Abstract
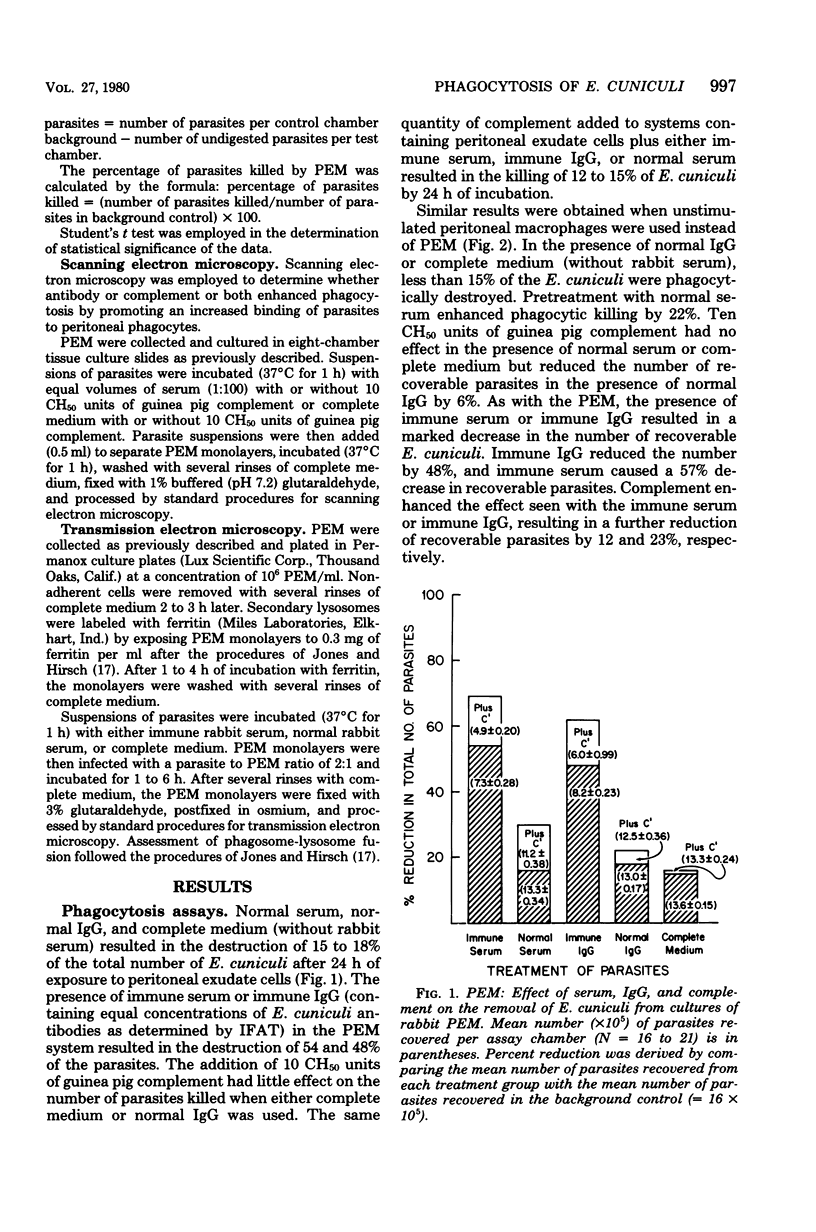
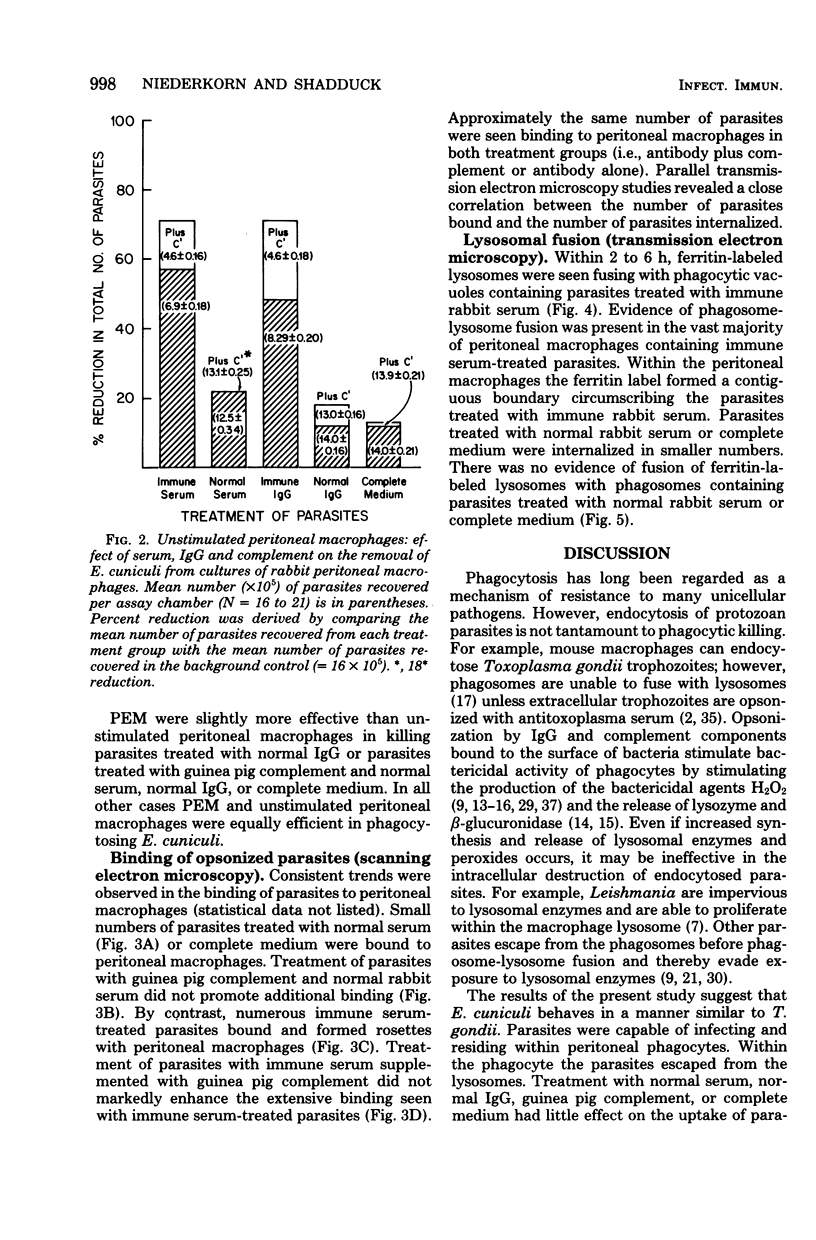
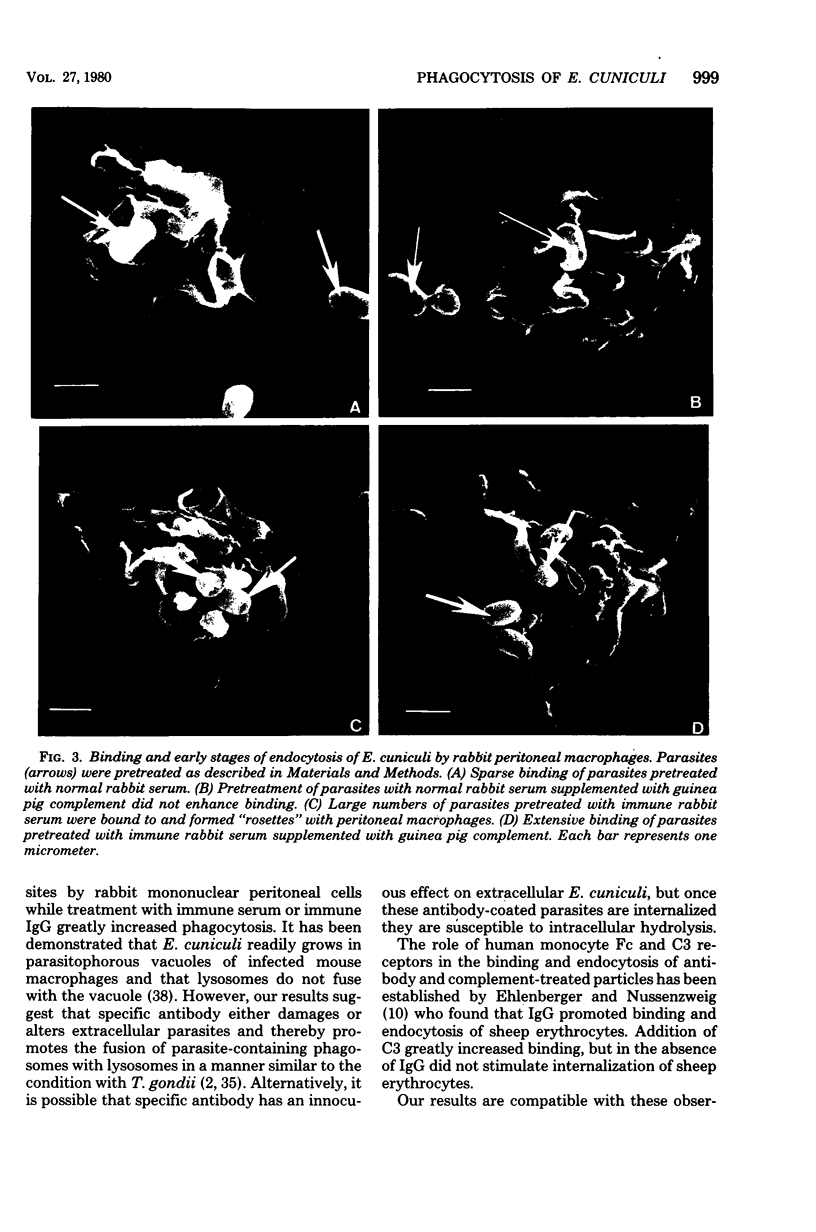
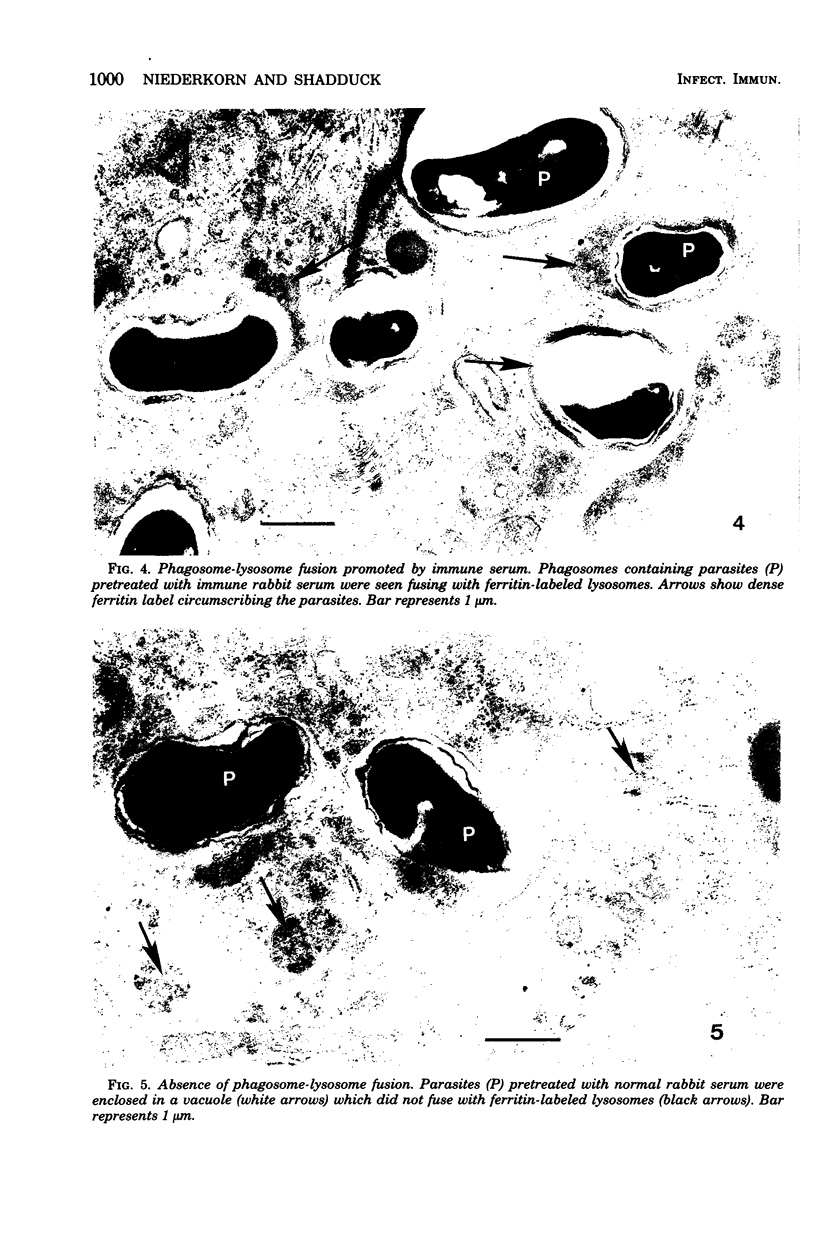
The capacity of mononuclear peritoneal macrophages to phagocytose Encephalitozoon cuniculi was tested in vitro. Normal rabbit serum or cell culture medium had little effect on the rate of removal of organisms by rabbit peritoneal macrophages. Treatment with immune rabbit serum or immune rabbit immunoglobulin G significantly (P less than 0.001) increased phagocytosis of E. cuniculi. Guinea pig complement was found to significantly (P less than 0.001) enhance the phagocytosis of antibody-treated E. cuniculi. With few exceptions, induced (peritoneal exudate) macrophages were no more effective than unstimulated (resident) macrophages in the phagocytosis of E. cuniculi. Secondary lysosomes labeled with ferritin were seen fusing with phagosomes containing immune rabbit serum-treated parasites. Phagosome-lysosome fusion was not observed when parasites were treated with either normal rabbit serum or culture medium. The results of the present study suggest a role for antibody enhancement of phagocytosis and intracellular killing as a mechanism of resistance to encephalitozoonosis in rabbits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Bautista S. C., Remington J. S. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin Exp Immunol. 1976 Dec;26(3):375–380. [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismanis J. E. Detection of latent murine nosematosis and growth of Nosema cuniculi in cell cultures. Can J Microbiol. 1970 Apr;16(4):237–242. doi: 10.1139/m70-044. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Radin A., Frosch M. Independent effects of IgG and complement upon human polymorphonuclear leukocyte function. J Immunol. 1976 Oct;117(4):1282–1287. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller L. D. Spontaneous Nosema cuniculi infection in laboratory rabbits. J Am Vet Med Assoc. 1969 Oct 1;155(7):1108–1114. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOWRIGHT W. An encephalitis-nephritis syndrome in the dog probably due to congenital encephalitozoon infection. J Comp Pathol. 1952 Apr;62(2):83–92. doi: 10.1016/s0368-1742(52)80008-6. [DOI] [PubMed] [Google Scholar]
- Pakes S. P., Shadduck J. A., Cali A. Fine structure of Encephalitozoon cuniculi from rabbits, mice and hamsters. J Protozool. 1975 Nov;22(4):481–488. doi: 10.1111/j.1550-7408.1975.tb05213.x. [DOI] [PubMed] [Google Scholar]
- Pakes S. P., Shadduck J. A., Olsen R. G. A diagnostic skin test for encephalitozoonosis (nosematosis) in rabbits. Lab Anim Sci. 1972 Dec;22(6):870–877. [PubMed] [Google Scholar]
- ROBINSON J. J. Common infectious disease of laboratory rabbits questionably attributed to Encephalitozoon cuniculi. AMA Arch Pathol. 1954 Jul;58(1):71–84. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinska M. A., Trager W., Lewengrub S. J., Gubert E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976 Jun 28;169(3):323–334. doi: 10.1007/BF00219605. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A. Nosema cuiculi: in vitro isolation. Science. 1969 Oct 24;166(3904):516–517. doi: 10.1126/science.166.3904.516. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A., Pakes S. P. Encephalitozoonosis (nosematosis) and toxoplasmosis. Am J Pathol. 1971 Sep;64(3):657–672. [PMC free article] [PubMed] [Google Scholar]
- Shadduck J. A., Watson W. T., Pakes S. P., Cali A. Animal infectivity of Encephalitozoon cuniculi. J Parasitol. 1979 Feb;65(1):123–129. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K., Suzuki N. Effects of immune lymphocyte products and serum antibody on the multiplication of Toxoplasma in murine peritoneal macrophages. Z Parasitenkd. 1976 Mar 31;49(1):11–23. doi: 10.1007/BF00445014. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C. Endocytic uptake of particles by mononuclear phagocytes and the penetration of obligate intracellular parasites. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):161–169. doi: 10.4269/ajtmh.1977.26.161. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]
- Wosu N. J., Olsen R., Shadduck J. A., Koestner A., Pakes S. P. Diagnosis of experimental encephalitozoonosis in rabbits by complement fixation. J Infect Dis. 1977 Jun;135(6):944–948. doi: 10.1093/infdis/135.6.944. [DOI] [PubMed] [Google Scholar]