Abstract
Development, tissue renewal and long term survival of multi-cellular organisms is dependent upon the persistence of stem cells that are quiescent, but retain the capacity to re-enter the cell cycle to self-renew, or to produce progeny that can differentiate and re-populate the tissue. Deregulated release of these cells from the quiescent state, or preventing them from entering quiescence, results in uncontrolled proliferation and cancer. Conversely, loss of quiescent cells, or their failure to re-enter cell division, disrupts organ development and prevents tissue regeneration and repair. Understanding the quiescent state and how cells control the transitions in and out of this state is of fundamental importance. Investigations into the mechanics of G1 arrest during the transition to quiescence continue to identify striking parallels between the strategies used by yeast and mammals to regulate this transition. When cells commit to a stable but reversible arrest, the G1/S genes responsible for promoting S phase must be inhibited. This process, from yeast to humans, involves the formation of quiescence-specific complexes on their promoters. In higher cells these so-called DREAM complexes of E2F4/DP/RBL/MuvB recruit the highly conserved histone deacetylase HDAC1, which leads to local histone deacetylation and repression of S phase-promoting transcripts. Quiescent yeast cells also show pervasive histone deacetylation by the HDAC1 counterpart Rpd3. In addition, these cells contain quiescence-specific regulators of G1/S genes: Msa1 and Msa2, which can be considered components of the yeast equivalent of the DREAM complex. Despite a lack of physical similarities, the goals and the strategies used to achieve a reversible transition to quiescence are highly conserved. This motivates a detailed study of this process in the simple model organism: budding yeast.
Keywords: SBF, E2F, RB, Rpd3, HDAC1, MuvB, Msa1, Msa2
The need to control proliferation and maintain a protective, reversible quiescent state is critical for survival of most organisms. The quiescent state of budding yeast shares many important features with that of higher cells (Valcourt, et al. 2012) and the cell cycle is fundamentally conserved (Tang 2016). As such, the strategies for arresting and maintaining this non-dividing quiescent state are likely to be shared. Cells enter quiescence from G1. This requires that the cell cycle machinery that promotes the G1 to S phase transition in proliferating cells must be eliminated or inactivated. In budding yeast, Swi4/Swi6 (or SBF) transcription complexes activate G1/S-specific genes, including cyclins, histones and other genes that promote the transition to S phase (Fig 1). These complexes are inactive in early G1 due to the binding of Whi5 (Costanzo, et al. 2004, de Bruin, et al. 2004). Whi5 and another complex component (Stb1) recruit the histone deacetylase (Rpd3) (Huang, et al. 2009, Takahata, et al. 2009, Wang, et al. 2009) to deacetylate surrounding nucleosomes and repress transcription. Derepression of these complexes occurs when the cyclin-dependent kinase complex Cln3/Cdk phosphorylates and releases Whi5. Swi6 also associates with a second DNA binding protein, Mbp1, which is related to Swi4 and binds to a similar but distinct DNA sequence (Koch, et al. 1993). Referred to as MBF, Mbp1/Swi6 confers late-G1 specific transcription on many genes involved in DNA replication and repair. This wave of late G1 transcription is critical for the timing and fidelity of DNA replication. If the G1 to S transition is accelerated, there are checkpoint proteins, including Rad53, that detect replication stress and become essential for delaying S phase and promoting DNA repair (Sidorova and Breeden 2002, Vallen and Cross 1999). This is achieved by Rad53-dependent phosphorylation and removal of the Nrm1 repressor from Mbp1/Swi6 target genes (de Bruin, et al. 2008).
Fig 1.
Striking parallels between the transition from proliferation and quiescence in budding yeast and metazoans In dividing yeast and metazoan cells, transcription factor complexes of Swi4 and Swi6 (or SBF) and E2F1, 2, or 3 and DP1 activate a set of genes that promote the transition to S phase. Whi5 and RB or RB-like (RBL) pocket proteins (p107 and p130) inhibit these complexes in G1, until Cyclin/Cdk kinases phosphorylate (P) and release them from the DNA binding complexes and allow transcription to procede. When cells exit the cell cycle, there are quiescence-specific complexes that form on these promoters to maintain a stable, but reversible G1 arrest. In metazoans, different quiescence-specific E2F and RB-like proteins bind. In yeast, there is no evidence of substitutes for Swi4 and Swi6. In these repressed complexes of both yeast and metazoans, additional proteins are recruited, including the highly conserved histone deacetylase complex of Rpd3(HDAC1)/Sin3/Sds3, which deactylates histones and represses transcription. These quiescence-specific complexes also include Msa1 and Msa2 in yeast and the MuvB complex in worms, flies and humans.
A strikingly similar strategy (Fig 1) is used in mammalian cells (reviewed in (Trimarchi and Lees 2002)). In cycling cells, there are several E2F/Dp1 protein complexes that activate transcription of G1/S genes. Many of their targets are the same as the G1/S genes in budding yeast (e.g. cyclins, histones, and replication proteins.) These transcription complexes are inactive in early G1 due to the binding of the RB or RB-like proteins (Nevins 1992), which, like Whi5, recruit histone deacetylase and repress target genes (Brehm, et al. 1998, Luo, et al. 1998). Removal of RB requires phosphorylation by cyclin D/Cdks. This enables E2F/Dp1 to induce transcription of their target genes, and promote the transition to S phase. As in budding yeast, replication stress activates a conserved DNA checkpoint pathway, which phosphorylates and releases negative regulators and enables cells to sustain the levels of G1/S transcripts required to maintain DNA integrity and promote survival (Bertoli, et al. 2013).
Defining one pathway to quiescence in budding yeast
The accumulation of evidence indicates that the general strategies of transcriptional control and checkpoint signaling at the G1 to S transition are fundamentally conserved. This motivates a detailed look at how this G1 to S transition is stably but reversibly halted when budding yeast transition from growth to quiescence. The advantage of studying these processes in a unicellular eukaryote is that these cells can be followed as they naturally enter quiescence, without any intervention or genetic manipulation. Wild yeast can survive on a simple mixture of inorganic salts and a soluable carbon source. When these cells are grown to saturation in rich glucose medium, they first limit for glucose at a point that is referred to as the diauxic shift (DS). However, even before they undergo the DS, they begin the transition from logarithmic growth to quiescence with a lengthening of G1 (Miles, et al. 2013). After two days, over 90% of the cells are arrested in G1 and they are full of stored glucose (Lillie and Pringle 1980). The last cell divisions, as cells approach stationary phase, are highly asymmetric and the physical growth of the daughter cells slows, resulting in a dramatic shift in the size of the cells (Li, et al. 2013) (Fig2A). Cell wall fortification also takes place (Shimoi, et al. 1998) and the cells destined to become quiescent become very dense, so they can be purified by sedimentation (Allen, et al. 2006). About 50% of cells become quiescent and they are primarily daughter cells. This process of G1 arrest, asymmetric cell division and cell wall fortification leads to the production of three distinct cell types (Li, et al. 2013), which can be distinguished by flow cytometry after 24 hours in culture (Fig. 2B.) The R1 cells are the very small daughter cells that result from the asymmetric cell divisions. The R2 cells are the non-quiescent mother cells, and the R3 cells are the dense daughter cells that can be purified. These R3 cells are uniformly G1 arrested and highly stress tolerant. In contrast, the cells that remain at the top of the gradient are more heterogeneous, less stress tolerant, and show signs of apoptosis (Allen, et al. 2006, Li, et al. 2009, Li, et al. 2013). A critical feature and defining characteristic of a quiescent cell is the ability to survive for long periods of time in the non-dividing state and then reverse the arrest and re-enter the cell cycle when conditions improve. These purified R3 cells can survive for over a year in water (unpubl.) From this state, they readily and synchronously return to the cell cycle (Allen, et al. 2006). The uniform G1 arrest, high stress tolerance, longevity in the non-dividing state and ready reversal of that state are all indicators that this R3 sub-population of a stationary phase culture is in a quiescent state.
Fig 2.
a Asymmetric cell divisions late in the transition to quiescence radically reduce the modal cell volume of post DS (blue) and log phase (black) cells b Cells grown for 24 hours differentiate into three very distinct cell types. R1 cells are the small daughters of the asymmetric cell division, R2 cells are the non-quiescent mothers, and R3 are the quiescent cells that can be purified by density gradient sedimentation. Log phase cells have uniform light scattering properties (see (Li, et al. 2013) for details.)
Conserved role for the Rpd3/HDAC1 histone deacetylase
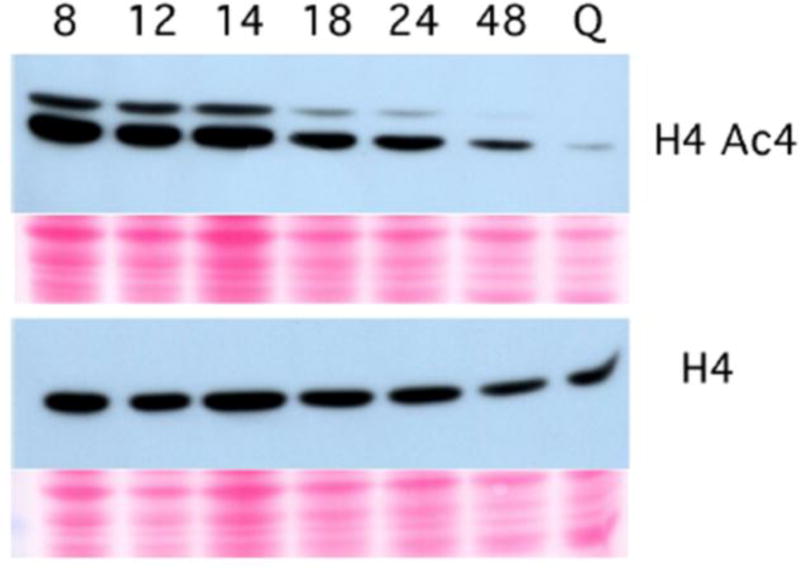
As budding yeast transition to quiescence, the Rad53 checkpoint kinase plays a measurable, protective role against Reactive Oxygen Species accumulation and apoptosis, and, just as in cycling cells, Rad53 becomes essential if the G1 to S transition is accelerated by overproduction of the G1 cyclin Cln3 (Miles, et al. 2013). CLN3 transcription is normally repressed as cells approach the diauxic shift (Barbet, et al. 1996, DeRisi, et al. 1997), and that repression is maintained in post-diauxic cells by Xbp1 (Miles, et al. 2013). Xbp1 is not expressed in log phase cells (Mai and Breeden 1997), but it is induced to high levels after the DS and it represses over 800 genes, including CLN3. Xbp1 recruits the histone deacetylase, Rpd3 (Tao, et al. 2011), which suggested that there might be a similarly global drop in histone acetylation. Figure 3 shows the drop in acetylation at four amino-terminal sites in histone H4 as cells transition from log phase, through the diauxic shift (14 hr) and into quiescence (Q). This drop in H4 acetylation and several other histone acetylations is dependent upon Rpd3 (McKnight, et al. 2015).
Fig 3.
Deacetylation of histone H4 is initiated after the diauxic shift. Immunoblots of yeast extracts probed with antibodies that bind the four conserved N-terminal acetylated lysines in histone H4 (upper) or total histone H4 (lower) over a time course from logarithmic growth, through the diauxic shift (at 14 hours) to 48 hours and then from quiescent (Q) cells purified from a seven day old stationary phase culture. Below each immunoblot is a ponceau S stain of protein in the same lane as a loading control.
Genome-wide analysis indicates that Rpd3 is recruited to thousands of promoters in quiescent cells. These include SBF-regulated promoters and the promoters of many other growth-related and cell cycle-specific genes (McKnight, et al. 2015). At these sites, Rpd3 causes hypo-acetylation of histones and a unique quiescence-specific chromatin state that is not elicited by abrupt starvation (McKnight, et al. 2015). The result is a genome-wide 30-fold transcriptional repression, which occurs long after the diauxic shift. Cells lacking Rpd3 produce very few quiescent cells and they are short lived, indicating that this Rpd3-dependent chromatin remodeling is required for the transition into quiescence and for longevity in the non-dividing state. Interestingly, rpd3 mutants significantly extend replicative life span (RLS), which is a measure of the number of cell divisions a single cell can undergo (Jiang, et al. 2002). One possible explanation for this extended RLS is that these cells continue to divide because they can’t respond to environmental changes that normally trigger the Rpd3-dependent transition to quiescence.
There is considerable evidence that Rpd3 plays a similar role in quiescence in metazoan cells. It is known that histone deacetylase (HDAC) inhibitors can deplete reservoirs of non-dividing leukemic stem cells (Zhang, et al. 2010) and reactivate latent HIV-infected cells (Margolis 2011, Savarino, et al. 2009, Shirakawa, et al. 2013). The Rpd3 histone deacetylase is highly conserved (Yang and Seto 2008) and its closest mammalian homolog is HDAC1 (Taunton, et al. 1996). Deletion of HDAC1 accelerates tumor development in epidermis (Winter, et al. 2013), and in T and B cells (Dovey, et al. 2010, Santoro, et al. 2013). Data suggest that HDAC1 functions as a tumor suppressor in normal cells and developing tumors, but established tumors are dependent upon its deacetylase activity (Heideman, et al. 2013). One possibility is that its role in established tumors is to maintain a population of quiescent cancer stem cells. Consistent with this, complexes of HDAC1 and associated proteins (e.g. mSin3b and Sds3) are recruited by RB-like proteins to repress E2F-regulated promoters specifically in quiescent cells (Fig1 and (Alland, et al. 2002, Rayman, et al. 2002).) Mouse embryonic fibroblasts lacking mSin3B divide normally under good growth conditions, but they are defective in exiting the cell cycle when signaled to terminally differentiate or enter quiescence (David, et al. 2008). These data support the view that HDAC1/Rpd3 performs a conserved function in preventing uncontrolled proliferation and conferring the quiescent state.
One obvious question is whether the Rpd3(HDAC1)-mediated repression of E2F-regulated promoters is solely responsible for the quiescence-promoting activity of these complexes, or if its role in the global repression of transcription also contributes. It is known that recruitment of Rpd3 to the SBF-targeted G1/S genes of budding yeast requires the presence of Whi5 and Stb1 (Sin Three Binding protein 1) in the SBF complexes (Takahata, et al. 2009). We have looked at whi5stb1 mutants and find that they produce wild type levels of quiescent cells (Miles, et al. 2016), while rpd3 mutants produce very few quiescent cells (McKnight, et al. 2015). This indicates that, at least in budding yeast, the global chromatin remodeling and transcriptional repression that Rpd3 mediates is critically important for the full transition to a quiescent state.
Novel roles for known cell cycle regulators
Quiescent cells have G1 DNA content, so it follows that the gene products that promote the transition from G1 to S phase should be eliminated or inhibited. Consistent with this, Cln3, which is rate limiting for the G1 to S transition in budding yeast (Cross 1988) reduces quiescent cell yields when it is overproduced and increases quiescent cell yields when it is absent (Miles, et al. 2013). In a survey of 26 mutants that affect the length of G1 during rapid growth, we found that there is a strong correlation between the length of G1 and the quiescent cell yield (Miles, et al. 2016). This suggests that, as cells enter quiescence from G1, some time is required to assemble the requisite complexes.
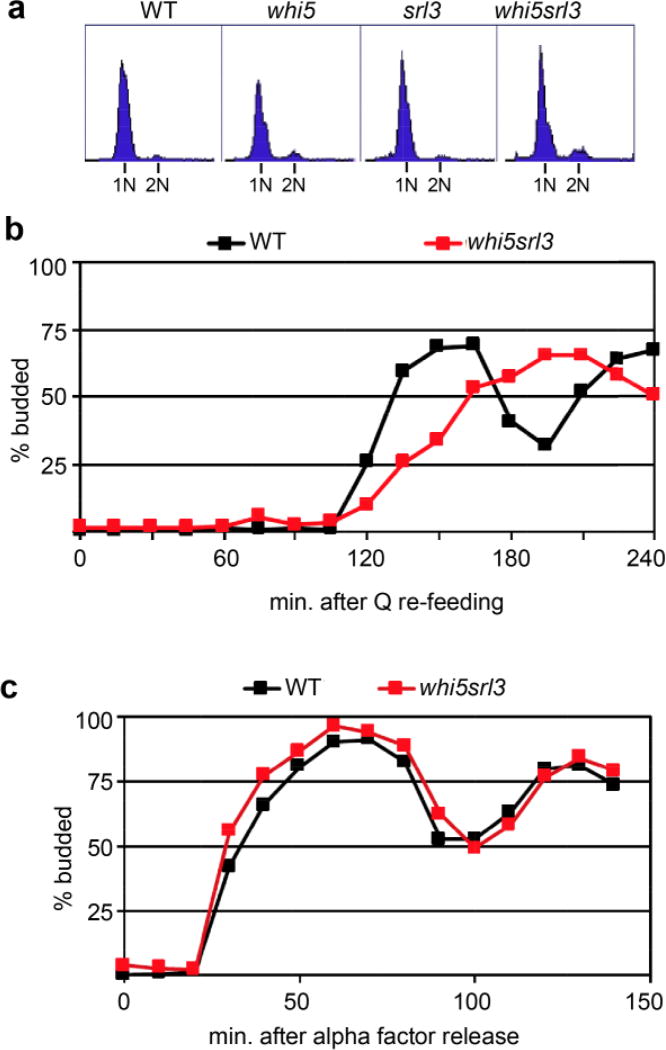
Interestingly, Whi5, the negative regulator of SBF and functional paralog of RB, is not absolutely required for the transition to quiescence, but it does influence the efficiency of the transition in and out of quiescence (Miles, et al. 2016). This phenotype is enhanced by elimination of the Whi5-related protein Srl3. Srl3 is a Suppressor of Rad53 Lethality when over-produced (Ouspenski, et al. 1999). Srl3 is induced by DNA damage and other forms of stress (Gasch, et al. 2000, Lee, et al. 2000, Ragni, et al. 2011). It has been shown to bind SBF transiently in DNA damage (Travesa, et al. 2013) and mediate the nuclear localization of Cln3 (Yahya, et al. 2014). The whi5srl3 mutant is slow to arrest in G1 (Miles, et al. 2016), but after seven days in culture, it achieves G1 arrest just like wild type (Fig 4A). It’s most pronounced defect is that it is very delayed in budding and DNA synthesis upon re-feeding quiescent cells ((Miles, et al. 2016) and Fig 4B). This role in speeding cell cycle re-entry is unexpected for negative regulators of the G1 to S transition and suggests novel functions for these proteins. One possible explanation is that Whi5 and/or Srl3 must be present on SBF target genes to enable their efficient activation by Cyclin3/Cdk at the appropriate time during the recovery cycle. The whi5srl3 mutant has no difficulty releasing from a G1 arrest induced by the mating pheromone alpha factor (Fig 4C), which indicates that there is a fundamental difference between these two types of cell cycle arrest and recovery. There is also a significantly longer lag before budding and DNA synthesis in the quiescent cell recovery cycle compared to release from alpha factor arrest.
Fig 4.
Whi5 and Srl3 mutants can arrest in G1 during the transition to quiescence, but they are required for efficient recovery from quiescence a Flow cytometry of DNA content after seven days in culture shows that all four strains are primarily arrested in G1 with 1N DNA content. b The timing of budding as purified quiescent cells re-enter the cell cycle showing the delay in whi5srl3 mutants. c The timing of budding upon release from G1 arrest by alpha factor is not delayed in the absence of Whi5 and Srl3. Note that wild type quiescent cells require two hours to initiate budding compared to the 30 minutes required for budding after release from alpha factor arrest (see (Miles, et al. 2016) for details.)
In contrast, loss of the SBF components Swi4 or Swi6, which activate G1/S transcripts and promote S phase, interferes with the transition to quiescence. These mutants never attain the cell densities of wild type cells at saturation and suffer significant cell death (Miles, et al. 2016). The finding that Swi4 and Swi6 are also critical for surviving the transition to quiescence suggested that there might be other quiescence-specific components associated with this SBF complex that would turn these activators into repressors when nutrients are limiting.
Novel quiescence-specific regulators of G1/S genes
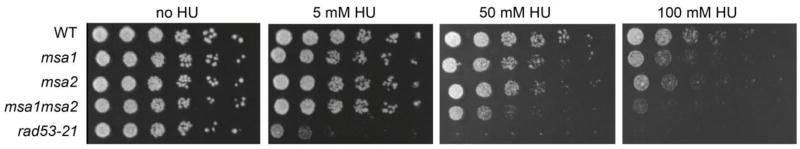
Two potential candidates for modulating G1/S transcription during the transition to quiescence are Msa1 and Msa2. These are structurally related proteins that associate with SBF and MBF, but their influence on SBF and MBF activity in cycling cells is minor (Ashe, et al. 2008). Msa1 suppresses some temperature sensitive DNA synthesis mutants, but is deleterious to several others when overproduced (Li, et al. 2008). Msa2 had no such activity, suggesting no redundancy of function. These authors identified 50 genes that were bound and regulated by Msa1 in cycling cells, but they had no clear connection to DNA synthesis or SBF activity. Msa1 also binds Dbf4, the regulatory subunit of the Dbf4/Cdc7 kinase, which is required for DNA replication and the replication stress checkpoint (Chen, et al. 2013). These data suggest a role for Msa1 in DNA replication or the DNA replication stress checkpoint. Consistent with this, both single mutants are sensitive to replication stress and the msa1msa2 double mutant is more sensitive (Fig. 5.) This indicates that these two proteins share some role, directly or indirectly, in DNA synthesis and/or the replication stress checkpoint. Msa1 and Msa2 also associate with Ste12 and Tec1 to bind and activate two genes involved in pseudohyphal development (van der Felden, et al. 2014). In osmotic stress, Msa1 and Whi5 are phosphorylated by Hog1 kinase and this leads to inhibition of SBF targets (Gonzalez-Novo, et al. 2015). These observations suggest that Msa1 and Msa2 are involved in coordinating cell division and development in response to environmental changes.
Fig 5.
Msa1 and Msa2 are required for survival during DNA replication stress Equal numbers of cells were plated on rich glucose plates in a dilution series on 0, 5, 50 and 100 mM hydroxyurea (HU). HU prevents production of DNA precursers and thereby causes replication stress (Koc, et al. 2004). Cells lacking Msa1 or Msa2 are sensitive to HU and cells lacking both are more sensitive, indicating they have a shared role in DNA replication and/or DNA stress tolerance. rad53-21 lacks the checkpoint function of the essential RAD53 gene (Allen, et al. 1994).
We have assayed Msa1 and Msa2 mutants for their roles in the transition to quiescence and find that they are both important during this transition (Miles, et al. 2016). The msa1msa2 cells are unable to arrest in G1 and they lose viability rapidly. Unlike wild type cells, they continue to increase in cell size, so they have also lost the ability to restrict physical growth during the transition to quiescence. Interestingly, the msa1msa2 cells that manage to arrest in G1 can form quiescent cells which have high stress tolerance and normal longevity. This indicates that the Msa proteins share a role in entry but not maintenance of the quiescent state. The single mutants have modest phenotypes, but in the absence of Rad53 checkpoint activity, they are also defective in G1 arrest and growth arrest. We conclude that Msa1 and Msa2 are important cell cycle regulators during the transition to quiescence and cells rely on the Rad53 checkpoint function when either protein is missing. We have carried out RNA deep sequencing with wild type, and the single and double msa mutants as cells transition from log phase into stationary phase. Msa1 and Msa2 have little influence on transcription in rapidly cycling cells. However, during the transition to quiescence, Msa1 and Msa2 bind and repress an important subset of SBF target genes, including SWI4, CLN2 and all the histone genes (Miles, et al. 2016). They also bind and activate transcription of many MBF target genes (Miles, et al. 2016). The Msa-activated MBF targets are enriched for genes that are involved in DNA repair and chromosome cohesion. The fact that Msa1 and Msa2 are important for the successful transition to quiescence suggest that some or all of these transcriptional changes are important for achieving a quiescent state.
Msa1 and Msa2 are present in the nucleus and bind SBF and MBF specifically during G1 phase of every cell cycle (Ashe, et al. 2008, Li, et al. 2008). Our working model is that this tight G1-specific regulation persists in cycling cells so that cells confronted with a waning nutrient supply or other stresses can respond immediately and reprogram their G1/S-specific transcription complexes from a proliferation-specific pattern to a protective, quiescence-specific pattern. Swi4/Swi6 and Mbp1/Swi6 provide critical docking sites for Msa1 and Msa2, which enable this reprogramming to take place. Here again, there is a striking parallel to the transcriptional regulation that promotes the transition from G1 to quiescence in metazoans (Fig 1.) In higher cells, entry into quiescence depends on the formation of novel “DREAM” complexes that serve to repress target genes that are E2F-activated in proliferating cells. This complex of DP1, RBL, E2F4 and MuvB was first identified in worms and flies (Ceol and Horvitz 2001, Korenjak, et al. 2004) and later found to carry out the same function in human cells (Litovchick, et al. 2007). The DREAM complex is required for entering quiescence and disruption of these complexes drives cells back into the cell cycle (Sadasivam and DeCaprio 2013). Msa1 and Msa2 appear to perform a similar function. They are not required in cycling cells, but they reprogram SBF and MBF activity during the transition to quiescence. In their absence, cells fail to arrest in G1 and lose viability. Msa1 and Msa2, as well as the two RB paralogs (Whi5 and Srl3) are also required for efficient recovery from quiescence. It will be interesting to determine what triggers this reprogramming and how the effected transcripts contribute to cell cycle exit and re-entry.
In summary, many of the key components of the cell duplication cycle (e.g. DNA replication, mitosis, mitochondrial fusion and fission) are functionally and structurally conserved to the extent that the mammalian gene product can sometimes replace its yeast homolog. Here we have highlighted the striking conservation of the regulatory strategies used to exit the cell cycle and enter a reversible quiescent state. This regulation primarily involves controlling the transcription of genes required to drive the transition to S phase. The transcription complexes involved are not related at the sequence level, but their targets are conserved, their mechanism of activation by cyclin-dependent kinases is conserved, and their histone deacetylase-dependent repression is conserved. More recently, quiescence-specific additions to these transcription complexes have been identified in yeast, flies, frogs and humans that promote and stabilize this protective, reversible resting state. It is reasonable to expect further similarities will be discovered. Now that we can study pure populations of quiescent yeast, we can expedite the understanding of this complex but conserved regulatory process.
Acknowledgments
We thank members of the Breeden lab for helpful comments on the manuscript. This work was supported by the National Institutes of Health, National Institute on Aging Grant R21-AG048595 to L.L.B.
References
- Alland L, David G, Shen-Li H, Potes J, Muhle R, Lee HC, Hou H, Jr, Chen K, DePinho RA. Identification of mammalian Sds3 as an integral component of the Sin3/histone deacetylase corepressor complex. Mol Cell Biol. 2002;22:2743–2750. doi: 10.1128/MCB.22.8.2743-2750.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C, Buttner S, Aragon AD, Thomas JA, Meirelles O, Jaetao JE, Benn D, Ruby SW, Veenhuis M, Madeo F, Werner-Washburne M. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J Cell Biol. 2006;174:89–100. doi: 10.1083/jcb.200604072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes & Dev. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- Ashe M, de Bruin RA, Kalashnikova T, McDonald WH, Yates JR, 3rd, Wittenberg C. The SBF- and MBF-associated protein Msa1 is required for proper timing of G1-specific transcription in Saccharomyces cerevisiae. J Biol Chem. 2008;283:6040–6049. doi: 10.1074/jbc.M708248200. [DOI] [PubMed] [Google Scholar]
- Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli C, Klier S, McGowan C, Wittenberg C, de Bruin RA. Chk1 inhibits E2F6 repressor function in response to replication stress to maintain cell-cycle transcription. Curr Biol. 2013;23:1629–1637. doi: 10.1016/j.cub.2013.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- Ceol CJ, Horvitz HR. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol Cell. 2001;7:461–473. doi: 10.1016/s1097-2765(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Chen YC, Kenworthy J, Gabrielse C, Hanni C, Zegerman P, Weinreich M. DNA replication checkpoint signaling depends on a Rad53-Dbf4 N-terminal interaction in Saccharomyces cerevisiae. Genetics. 2013;194:389–401. doi: 10.1534/genetics.113.149740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, Nishikawa JL, Tang X, Millman JS, Schub O, Breitkreuz K, Dewar D, Rupes I, Andrews B, Tyers M. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell. 2004;117:899–913. doi: 10.1016/j.cell.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Cross FR. DAF1, a mutant gene affecting size control, pheromone arrest, and cell cycle kinetics of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4675–4684. doi: 10.1128/mcb.8.11.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Grandinetti KB, Finnerty PM, Simpson N, Chu GC, Depinho RA. Specific requirement of the chromatin modifier mSin3B in cell cycle exit and cellular differentiation. Proc Natl Acad Sci U S A. 2008;105:4168–4172. doi: 10.1073/pnas.0710285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin RA, Kalashnikova TI, Aslanian A, Wohlschlegel J, Chahwan C, Yates JR, 3rd, Russell P, Wittenberg C. DNA replication checkpoint promotes G1-S transcription by inactivating the MBF repressor Nrm1. Proc Natl Acad Sci U S A. 2008;105:11230–11235. doi: 10.1073/pnas.0801106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin RA, McDonald WH, Kalashnikova TI, Yates J, 3rd, Wittenberg C. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell. 2004;117:887–898. doi: 10.1016/j.cell.2004.05.025. [DOI] [PubMed] [Google Scholar]
- DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- Dovey OM, Foster CT, Cowley SM. Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc Natl Acad Sci U S A. 2010;107:8242–8247. doi: 10.1073/pnas.1000478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Novo A, Jimenez J, Clotet J, Nadal-Ribelles M, Cavero S, de Nadal E, Posas F. Hog1 Targets Whi5 and Msa1 Transcription Factors To Downregulate Cyclin Expression upon Stress. Mol Cell Biol. 2015;35:1606–1618. doi: 10.1128/MCB.01279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman MR, Wilting RH, Yanover E, Velds A, de Jong J, Kerkhoven RM, Jacobs H, Wessels LF, Dannenberg JH. Dosage-dependent tumor suppression by histone deacetylases 1 and 2 through regulation of c-Myc collaborating genes and p53 function. Blood. 2013;121:2038–2050. doi: 10.1182/blood-2012-08-450916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Kaluarachchi S, van Dyk D, Friesen H, Sopko R, Ye W, Bastajian N, Moffat J, Sassi H, Costanzo M, Andrews BJ. Dual regulation by pairs of cyclin-dependent protein kinases and histone deacetylases controls G1 transcription in budding yeast. PLoS Biol. 2009;7:e1000188. doi: 10.1371/journal.pbio.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Wawryn J, Shantha Kumara HM, Jazwinski SM. Distinct roles of processes modulated by histone deacetylases Rpd3p, Hda1p, and Sir2p in life extension by caloric restriction in yeast. Experimental gerontology. 2002;37:1023–1030. doi: 10.1016/s0531-5565(02)00064-5. [DOI] [PubMed] [Google Scholar]
- Koc A, Wheeler LJ, Mathews CK, Merrill GF. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J Biol Chem. 2004;279:223–230. doi: 10.1074/jbc.M303952200. [DOI] [PubMed] [Google Scholar]
- Koch C, Moll T, Neuberg M, Ahorn H, Nasmyth K. A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science. 1993;261:1551–1557. doi: 10.1126/science.8372350. [DOI] [PubMed] [Google Scholar]
- Korenjak M, Taylor-Harding B, Binne UK, Satterlee JS, Stevaux O, Aasland R, White-Cooper H, Dyson N, Brehm A. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell. 2004;119:181–193. doi: 10.1016/j.cell.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Lee SE, Pellicioli A, Demeter J, Vaze MP, Gasch AP, Malkova A, Brown PO, Botstein D, Stearns T, Foiani M, Haber JE. Arrest, adaptation, and recovery following a chromosome double-strand break in Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 2000;65:303–314. doi: 10.1101/sqb.2000.65.303. [DOI] [PubMed] [Google Scholar]
- Li JM, Tetzlaff MT, Elledge SJ. Identification of MSA1, a cell cycle-regulated, dosage suppressor of drc1/sld2 and dpb11 mutants. Cell Cycle. 2008;7:3388–3398. doi: 10.4161/cc.7.21.6932. doi: 6932 [pii] [DOI] [PubMed] [Google Scholar]
- Li L, Lu Y, Qin LX, Bar-Joseph Z, Werner-Washburne M, Breeden LL. Budding yeast SSD1-V regulates transcript levels of many longevity genes and extends chronological life span in purified quiescent cells. Mol Biol Cell. 2009;20:3851–3864. doi: 10.1091/mbc.E09-04-0347. [pii] 10.1091/mbc.E09-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Miles S, Melville Z, Prasad A, Bradley G, Breeden LL. Key events during the transition from rapid growth to quiescence in budding yeast require posttranscriptional regulators. Mol Biol Cell. 2013;24:3697–3709. doi: 10.1091/mbc.E13-05-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Pringle JR. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980;143:1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–551. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- Mai B, Breeden L. Xbp1, a stress-induced transcriptional repressor of the saccharomyces cerevisiae Swi4/Mbp1 family. Mol Cell Biol. 1997;17:6491–6501. doi: 10.1128/mcb.17.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6:25–29. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight JN, Boerma JW, Breeden LL, Tsukiyama T. Global Promoter Targeting of a Conserved Lysine Deacetylase for Transcriptional Shutoff during Quiescence Entry. Mol Cell. 2015;59:732–743. doi: 10.1016/j.molcel.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles S, Croxford MW, Abeysinghe AP, Breeden LL. Msa1 and Msa2 Modulate G1- Specific Transcription to Promote G1 Arrest and the Transition to Quiescence in Budding Yeast. PLoS genetics. 2016;12:e1006088. doi: 10.1371/journal.pgen.1006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles S, Li L, Davison J, Breeden LL. Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State. PLoS genetics. 2013;9:e1003854. doi: 10.1371/journal.pgen.1003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Ouspenski II, Elledge SJ, Brinkley BR. New yeast genes important for chromosome integrity and segregation identified by dosage effects on genome stability. Nucleic Acids Res. 1999;27:3001–3008. doi: 10.1093/nar/27.15.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni E, Piberger H, Neupert C, Garcia-Cantalejo J, Popolo L, Arroyo J, Aebi M, Strahl S. The genetic interaction network of CCW12, a Saccharomyces cerevisiae gene required for cell wall integrity during budding and formation of mating projections. BMC Genomics. 2011;12:107. doi: 10.1186/1471-2164-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman JB, Takahashi Y, Indjeian VB, Dannenberg JH, Catchpole S, Watson RJ, te Riele H, Dynlacht BD. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 2002;16:933–947. doi: 10.1101/gad.969202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam S, DeCaprio JA. The DREAM complex: master coordinator of cell cycle- dependent gene expression. Nat Rev Cancer. 2013;13:585–595. doi: 10.1038/nrc3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro F, Botrugno OA, Dal Zuffo R, Pallavicini I, Matthews GM, Cluse L, Barozzi I, Senese S, Fornasari L, Moretti S, Altucci L, Pelicci PG, Chiocca S, Johnstone RW, Minucci S. A dual role for Hdac1: oncosuppressor in tumorigenesis, oncogene in tumor maintenance. Blood. 2013;121:3459–3468. doi: 10.1182/blood-2012-10-461988. [DOI] [PubMed] [Google Scholar]
- Savarino A, Mai A, Norelli S, El Daker S, Valente S, Rotili D, Altucci L, Palamara AT, Garaci E. "Shock and kill" effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology. 2009;6:52. doi: 10.1186/1742-4690-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoi H, Kitagaki H, Ohmori H, Iimura Y, Ito K. Sed1p is a major cell wall protein of Saccharomyces cerevisiae in the stationary phase and is involved in lytic enzyme resistance. J Bacteriol. 1998;180:3381–3387. doi: 10.1128/jb.180.13.3381-3387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21:277–285. doi: 10.1016/j.tim.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova J, Breeden LL. Precocious S-phase entry in budding yeast prolongs replicative state and increases dependence upon Rad53 for viability. Genetics. 2002;160:123–136. doi: 10.1093/genetics/160.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata S, Yu Y, Stillman DJ. The E2F functional analogue SBF recruits the Rpd3(L) HDAC, via Whi5 and Stb1, and the FACT chromatin reorganizer, to yeast G1 cyclin promoters. EMBO J. 2009;28:3378–3389. doi: 10.1038/emboj.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. Model Organisms for Studying the Cell Cycle. Methods Mol Biol. 2016;1342:21–57. doi: 10.1007/978-1-4939-2957-3_2. [DOI] [PubMed] [Google Scholar]
- Tao R, Chen H, Gao C, Xue P, Yang F, Han JD, Zhou B, Chen YG. Xbp1-mediated histone H4 deacetylation contributes to DNA double-strand break repair in yeast. Cell Res. 2011:cr201158. doi: 10.1038/cr.2011.58. [pii] 10.1038/cr.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- Travesa A, Kalashnikova T, de Bruin R, Cass SR, Chahwan C, Lee DE, Lowndes N, Wittenberg C. Repression of G1/S transcription is mediated via interaction of the GTB motif of Nrm1 and Whi5 with Swi6. Mol Cell Biol. 2013 doi: 10.1128/MCB.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Valcourt JR, Lemons JM, Haley EM, Kojima M, Demuren OO, Coller HA. Staying alive: metabolic adaptations to quiescence. Cell Cycle. 2012;11:1680–1696. doi: 10.4161/cc.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen EA, Cross FR. Interaction Between the MEC1-Dependent DNA synthesis Checkpoint and G1 Cyclin Function in Saccaromyces Cerevisiae. Genetics. 1999;151:459–471. doi: 10.1093/genetics/151.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Felden J, Weisser S, Bruckner S, Lenz P, Mosch HU. The transcription factors Tec1 and Ste12 interact with coregulators Msa1 and Msa2 to activate adhesion and multicellular development. Mol Cell Biol. 2014;34:2283–2293. doi: 10.1128/MCB.01599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Carey LB, Cai Y, Wijnen H, Futcher B. Recruitment of Cln3 cyclin to promoters controls cell cycle entry via histone deacetylase and other targets. PLoS Biol. 2009;7:e1000189. doi: 10.1371/journal.pbio.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M, Moser MA, Meunier D, Fischer C, Machat G, Mattes K, Lichtenberger BM, Brunmeir R, Weissmann S, Murko C, Humer C, Meischel T, Brosch G, Matthias P, Sibilia M, Seiser C. Divergent roles of HDAC1 and HDAC2 in the regulation of epidermal development and tumorigenesis. EMBO J. 2013;32:3176–3191. doi: 10.1038/emboj.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahya G, Parisi E, Flores A, Gallego C, Aldea M. A Whi7-anchored loop controls the G1 Cdk-cyclin complex at start. Mol Cell. 2014;53:115–126. doi: 10.1016/j.molcel.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Strauss AC, Chu S, Li M, Ho Y, Shiang KD, Snyder DS, Huettner CS, Shultz L, Holyoake T, Bhatia R. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer cell. 2010;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]