dependence on strain and age of mouse for the induction of paralysis than did the ADPE agent. The LDV isolates and the ADPE agent exhibited indistinguishable morphologies, buoyant densities, structural protein patterns, and virion ribonucleic acid sedimentation rates. Furthermore, they displayed strong antigenic cross-reactivity, as determined by cross-protection in vivo and by radioimmunoassay.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain L., Croft P. B., Wilkinson M. Motor neurone disease as a manifestation of neoplasm (with a note on the course of classical motor neurone disease). Brain. 1965 Sep;88(3):479–500. doi: 10.1093/brain/88.3.479. [DOI] [PubMed] [Google Scholar]
- Brinton-Darnell M., Collins J. K., Plagemann P. G. Lactate dehydrogenase-elevating virus replication, maturation, and viral RNA synthesis in primary mouse macrophage cultures. Virology. 1975 May;65(1):187–195. doi: 10.1016/0042-6822(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Brinton-Darnell M., Plagemann P. G. Structure and chemical-physical characteristics of lactate dehydrogenase-elevating virus and its RNA. J Virol. 1975 Aug;16(2):420–433. doi: 10.1128/jvi.16.2.420-433.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. J., Jr, Parker J. C. Murine virus contaminants of leukemia viruses and transplantable tumors. J Natl Cancer Inst. 1972 Oct;49(4):1139–1143. [PubMed] [Google Scholar]
- Crispens C. G., Jr Genetic control of the response of SJL-J mice to LDH virus infection. Arch Gesamte Virusforsch. 1972;38(2):225–227. doi: 10.1007/BF01249673. [DOI] [PubMed] [Google Scholar]
- Crispens C. G., Jr Studies on the response of SJL-J mice to infection with the lactate dehydrogenase virus. Arch Gesamte Virusforsch. 1971;35(2):177–182. doi: 10.1007/BF01249708. [DOI] [PubMed] [Google Scholar]
- Duffey P. S., Lukasewycz O. A., Martinez D., Murphy W. H. Pathogenetic mechanisms in immune polioencephalomyelitis: quantitative evaluation of protective and pathogenic effects of lymphoid cells. J Immunol. 1976 May;116(5):1332–1336. [PubMed] [Google Scholar]
- Duffey P. S., Martinez D., Abrams G. D., Murphy W. H. Pathogenetic mechanisms in immune polioencephalomyelitis: induction of disease in immunosuppressed mice. J Immunol. 1976 Feb;116(2):475–481. [PubMed] [Google Scholar]
- Henson R. A., Hoffman H. L., Urich H. Encephalomyelitis with carcinoma. Brain. 1965 Sep;88(3):449–464. doi: 10.1093/brain/88.3.449. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Allison A. C., Harvey J. J. Immune complexes in mice infected neonatally with Moloney leukaemogenic and murine sarcoma viruses. Nature. 1969 Aug 16;223(5207):739–740. doi: 10.1038/223739a0. [DOI] [PubMed] [Google Scholar]
- Homburger H., Abrams G. D., Lawton J. W., Murphy W. J. Histopathology of serum-transmitted immune polioencephalomyelitis in C58 mice. Proc Soc Exp Biol Med. 1973 Dec;144(3):979–982. doi: 10.3181/00379727-144-37724. [DOI] [PubMed] [Google Scholar]
- Lawton J. W., Murphy W. H. Histopathology in immune polioencephalomyelitis in C58 mice. Arch Neurol. 1973 Jun;28(6):367–370. doi: 10.1001/archneur.1973.00490240027002. [DOI] [PubMed] [Google Scholar]
- MANCALL E. L., ROSALES R. K. NECROTIZING MYELOPATHY ASSOCIATED WITH VISCERAL CARCINOMA. Brain. 1964 Dec;87:639–656. doi: 10.1093/brain/87.4.639. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MURPHY W. H., Jr, WIENS A. L., WATSON D. W. Impairment of innate resistance by triiodothyronine. Proc Soc Exp Biol Med. 1958 Oct;99(1):213–215. doi: 10.3181/00379727-99-24299. [DOI] [PubMed] [Google Scholar]
- Martinez D. Histocompatibility-linked genetic control of susceptibility to age-dependent polioencephalomyelitis in mice. Infect Immun. 1979 Jan;23(1):45–48. doi: 10.1128/iai.23.1.45-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D., Wolanski B., Tytell A. A., Devlin R. G. Viral etiology of age-dependent polioencephalomyelitis in C58 mice. Infect Immun. 1979 Jan;23(1):133–139. doi: 10.1128/iai.23.1.133-139.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascoli C. C., Ittensohn O. L., Villarejos V. M., Arguedas J. A., Provost P. J., Hilleman M. R. Recovery of hepatitis agents in the marmoset from human cases occurring in Costa Rica. Proc Soc Exp Biol Med. 1973 Jan;142(1):276–282. doi: 10.3181/00379727-142-37005. [DOI] [PubMed] [Google Scholar]
- Michaelides M. C., Schlesinger S. Structural proteins of lactic dehydrogenase virus. Virology. 1973 Sep;55(1):211–217. doi: 10.1016/s0042-6822(73)81023-2. [DOI] [PubMed] [Google Scholar]
- Murphy W. H., Tam M. R., Lanzi R. L., Abell M. R., Kauffman C. Age dependence of immunologically induced central nervous system disease in C58 mice. Cancer Res. 1970 Jun;30(6):1612–1622. [PubMed] [Google Scholar]
- NOTKINS A. L. LACTIC DEHYDROGENASE VIRUS. Bacteriol Rev. 1965 Jun;29:143–160. doi: 10.1128/br.29.2.143-160.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOTKINS A. L., SCHEELE C. IMPAIRED CLEARANCE OF ENZYMES IN MICE INFECTED WITH THE LACTIC DEHYDROGENASE AGENT. J Natl Cancer Inst. 1964 Oct;33:741–749. [PubMed] [Google Scholar]
- NOTKINS A. L., SHOCHAT S. J. Studies on the multiplication and the properties of the lactic dehydrogenase agent. J Exp Med. 1963 May 1;117:735–747. doi: 10.1084/jem.117.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L. Enzymatic and immunologic alterations in mice infected with lactic dehydrogenase virus. Am J Pathol. 1971 Sep;64(3):733–746. [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lactic dehydrogenase virus-induced immune complex type of glomerulonephritis. J Immunol. 1971 May;106(5):1260–1263. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAGEMANN P. G., WATANABE M., SWIM H. E. Plasma lactic dehydrogenase-elevating agent of mice: effect on levels of additional enzymes. Proc Soc Exp Biol Med. 1962 Dec;111:749–754. doi: 10.3181/00379727-111-27911. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IDENTIFICATION OF WM1 AS LDH VIRUS, AND ITS RECOVERY FROM WILD MICE IN MARYLAND. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1015–1019. doi: 10.3181/00379727-116-29436. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Propagation of lactic dehydrogenase-elevating virus in cell culture. Proc Soc Exp Biol Med. 1966 Apr;121(4):1147–1152. doi: 10.3181/00379727-121-30991. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. D., Hayashi K., Notkins A. L. Comparison of direct and indirect solid-phase microradioimmunoassays for the detection of viral antigens and antiviral antibody. Appl Microbiol. 1973 Apr;25(4):567–573. doi: 10.1128/am.25.4.567-573.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson K. E., Mahy B. W. Lactic dehydrogenase virus. Virol Monogr. 1975;(13):1–121. doi: 10.1007/978-3-7091-8378-6_1. [DOI] [PubMed] [Google Scholar]
- Santoli D., Moretta L., Lisak R., Gilden D., Koprowski H. Imbalances in T cell subpopulations in multiple sclerosis patients. J Immunol. 1978 Apr;120(4):1369–1371. [PubMed] [Google Scholar]
- Snodgrass M. J., Lowrey D. S., Hanna M. G., Jr Changes induced by lactic dehydrogenase virus in thymus and thymus-dependent areas of lymphatic tissue. J Immunol. 1972 Apr;108(4):877–892. [PubMed] [Google Scholar]
- Walton J. N., Tomlinson B. E., Pearce G. W. Subacute "poliomyelitis" and Hodgkin's disease. J Neurol Sci. 1968 May-Jun;6(3):435–445. doi: 10.1016/0022-510x(68)90029-4. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Narayan O. Virologic studies of progressive multifocal leukoencephalopathy. Prog Med Virol. 1974;18(0):229–240. [PubMed] [Google Scholar]




