Abstract
Rationale
Intracoronary infusion of bone marrow mononuclear cells (BM-MNCs) after acute myocardial infarction (AMI) has led to limited improvement in left ventricular (LV) function. Although experimental AMI models have implicated cytokine-related impairment of progenitor cell function, this response has not been investigated in humans.
Objective
To test the hypothesis that peripheral blood (PB) cytokines predict bone marrow (BM) endothelial progenitor cell (EPC) colony outgrowth and cardiac function after AMI.
Methods and Results
BM and PB samples were collected from 87 participants 14–21 days after AMI and BM from healthy donors was used as a reference. Correlations between cytokine concentrations and cell phenotypes, cell functions, and post-AMI cardiac function were determined. PB IL-6 negatively correlated with endothelial colony forming cell (ECFC) colony maximum in the BM of AMI patients (estimate ± SE (ESE) −0.13±0.05, P=0.007). BM from healthy individuals showed a dose-dependent decrease in ECFC colony outgrowth in the presence of exogenous IL-1β or IL-6 (P <0.05). Blocking the IL-1R or IL-6R reversed cytokine impairment. In AMI study participants, the angiogenic cytokine platelet-derived growth factor BB glycoprotein (PDGF-BB) correlated positively with BM-derived CFU-EC colony maximum (ESE 0.01 ± 0.002, P<0.001), multipotent mesenchymal stromal cell (MSC) colony maximum (ESE 0.01±0.002, P=0.002) in BM, and MSC colony maximum in PB (ESE 0.02±0.005, P<0.001).
Conclusions
Two weeks after AMI, increased PB PDGF-BB was associated with increased BM function, while increased IL-6 was associated with BM impairment. Validation studies confirmed inflammatory cytokine impairment of BM that could be reversed by blocking IL-1R or IL-6R. Together, these studies suggest that blocking IL-1 or IL-6 receptors may improve the regenerative capacity of BM cells after AMI.
Clinical Trial Registrations
clinicaltrials.gov Identifiers: NCT00684060
Keywords: Inflammatory cytokines, angiogenic cytokines, acute myocardial infarction, bone marrow, stem and progenitor cells, cell therapy, growth factors
Subject Terms: Growth Factors/Cytokines, Cell Therapy, Inflammation, Vascular Biology
INTRODUCTION
Despite advances in cardiac care including prompt reperfusion therapy, acute myocardial infarction (AMI) remains the most common cause of cardiovascular morbidity and mortality1, 2. Cell therapy is being investigated as a therapeutic strategy for AMI; however, results have been inconsistent as most clinical studies evaluating autologous cell therapy in patients after AMI have reported limited improvement in cardiac function3–7. Therefore, it is important to elucidate the factors that influence the efficacy of cell therapy in AMI since a better understanding may improve treatment results.
Reasons for lack of improvement after autologous bone marrow (BM) cell therapy may relate, in part, to the capacity of the injected cells and/or the myocardial microenvironment receiving the cells. In prior studies, we found that after AMI the BM cell function was markedly impaired8. Also, there was a significant association between improved cardiac function after autologous BM cell injection and BM-derived vasculogenic colony forming capacity and percentages of BM-derived stem/progenitor (CD34+), endothelial (CD31+) and inflammatory/monocytic (CD11b+) cells9, 10. Although various cell types have been implicated, the potential of other factors (e.g., pro-inflammatory cytokines, angiogenic factors and sympathetic nervous system)8, 11 to influence BM and peripheral blood cell composition and function have not been explored. Therefore, we hypothesized that the angiogenic and inflammatory response after AMI may negatively impact BM cell contribution to the repair of damaged myocardium. Although it is known that AMI increases the number and activation state of inflammatory cells in circulation, heart,12 and spleen,5, 13 only one preclinical report supported our hypothesis, and patient data were lacking.14 Thus, using banked specimens and clinical trial data from the Cardiovascular Cell Therapy Research Network (CCTRN) LateTIME study; we measured circulating cytokines and examined their association with BM function after AMI.
METHODS
Study population
Participants in the Cardiovascular Cell Therapy Research Network (CCTRN) Late-TIME study provided additional written consent to donate BM and peripheral blood (PB) for further analyses.3 The Institutional Review Boards (IRBs) of the CCTRN clinical trial sites approved the clinical study and the University of Florida IRB approved this experimental study. Participants who experienced a large AMI (LVEF < 45% despite successful reperfusion) within the previous 14 to 21 days were eligible. A total of 87 AMI patients provided BM and PB samples. Samples were shipped to the CCTRN Biorepository Core Laboratory. Plasma cytokine measurements, cell phenotyping and cell function assays were performed on PB and BM samples.15 Echocardiogram and cardiac magnetic resonance imaging (CMRI) studies were performed within 24 hours of the BM aspiration and repeated six months after BM cell injection as described in detail elsewhere.3, 16
Peripheral blood cytokine measurements
Cytokines from PB plasma were quantified using bead array technique (Bio-Plex ®, Bio-Rad Laboratories, Hercules, California). Analytes included angiogenic or arteriogenic growth factors (basic fibroblast growth factor [bFGF], platelet-derived growth factor BB glycoprotein [PDGF-BB], vascular endothelial growth factor [VEGF], stromal cell-derived factor [SDF-17agr;]), pro-inflammatory cytokines (eotaxin, G-CSF, granulocyte-macrophage colony-stimulating factor [GM-CSF], IFN γ, IL-17agr;, IL-1β, IL-2, IL-6, IL-7, IL-8, IL-9, IL-12, IL-15, IL-17, tumor necrosis factor [TNF]-7agr;, MCP-1, chemokine (C-C motif) ligand 5 [RANTES]) and anti-inflammatory cytokines (IL-1RA, IL-4, IL-5, IL-10, IL-13). The assay was performed according to the manufacturer instructions. Patient samples and standards were assayed in duplicate. Cytokine measurements that were below the lower limit of detection (LOD), based on kit validation studies, were assigned the lowest rank or categorized in a low group.
Bone marrow and peripheral blood mononuclear progenitor colony assays
BM and PB mononuclear cell function were evaluated by standardized ECFC assay, CFU-EC assay and multipotent mesenchymal stromal cell (MSC) colony assay, as previously described.8, 15 CFU-EC colony formations were enumerated every day for 14 days. ECFC and MSC colonies were enumerated every week for 4 weeks. The assays were performed in triplicate. The maximal number of colonies per plate was used for analyses.
In vitro cytokine treatment of healthy donor bone marrow cells
To evaluate the direct effect of exogenous cytokines on BM endothelial colony outgrowth (ECFC assay), 5 million BM cells each from three healthy donors were plated in EGM-2 media on fibronectin dishes. The cells were incubated at 37°C in a fully humidified atmosphere with 5% CO2 for 24 hours. The adherent cells were trypsinized and removed from the fibronectin dishes and then plated in 6 well dishes in the amount of 1 million cells/well. Then the cells were cultured separately, in the presence of increasing concentrations of IL-6 (0, 1, 10, 100 μg/mL or 38.46 nM, 384.6 nM and 3.85 μM), IL-1α (55.5 μM, 555.5 μM and 5.5 mM) and IL-1β (57.1 μM, 571.4 μM and 5.7 mM). ECFC colony formations were enumerated every other day for 2 weeks. The assay was performed in triplicate. The maximum numbers of colonies per well were used for analyses. In three separate sets of experiments, BM ECFC colonies were treated in triplicate with 1 μg/mL of IL-6 and then given 1 μg/mL IL-6 receptor antagonist (IL-6Ra); 1 μg/mL of each of IL-1α and IL-1β and then given 1, 0.1 and 0.5 ng/mL of IL-1 receptor antagonist.17 Cytokine concentrations and antagonist concentrations were chosen based on available prior reports.18 Colony formations were enumerated daily for 2 weeks, in a manner identical to that described above, and the maximal numbers of colonies per well were used for analyses.
Statistical analyses
Because cytokines have lower and upper limits of detectability, rank methods were used for ordinal associations between cytokines and outcomes of interest (i.e., clinical and laboratory data). Spearman rank correlation coefficients were calculated to evaluate associations between the level of cytokines in the PB and colony counts for both BM and PB. Spearman rank correlation coefficients were also calculated for determining associations between PB cytokine levels and cardiac function tests (echocardiogram and cardiac magnetic resonance imaging [CMRI]). Wilcoxon tests were used to determine associations with binary demographic variables.
Multivariable regression analysis was used to evaluate associations between all angiogenesis, pro-inflammatory cytokines, anti-inflammatory cytokines and colony assays. To confirm our results, using a different approach we selected the significant cytokines (p≤0.2) in correlation with a colony forming cell assay and then ran the multiple regression model between the selected cytokines and each colony forming cell. In addition, sensitivity analyses19, 20 were conducted to test our primary multivariable method with 3 possible approaches to manage cytokine concentrations that were below the lower limit of detection (LOD), based on the kit validation. For the first sensitivity analysis we used the actual LOD, for the second sensitivity analysis we used half of LOD, and for the third sensitivity analysis we used a random number between 0 and the LOD for study participants whose values were below the LOD. To compare colony counts after treating BM-MNC with IL-6 in our experimental study, nonparametric tests were used to calculate the statistical significance of differences between 2 groups of paired data (Wilcoxon signed-rank test). The level of statistical significance was set at P<0.05 to detect any significant associations. A Mann-Whitney U test was used to compare the cardiac function improvement between baseline and 6 months follow-up in two groups of participants: upper quartile of IL-6 and IL-10 (IL-6 hi IL-10 hi) versus upper quartile of IL-6 and lower quartile of IL-10 (IL-6 hi IL-10 lo).
Data were analyzed using SAS 9.3. No error controls were used for this study, as false negatives are at least as serious as false positives when screening for promising research leads. This report selectively details the significant associations.
RESULTS
Patient characteristics
The complete details regarding patient characteristics in the LateTIME trial have been reported3, 16. In brief, mean age was 57 years, 83% were men, 86% were white, mean body mass index was 27.8 kg/m2, and there were high proportions of study participants with cardiac risk factors including smoking (59%), hypertension (53%), and hyperlipidemia (70%).
Cytokine levels in peripheral blood after acute myocardial infarction
Peripheral blood cytokine concentrations from AMI participants are summarized in Table 1 and juxtaposed to prior reported cytokine concentrations from healthy individuals. Of note, the median concentrations of inflammatory cytokines known to impair the regenerative capacity of BM after AMI were 11.2 pg/mL for IL-6, 1.4 pg/mL for IL-1α, and 3.2 pg/mL for IL-1β. Among the cytokines, IL-1α, IL-1β, IFNγ, IL-4 and IL-5 had the highest number of concentrations below the lower limit of detection (LOD). IL-1α had 73 (83.9%), IFNγ had 70 (80.4%), IL-1β had 69 (79.3%), IL-4 had 60 (68.97%) and IL-5 had 62 (71.26%) participants whose values were below the LOD.
TABLE 1.
Cytokine Concentrations in Peripheral Blood of Participants 2–3 Weeks After Acute Myocardial Infarction
| Cytokine | Median (Q1-Q3), Concentration (pg/mL), [%]≤LOD 2–3 weeks after AMI N=87* Missing N=8 | Median (Q1-Q3) Concentration (pg/mL) In a Prior Report of Healthy Subjects** 41 Adult≥18 years N=35 | P-value |
|---|---|---|---|
| FGF | 134 (65.6–218.52), [10.34] | 41.7 (33.2–49.5) | <0.0001 |
| Eotaxin | 63.3 (16.5–104.09), [32.1] | 53.2 (31.9–112.2) | <0.0001 |
| G-CSF | 66.9 (16.20–205.3), [11.5] | 45.5 (34–53.6) | 0.02 |
| GM-CSF | 74.9 (4.3–136.9), [41.4] | 38.3 (26.3–63.8) | <0.0001 |
| IFNγ | 27.2 (3.94–53.64), [80.4] | 150.2 (128.3–166.2) | <0.0001 |
| IL-1α | 0 (0–0.74), [83.9] | <1.4 (N/A) | N/A |
| IL-1β | 0.24 (0–1.4), [79.3] | <3.2 (N/A) | N/A |
| IL-1RA | 84.3 (19.6–214.4), [45.9] | 129.6 (101.2–172) | 0.13 |
| IL-2 | 39.1 (19.6–70.6), [2.2] | 14 (9.4–15.9) | <0.0001 |
| IL-4 | 1.2 (0.43–2.02), [68.9] | 7.05 (0–7.5) | 0.20 |
| IL-5 | 1.1 (0–2.44), [71.2] | <1.3 (N/A) | N/A |
| IL-6 | 11.2 (3.78–23.5), [13.7] | 11.03 (9.1–14.01) | 0.41 |
| IL-7 | 3.1 (1–9.6), [52.8] | 13.5 (11–17.1) | 0.008 |
| IL-8 | 22.2 (14.4–39.3), [1.1] | 29.3 (24.4–35.9) | 0.20 |
| IL-9 | 28.9 (5.3–51.9), [16.09] | 23.3 (16.3–31.3) | 0.10 |
| IL-10 | 18 (5.5–46.5), [13.7] | 12.6 (8.5–16.7) | <0.001 |
| IL-12p70 | 40.7 (6.08–137.3), [11.4] | 34.8 (19.6–56.3) | 0.07 |
| IL-13 | 3.3 (0.06–7.6), [47.1] | 14.34 (10.7–17.5) | 0.20 |
| IL-15 | 26.6 (0–64.5), [25.2] | <2.1 (N/A) | N/A |
| IL-17a | 84.2 (21.9–201.05), [12.6] | 123.3 (94.15–137.6) | 0.69 |
| MCP-1 | 77.5 (40–119), [6.8] | 41.5 (20.1–78.9) | N/A |
| MCP-1a | 8.4 (3.5–14.1), [5.7] | 7.09 (N/A) | N/A |
| MCP-1b | 86 (60–117), [0] | 72.95 (N/A) | N/A |
| VEGF | 26.3 (2–44), [25.2] | 61.6 (32–118.9) | 0.001 |
| PDGF-BB | 314.5 (122.2–642.5), [0] | 6679 (4460–8935) | <0.0001 |
| RANTES | 3518.7 (1574.5–6697.5), [0] | 5839 (5154–6089) | <0.0001 |
| TNFα | 16.7 (3.5–33.5), [31.03] | 31.07 (25.3–34.2) | 0.37 |
| SDF-1α | 99.9 (0–191), [25.2] | 22.4 (16.06–24.6) | <0.0001 |
FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP1, monocyte chemo attractant protein-1; VEGF, vascular endothelial growth factor; PDGF-BB, platelet-derived growth factor BB glycoprotein; RANTES, chemokine (C-C motif) ligand 5; SD, standard deviation; SDF-1, stromal cell-derived factor 1; VEGF, vascular endothelial growth factor; N/A, not available.
No result was obtained for 8 of 2436 samples tested and these missing values randomly distributed across the list of measured cytokines.
Personal communication from Giulio Kleiner on behalf of authors of reference 33.
Angiogenic cytokines associated with bone marrow or peripheral blood cell function after AMI
Multiple regression models were used to assess correlations between angiogenic or arteriogenic cytokines (bFGF, PDGF-BB, VEGF, SDF-1α) and BM or PB progenitor cell colony capacities. Among angiogenic cytokines, only PDGF-BB positively correlated with BM-derived CFU-EC colony number (estimate ± standard error [SE; ESE]) 0.01 ± 0.002, P<0.001, multivariable R2:0.22), BM-derived MSC colony number (ESE:0.01 ± 0.002, P=0.002, multivariable R2:0.17) and PB-derived MSC colony number (ESE:0.02 ± 0.005, P< 0.001, multivariable R2:0. 25) from patients after AMI (Table 2). Sensitivity analyses confirmed the significant positive associations between PB PDGF-BB concentration and BM-derived CFU-EC colony number, BM-derived MSC colony number and PB-derived MSC colony number. To confirm our finding, in a second approach, we selected the significant angiogenic cytokines in correlation with colony forming assays and ran the multiple regression models between the selected cytokines and each of the colony-forming assays, separately. This second approach confirmed the significant positive association of PDGF-BB with MSC colony maximum from BM (ESE: −0.01 ± 0.002, P=0.02).
TABLE 2.
Multiple Regressions Among Angiogenic Cytokines and Cell Function of Patients with History of Recent Acute Myocardial Infarction
| Cell Function | Angiogenesis Cytokine | Estimate | Standard Error | P-value | R2 |
|---|---|---|---|---|---|
| CFU-EC colony number maximum from bone marrow | FGF | 0.0001 | 0.004 | 0.98 | 0.22 |
| PDGF-BB | 0.01 | 0.002 | <0.001* | ||
| VEGF | -0.05 | 0.02 | 0.054 | ||
| SDF-1a | -0.0002 | 0.005 | 0.97 | ||
| MSC colony number maximum from bone marrow | |||||
| FGF | -0.004 | 0.004 | 0.30 | 0.17 | |
| PDGF-BB | 0.01 | 0.002 | 0.002* | ||
| VEGF | 0.02 | 0.03 | 0.53 | ||
| SDF-1α | 0.002 | 0.005 | 0.69 | ||
| MSC colony number maximum from peripheral blood | |||||
| FGF | 0.005 | 0.01 | 0.64 | 0.25 | |
| PDGF-BB | 0.02 | 0.005 | <0.001* | ||
| VEGF | -0.09 | 0.07 | 0.17 |
CFUEC, colony forming unit-endothelial colony; FGF, fibroblast growth factors; MSC, mesenchymal stromal cell; PDGF-BB, platelet-derived growth factor BB glycoprotein; SDF-1α, stromal cell-derived factor 1 alpha; VEGF; vascular endothelial growth factor.
Pro-inflammatory cytokines associated with bone marrow cell function after AMI
Multiple regression analysis was used to model the relationship between pro-inflammatory cytokines (eotaxin, G-CSF, GM-CSF, IFNγ, IL-12, IL-15, IL-17a, IL-1B, IL-1A, IL-2, IL-6, IL-7, IL-8, IL-9, TNFα, MCP-1, RANTES) with each of the endothelial and mesenchymal cell assays. Participants with higher IL-6 after AMI showed larger reductions in endothelial progenitor cell capacity in BM (ECFC colony number) (ESE: −0.13 ± 0.05, P=0. 007, multivariable R2:0.60). Additionally, IL-12p70 was negatively associated with ECFC colony of BM (ESE: −0.01 ± 0.007 P=0. 050, multivariable R2:0.60) (Table 3). Moreover, after adjusting for age, sex, BMI, history of smoking and the time of BM cell therapy after AMI, participants with higher IL-6 had larger reductions of ECFC colony count in BM (ESE: −0.15 ± 0.02, P< 0.001, multivariable R2:0.60). Sensitivity analyses confirmed that IL-6 was negatively associated with endothelial progenitor cell capacity in BM. Again, to confirm results, we used a second approach that selected the significant inflammatory cytokines in correlation with colony forming assays and ran the multiple regression models between the selected cytokines and endothelial assay. The second approach confirmed the significant negative association of IL-6 with ECFC colony maximum from BM (ESE: −0.13 ± 0.04, P=0.003, standardized coefficient: −3.1).
TABLE 3.
Associations Between Pro-Inflammatory Cytokines and Endothelial Colony Forming Cell Colony Number from Bone Marrow of Patients with History of Recent Acute Myocardial Infarction
| Parameter Estimates | R-Square: 0.60 | |||
|---|---|---|---|---|
|
| ||||
| Pro-Inflammatory Cytokines | Estimate | Std Error | Standardized Coefficient | Prob>|t| |
| Eotaxin | -0.01 | 0.02 | -0.5 | 0.60 |
| G-CSF | 0.001 | 0.009 | 0.11 | 0.87 |
| GM-CSF | 0.004 | 0.01 | 0.31 | 0.75 |
| IFNγ | 0.04 | 0.01 | 3.3 | 0.001* |
| IL12p70 | -0.01 | 0.007 | -2.06 | 0.05* |
| IL-15 | -0.05 | 0.03 | -1.58 | 0.12 |
| IL-17a | -0.003 | 0.006 | -0.57 | 0.57 |
| IL-1β | 0.05 | 0.09 | 0.56 | 0.58 |
| IL-2 | 0.04 | 0.03 | 1.26 | 0.22 |
| IL-6 | -0.13 | 0.05 | -2.28 | 0.007* |
| IL-7 | 0.05 | 0.08 | 0.64 | 0.53 |
| IL-8 | 0.04 | 0.02 | 1.85 | 0.07 |
| IL-9 | 0.007 | 0.01 | 0.73 | 0.47 |
| TNFα | 0.03 | 0.02 | 1.26 | 0.21 |
| RANTES | -7.601e-6 | 6.832e-5 | -0.4 | 0.67 |
| MCP-1 | 0.18 | 0.14 | 1.22 | 0.23 |
Significantly correlated with ECFC colony maximum in bone marrow.
G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNγ, interferon gamma; IL, interleukin; TNFα, tumor necrosis factor alpha; RANTES, regulated on activation, normal T cell expressed and secreted (C-C motif ligand 5, CCL5); MCP-1, monocyte chemo-attractant protein 1 (C-C motif ligand 2, CCL2)
Association of cytokines and baseline cardiac function
Per LateTIME trial protocol, baseline cardiac functions were measured on the same day of PB collection. Cardiac function measurements included left ventricular ejection fraction (LVEF), end diastolic volume (LVEDV), and end systolic volume (LVESV). We examined the extent that each PB cytokine correlated with baseline cardiac function measure. We found that the level of IL-9 negatively correlated with LVEF and positively correlated with LVEDV (Table 4), suggesting an association between the pro-inflammatory cytokine IL-9 and worse cardiac function. Other cytokines, such as GM-CSF, G-CSF and IL-17a positively associated with cardiac function measures. There was no significant association between IL-6 and cardiac function (Table 4).
TABLE 4.
Correlations Between Peripheral Blood Cytokine Concentrations and Baseline Cardiac Function 2–3 Weeks After AMI.
| Cardiac Function Test | Cytokine | Spearman, r | P-value |
|---|---|---|---|
| Cardiac MRI | |||
| LVEF | IL-9 | -0.29 | 0.01 |
| LVEDV | GM-CSF | 0.25 | 0.02 |
| Echocardiogram | |||
| LVEF | IL-9 | -0.32 | 0.004 |
| LVEDV | IL-9 | 0.36 | 0.001 |
| G-CSF | 0.25 | 0.03 | |
| IL-17a | 0.31 | 0.005 | |
| LVESV | G-CSF | 0.26 | 0.01 |
| IL-17a | 0.29 | 0.01 | |
LVEF, left ventricular ejection fraction; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume. Significant correlations were selected to report.
Focused analysis on IL-6 and cardiac function improvement
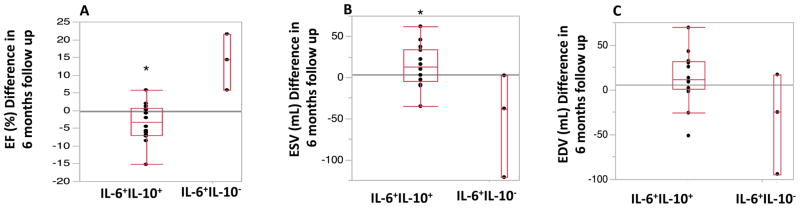
Given that IL-6 was negatively associated with BM colony outgrowth impairment after AMI, we hypothesized that higher IL-6 level may correlate with reduced improvement in cardiac function after BM cell or placebo therapy. Therefore, a focused analysis was performed to compare cardiac function at 6 months follow-up in the subsets of participants with high IL-6 and high IL-10 (upper quartile (Q3) of IL-6 and IL-10) in comparison with a group of study subjects with high IL-6 and low IL-10 (upper quartile (Q3) of IL-6 and lower quartile (Q1) of L-10).21 Sex, age and cardiovascular risk factors such as diabetes mellitus, hypertension, and smoking were not significantly different (P > 0.05) between IL-6 hi IL-10 hi and IL-6 hi IL-10 lo groups. The difference between baseline and 6 months follow-up of LV functions including LVEF, LVEDV, and LVESV measured by cardiac MRI were compared in IL-6 hi IL-10 hi versus IL-6 hi IL-10 lo study participants. LVEF changes were significantly lower in a group with high level of IL-6 and IL-10 at 6 months follow up (-3.41 ± 5.3 vs. 13.84 ± 7.8, P=0.01). Although LVEDV changes after BM cell or placebo therapies were not significantly different between the two IL-6 subgroups, participants with IL-6 hi IL-10 hi showed an increase in LVESV (+14.6 mL ± 25.3 mL) at 6 months follow up compared with study participant with IL-6 hi IL-10 lo (-52.4 mL ± 63.19 mL, P=0.03) (Figure 1).
Figure 1. Differences in Cardiac Functional Parameters (Measured by cardiac MRI) at baseline and 6 month follow-up Versus Peripheral Blood Concentrations of Interleukin 6 and Interleukin 10.
The differences of cardiac MRI (A) ejection fraction (EF), (B) left ventricular end systolic volume (LVESV) and (C) left ventricular end diastolic volume (LVEDV) at baseline immediately prior to cell therapy and six months follow up are plotted according to peripheral blood IL-6 and IL-10 levels. Participants with high IL-6 and IL-10 concentration in PB (IL-6 hi IL-10 hi ) showed significantly lower LVEF changes at 6 months follow up (-3.41 ± 5.3 vs. 13.84 ± 7.8, P=0.01). Moreover study participants with IL-6 hi IL-10 hi showed an increase in LVESV (+14.6 mL ± 25.3 mL) at 6 months follow up compared with those with IL-6 hi IL-10 lo (-52.4 mL ± 63.19 mL, P=0.03). (* P<0.05).
Focused analysis on IL-6 and IL-10 on bone marrow or peripheral blood cell function after AMI
A prior study of STEMI patients, showed that simultaneous elevation of IL-6 and IL-10 levels identified patients with poor clinical outcomes.21 Our study extends the importance of IL-6 and IL-10 on bone marrow impairment after AMI. Therefore, we examined the ratio of IL6/IL-10, in addition to IL-6 and IL-10 alone. Univariate analysis of the individual IL-6 and IL-10 concentrations detected no significant associations with BM and PB cell function. However, multivariable analysis of IL-10, IL-6 and IL-6/IL-10 ratio with each of BM or PB colony numbers showed that IL-6 negatively associated with MSC colony number maximum from PB (standard coefficient −2.4, P=0.01) and IL-10 positively associated with MSC colony numbers from PB (standard coefficient 3.4, P=0.001, R2= 0.2) (Table 5).
TABLE 5.
Multiple Regression analysis of IL-6 and IL-10 with Bone Marrow or Peripheral Blood Cell Functions
| Bone Marrow | Peripheral Blood | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFU-EC colony number maximum |
ECFC colony number maximum |
MSC colony number maximum |
CFU-EC colony number maximum |
ECFC colony number maximum |
MSC colony number maximum |
|||||||
| Standardized Coefficient |
P | Standardized Coefficient |
P | Standardized Coefficient |
P | Standardized Coefficient |
P | Standardized Coefficient |
P | Standardized Coefficient |
P | |
| IL-6 | -1.32 | 0.2 | 1.12 | 0.2 | -1.76 | 0.08 | -0.05 | 0.9 | -0.4 | 0.6 | -2.4 | 0.01* |
| IL-10 | 1.99 | 0.05 | -0.39 | 0.7 | 1.97 | 0.05 | 0.38 | 0.7 | 0.2 | 0.8 | 3.4 | 0.001* |
| IL-6/IL-10 ratio | -0.07 | 0.9 | 2.79 | 0.007 | -0.13 | 0.9 | 0.15 | 0.8 | 0.00 | 0.9 | -0.15 | 0.8 |
| R2 | 0.07 | … | 0.2 | 0.07 | 0.008 | …. | 0.007 | 0.2 | …. | |||
Reversal of IL-6 impairment of BM-derived endothelial progenitor colony formation
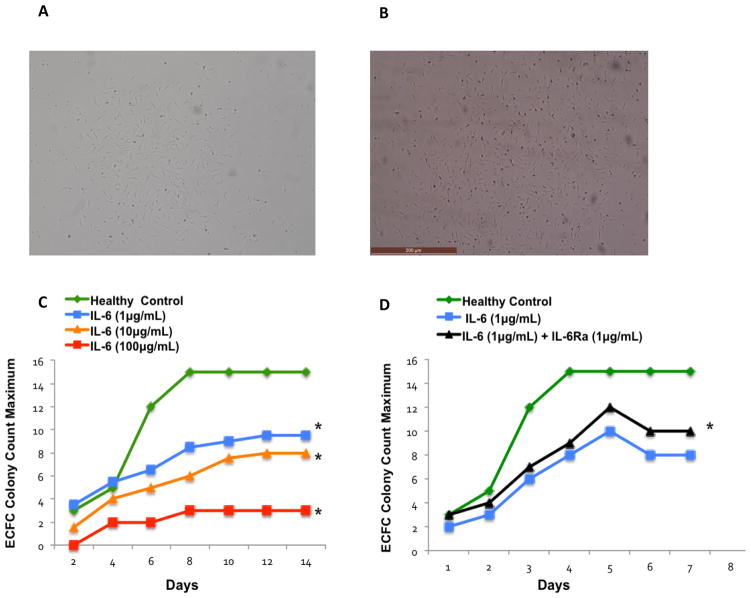
Having observed that IL-6 levels correlated with reduced BM endovascular colony outgrowth in AMI patients, we next sought to confirm the associative findings. To this end, BM samples from three healthy individuals were cultured in ECFC assay and treated with increasing concentrations of IL-6. ECFC colony numbers were enumerated every other day for 14 days. BM in control conditions generated ECFC colonies as expected (Figure 2A). However, when cultures were exposed to IL-6, the number of ECFC colonies decreased in a dose-dependent fashion (Figure 2C). ECFC colony counts were significantly decreased after exposure to 1 μg/mL of IL-6 (mean±SE: 7.42±1.17 P=0.014), 10 μg/mL of IL-6 (mean±SE: 5.71±1.18 P=0.002), and100 μg/mL of IL-6 (mean±SE: 2.28±1.60, P=0.001) in comparison with the healthy reference group (mean±SE: 11.42 ±1.97, Figure 2C). These results support the finding that IL-6 exerted an inhibitory action on BM-derived endothelial progenitor cell colony outgrowth in patients with AMI.
Figure 2. IL-6 Receptor Antagonist Improved Bone Marrow-Derived Vasculogenic Colony Outgrowth * P-value<0.05.
(A) A representative phase-contrast micrograph of an ECFC colony without IL-6 treatment. (B) A representative phase-contrast micrograph of an ECFC colony after IL-6 treatment. (C) BM mononuclear cells from three healthy individuals were treated with increasing concentrations of IL-6. The cells were cultured in ECFC assays and colony numbers were enumerated every other day for 14 days. IL-6 produced a dose-dependent impairment of BM endovascular colony outgrowth. ECFC colony count maximum was decreased after exposure to 1 μg/mL of IL-6 (mean±SD: 7.42±2.31, P=0.01), 10 μg/mL of IL-6 (mean±SD: 5.71±2.41, P=0.002) and 100 μg/mL of IL-6 (mean±SD: 2.28±1.11, P=0.001) vs. healthy control group (mean±SD: 11.42 ±4.43). (D) Colony numbers of the BM-MNCs, treated with 1 μg/mL of IL-6 in ECFC assays, were compared with ECFC colony counts after adding 1 μg/mL IL-6Ra. ECFC colony count maximum significantly increased after exposure to IL-6Ra (mean±SD: 7±3.64, P=0.003) vs. control group (mean±SD: 5.8 ±3.34). IL-6Ra improved BM-derived vasculogenic capacity.
We next investigated whether the inhibitory effects of IL-6 on the BM could be reversed. We added an IL-6 receptor antagonist (IL-6Ra) to BM ECFC cultures that were spiked with IL-6. Again, in conditions with no IL-6Ra, IL-6 reduced the number of BM ECFC colonies compared with untreated controls (Figure 2D). However, the addition of 1 μg/mL of IL-6Ra resulted in increased BM ECFC colonies compared with BM treated with 1 μg/mL of IL-6 and no IL-6Ra (mean±SE: 7±0.2 vs. 5.8 ±1.49, P=0.003) (Figure 2D). These results support the notion that IL-6Ra can rescue BM that has been compromised by IL-6, although the reversal was partial.
Interleukin-1 receptor antagonism improves BM-derived endothelial progenitor colony formation
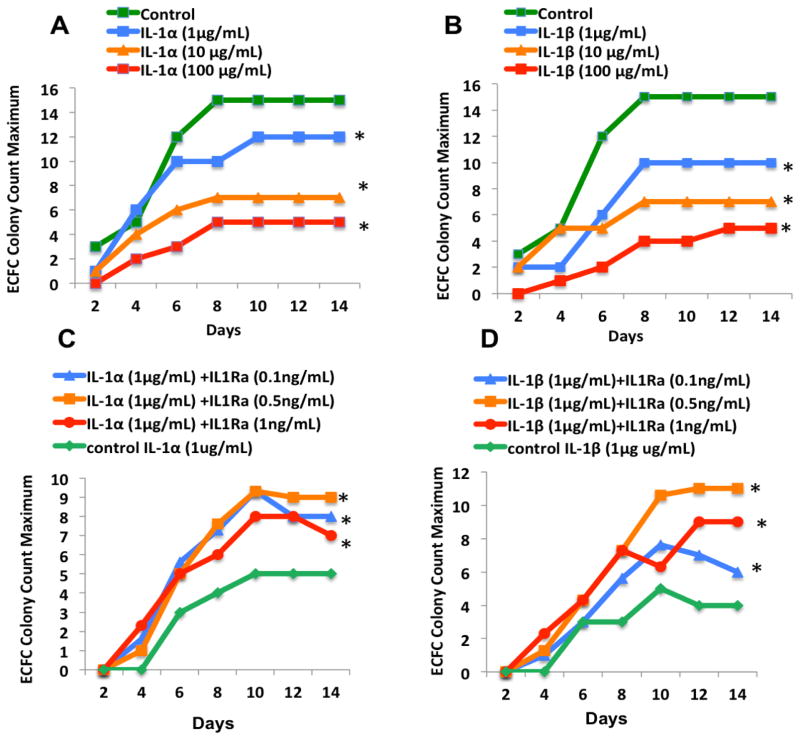
Given IL-6 signaling downstream of IL-1 receptor and the known sequential appearance of IL-1β and IL-6 after AMI, we next sought to examine the effects of IL-17agr;/β on BM-derived vasculogenic colony outgrowth. We observed that BM-MNC colony formation was significantly decreased after exposure to either IL-1α or IL-1β in a dose-dependent manner. ECFC colony counts, after treating with 1μg of IL-1α (8.71±5.5 mean±SE, P=0.01) or 1μg of IL-1β (6±4, P<0.001) were significantly lower compared with the healthy control group without exposure to the cytokine (mean: 11.42±1.97)(Figure 3A and 3B).
Figure 3. Interleukin-1 receptor antagonist (IL-1Ra) improved endothelial colony forming cells (ECFC) outgrowth (* P < 0.05).
Healthy BM-MNCs were cultured in ECFC assay in presence of increasing concentrations of (A) IL-1α and (B) IL-1β (1, 10, or 100 μg/mL). Colony formations enumerated for 2 weeks, and the maximal number of colonies per plate was used for analyses. EFCF colony counts significantly decreased after exposure to IL-1α or IL-1β in a dose-dependent manner. Colony numbers of Healthy BM-MNCs treated with 1μg/mL IL-1α (C) or 1μg/mL IL-1β (D) as a control group, were compared with ECFC colony counts after exposing to increasing concentrations of IL-1Ra (0.1, 0.5, 1ng/mL). EFCF colony outgrowth improved significantly after treating with IL-1Ra
We next examined whether inhibition of IL-1R could improve the ECFC colony outgrowth. We treated the BM-MNCs, separately with either IL-1α or IL-1β in a control group and compared the colony formation after the addition of incrementally increasing doses of IL-1R antagonist (IL-Ra). We observed that the ECFC colony outgrowth significantly improved with IL-1Ra compared with the control group (Figure 3C and 3D).
DISCUSSION
In this study, we report the levels of circulating angiogenic and inflammatory cytokines in patients 2–3 weeks after AMI. We found that patients with increased PB levels of the angiogenic cytokine PDGF-BB exhibited increased BM-derived endothelial and mesenchymal colony outgrowth. In contrast, AMI participants with increased PB levels of the inflammatory cytokine, IL-6, showed decreased BM-derived endothelial progenitor cell outgrowth. These results support our hypothesis that cytokine signaling after AMI alters BM phenotype and function, a phenomenon that may underlie the mixed clinical response to autologous BM cells after AMI.
Following AMI, dying cardiomyocytes trigger a systemic inflammatory response through activation of the nuclear factor-κB pathway, which induces release of many pro-inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α.22 The sequential appearance of IL-1β and IL-6 is known to initiate and regulate the inflammatory response after AMI.23–25 Our results indicate that participants with higher IL-6 after AMI show larger reductions in endothelial progenitor cell capacity in BM. Moreover, our experimental study supported a role of IL-6 in the impairment of BM-derived vasculogenic capacity. Although our results are consistent with an important function of IL-6 late after AMI in impairment of autologous BM, we found that IL-1α and IL-1β levels in the peripheral blood were low. Given that previous rat studies have shown higher levels of circulating cytokines early (hours-to-days) after AMI compared with late (weeks) after AMI,26 it is possible that IL-1α and IL-1β levels in patients may be higher at earlier time points (days) after AMI and then abate later (weeks). Serial measurements of PB cytokines in humans beginning early after AMI will be necessary examine this possibility.
In a prior observational study of ST segment elevated AMI patients, the combined analysis of the circulating pro-inflammatory cytokine, IL-6, and the circulating anti-inflammatory cytokine, IL-10, identified high-risk patients with worse clinical outcomes.21 Patients with high IL-6 and high IL-10 in PB had an increased risk of LV systolic dysfunction at hospital discharge and an increased risk of death at 6-months. The current study confirms and extends the importance of IL-6 as a potential prognostic risk factor and potential target for reversing bone marrow impairment after AMI.
Although no significant associations were found between IL-6 with baseline EF or IL-6 with EF after cell therapy, the concept that IL-6 as a pro-inflammatory cytokine may be associated with greater reduction in regenerative capacity of BM is worthy of further study. Another important consideration is the appropriateness of EF as an outcome of interest. It is possible that LVEF, LVESV and LVEDV are not appropriate outcome measurements. Other measures of cardiac function such as left ventricular global function index 27; return of functional capacity, cardiac symptoms, and MACE events might be more helpful in defining significant correlations between cytokines and cardiac function.
BMC therapy after AMI has shown minimal to no improvement in left ventricular function after reperfusion.5 There is much controversy about autologous BMC therapy with different views. Preclinical studies indicate that locally delivered BMCs can regenerate de novo myocardium.28 Further, parabiosis experiments suggest that stem/progenitor cell regeneration of the infarcted myocardium may occur in the early time period after intramyocardial injection; however, over time, most of the regenerated cells either die or migrate elsewhere.29 In a recent cell therapy experiment, 14 cytokines released from the infarcted myocardium were implicated as impairing the regenerative potential of bone marrow cells. Thus, the cardiovascular cell therapy community has struggled to reconcile experimental results with clinical trial experience. In this study, we sought reasons for the lack of cardiac function improvement after autologous bone marrow cell therapy and used LateTIME data for this exploration. Results of this study support the concept that inflammatory cytokines released after AMI impair the reparative ability of the BM, which may explain the lack of efficacy seen in autologous BM cell therapy studies. Our data suggest that PDGF-BB may be a biomarker for positive response to cell therapy and IL-6 may be a key offender two weeks after AMI.
However, we also found that blocking IL-6 or IL-1 receptors using an IL-6 receptor antagonist or IL-1 receptor antagonist in vitro mitigated the inflammatory cytokines’ repressive effects on BM. These findings may be clinically relevant since inhibitors to these cytokines are already FDA approved and could be applied after AMI with the intent of improving the regenerative capacity of autologous BM cells.
Prior experimental studies of IL-6R inhibition in rodent models of acute MI have generated mixed results. Whereas a first study of IL-6R inhibition in acute MI showed suppression of myocardial inflammation, improvement in LV function and lengthened survival time, a follow-up study showed worsened LV function but no histologic changes after IL-6R inhibition.30, 31 There were significant methodological differences between the two pre-clinical studies, with the first using single dose of IL-6R antibodies in a mouse coronary ligation model assessed by echocardiography and the second using repeated dosing of IL-6R antibodies in an ischemia-reperfusion mouse model assessed by cardiac nuclear magnetic resonance (NMR) imaging. Common to both studies were reductions in inflammatory cytokines after acute MI. However, it is possible that a single dose of IL-6R-antagonist after acute MI neutralized the acute increase of IL-6 and prevented LV remodeling, whereas prolonged IL-6R-antagonism neutralized IL-6 in the acute setting but also prevented wound healing in later phases after acute MI. In clinical translation, a recent phase I clinical trial of a single dose of IL-6 receptor antagonist, tocilizumab, prior to coronary angiography in patients with non-ST-elevated MI (NSTEMI) suppressed the levels of PCI-related inflammatory biomarkers after acute MI and secondarily reduced troponin release.32 This early phase clinical trial demonstrated the feasibility of single dose IL-6R antagonism in patients with acute MI. It is noted that this study was not designed to examine efficacy, but serves as the basis for designing and executing such phase II and III clinical trials of IL-6R antagonism in patients with acute MI.
The cytokine IL-1β is best known for its role in inflammation, but it also participates in a host of other physiologic processes including the regulation of BM hematopoietic stem and progenitor cells (HSPCs), which express IL-1R.33 IL-1β mobilizes HSPCs from the bone marrow by enhancing transendothelial migration between or through BM endothelial cells.34 IL-1β also induces IL-6 secretion, stimulates prostaglandin production, alters HSPC differentiation and at certain doses can impair HSPC colony outgrowth and self-renewal.35 Results from this report extend prior findings by showing that IL-1β impairs BM-derived vasculogenic activity. We also demonstrate that this impairment can be mitigated by IL-1RA. Already, investigators have attempted to block IL-1 in patients with cardiovascular diseases. A placebo-controlled, randomized study of the recombinant IL-1RA, anakinra, in 30 patients with STEMI showed that anakinra significantly reduced circulating C-reactive protein (CRP) levels and reduced the proportion of STEMI patients who developed heart failure.36, 37 Another placebo-controlled, randomized phase II clinical trial of anakinra in patients with non-STEMI acute MI showed significant reduction in circulating C-reactive protein levels.38 Although this prior study was not powered to assess clinical outcomes, there was an increase in the MACE composite for the anakinra-treated group, primarily driven by recurrent MI. In both anakinra studies, the treatment dose (100 mg/day) and duration (14 days) clearly blunted the acute inflammatory reaction after MI. Yet to be determined is whether a shorter course of IL-1 blockade adequately diminishes deleterious immune reactivity after acute MI while avoiding late adverse effects. Another strategy of blocking IL-1 activity is via a soluble decoy receptor, rilonacept, which binds and neutralizes IL-1β, IL-1α and IL-1RA. This drug is FDA approved for use in patients with IL-1β-driven inflammatory disorders such as cryopyrin-associated periodic syndrome (CAPS), including the NLRP3-mutant diseases of familial cold auto-inflammatory syndrome (FCAS) and Muckle-Wells Syndrome (MWS). In an experimental model of acute MI, mice treated with a murine IL-1 decoy receptor (IL-1 Trap) had more favorable cardiac remodeling and reduced infarct sizes than saline-control animals.39 There are no published studies rilonacept in patients with cardiovascular disease. Another strategy for IL-1 blockade uses the monoclonal anti-human IL-1β antibodies, canakinumab, which is approved for the use in patients with CAPS. Canakinumab is currently being investigated in a large, multi-national, placebo-controlled, randomized clinical trial involving patients with acute MI and persistent elevation of high-sensitivity CRP levels (CANTOS trial, NCT01327846).40 With specific regard to cardiovascular cell therapy trial design, it is envisioned that harvested autologous bone marrow cells would be incubated ex vivo with lower dose and shorter exposure time to the cytokine receptor antagonist prior to administration back to the patient.
When interpreting our results, a few considerations are required. First, the timing of cytokine sampling in this study was 2–3 weeks after AMI. At this time point, acute phase reactants have waned. Thus, the important biology that we present in this report is only part of the post-MI picture. Second, our in vitro experiments with IL-6 and IL-1β contained higher concentrations compared to the cytokine concentrations detected in patient PB 2–3 weeks after AMI. The primary intent of the laboratory experiments was to establish a proof of concept whether IL-6 or IL-1β reduced, increased or had no effect on bone marrow-derived colony outgrowth. Agnostic to the effects, our results are clearly consistent with a dose-dependent impairments of bone marrow colony capacity. One major caveat is that our experiments were not designed to define absolute cytokine concentrations relative to bone marrow function. Local concentrations of IL-6 and IL-1α/β after MI in either cardiac tissue or bone marrow are unknown and were not sampled in this study. Furthermore, because our PB sampling was in the subacute phase after MI, we may have missed the peak PB concentrations of IL-6 and IL-1β. Although our results support the overarching concept of IL-6 or IL-1β cytokine impairment of autologous bone marrow cells after AMI, any extrapolation from culture dish to human application would be fraught with assumptions and deserving of designed for biological scaling.
Limitations
This study examined baseline cytokine levels from patients recruited to the LateTIME study. The clinical trial was not designed nor powered to define absolute cytokine levels or determine exact dose and schedule of cytokine receptor antagonists. Results from this study are proof of concept and not intended to direct therapeutic decision-making. Additionally, several PB cytokines were at concentrations below the limit of assay detection. Given these low concentrations and to further appraise our multiple regression analysis strategy, we conducted sensitivity analyses at three levels, which confirmed the significant association of IL-6 and BM-derived vasculogenic colony outgrowth.
Conclusions
Two to three weeks after AMI, circulating cytokine concentrations associated with BM function and response to autologous BM cell therapy. PDGF-BB positively correlated with BM-derived endovascular clonogenic capacity. IL-6 and IL-1β adversely impacted BM colony outgrowth, which may have contributed to the lack of efficacy seen in autologous BM cell therapy studies. Blocking the IL-6 receptor or IL-1 receptor restored BM endovascular function, suggesting a potential new therapeutic strategy for restoring autologous BM function after AMI.
NOVELTY AND SIGNIFICANCE.
What Is Known?
Bone marrow (BM) contains stem and progenitor cells capable of regenerating blood vessels in response to ischemia and inflammation.
Experimental studies showed BM cell (BMC) therapy improved left ventricular (LV) function in animal models of acute myocardial infarction (AMI). However, only minimal LV function improvement has been reported after BMC therapy in AMI patients.
While experimental AMI models have implicated cytokine-related impairment of BM cell regeneration, such a response has yet to be studied in humans.
What New Information Dose This Article Contribute?
Patients with higher peripheral blood (PB) levels of IL-6 two to three weeks after AMI had impaired BM vasculogenic capacity.
Patients with higher PB levels of platelet-derived growth factor BB protein (PDGF-BB) two to three weeks after AMI had improved BM vasculogenic capacity.
IL-6 and IL-1β impaired BM vasculogenic capacity, which could be reversed by blocking IL-6R and IL-1R, respectively.
Bone marrow contains cells capable of regenerating the heart after acute myocardial infarction in experimental models. However, in BM cell therapy clinical trials, human patients have shown minimal functional improvement. Experimental evidence suggested that inflammatory cytokines may impair the cardiac repair capacity of BM cells. Here, we examined the peripheral blood cytokine levels from a large number of patients two to three weeks after acute MI. We report significant associations between cytokine levels and bone marrow function. Our study identified IL-6, IL-1β and PDGF-BB as important cytokines that may regulate autologous BM cell repair of ischemic heart disease in patients. The impairment of IL-6 and IL-1 β can be reversed by blocking IL-6R and IL-1R, respectively. Our study provides a mechanistic understanding of the mixed results seen with autologous BM cell clinical trials and suggests methods of improving this application.
Acknowledgments
SOURCES OF FUNDING
This study was supported by National Institutes of Health (NIH) network grants (National Heart, Lung, and Blood Institute [NHLBI] UM1 HL087318-08); NIH R01 to Pepine and Cogle (NHLBI R01 HL091005). MS was supported by an NIDDK training grant to the Division of Nephrology, Hypertension, and Renal Transplantation at the University of Florida (T32DK104721).
The authors acknowledge the contributions of Curtis G Gravance, PhD, MS, for his editing of the manuscript and James Colee for his assistance with biostatistics.
Nonstandard Abbreviations and Acronyms
- AMI
Acute myocardial infarction
- BM
Bone marrow
- BMC
Bone marrow cell
- CCTRN
Cardiovascular Cell Therapy Research Network
- CFC
Colony forming cell
- CFU
Colony forming unit
- ECFC
Endothelial colony forming cell
- CFUEC
Colony forming unit-endothelial colony
- CFU-F
Colony forming unit fibroblast
- ECG
Electrocardiogram
- EDV
End diastolic volume
- EVD-D
End diastolic volume difference
- EGM-2
Endothelial growth media-2
- EF
Ejection fraction
- ESE
Estimate ± standard error
- ESV
End systolic volume
- FGF
Fibroblast growth factors
- G-CSF
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- LV
Left ventricular
- LVD
Left ventricular dysfunction
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- MNC
Mononuclear cell
- MRI
Magnetic resonance imaging
- MSC
Mesenchymal stromal cell
- PB
Peripheral blood
- PDGF-BB
Platelet-derived growth factor BB dimeric glycoprotein
- RANTES
Chemokine (C-C motif) ligand 5
- SD
Standard deviation
- SDF-1
Stromal cell-derived factor 1
- STEMI
ST elevation AMI
- VEGF
Vascular endothelial growth factor
Footnotes
DISCLOSURES
The authors have no disclosures of conflict of interest. Dr. Joshua Hare reports equity ownership and board membership in Vestion. Vestion did not fund or participate in this research. The opinions expressed by one of the authors (RFE) do not necessarily reflect those of the National Institutes of Health, the National Heart, Lung, and Blood Institute, or the US Department of Health and Human Services.
References
- 1.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. The New England journal of medicine. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 3.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy R. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The latetime randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research N. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye LA, Surder D, Corti R, Huikuri H, Miettinen J, Wohrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G Investigators A. Meta-analysis of cell-based cardiac studies (accrue) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–1360. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;9:CD006536. doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cogle CR, Wise E, Meacham AM, Zierold C, Traverse JH, Henry TD, Perin EC, Willerson JT, Ellis SG, Carlson M, Zhao DX, Bolli R, Cooke JP, Anwaruddin S, Bhatnagar A, da Graca Cabreira-Hansen M, Grant MB, Lai D, Moye L, Ebert RF, Olson RE, Sayre SL, Schulman IH, Bosse RC, Scott EW, Simari RD, Pepine CJ, Taylor DA Cardiovascular Cell Therapy Research N. Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: Bm cd34, cd11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115:867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutt RC, Trachtenberg BH, Cooke JP, Traverse JH, Henry TD, Pepine CJ, Willerson JT, Perin EC, Ellis SG, Zhao DX, Bhatnagar A, Johnstone BH, Lai D, Resende M, Ebert RF, Wu JC, Sayre SL, Orozco A, Zierold C, Simari RD, Moye L, Cogle CR, Taylor DA Cardiovascular Cell Therapy Research N. Bone marrow characteristics associated with changes in infarct size after stemi: A biorepository evaluation from the cctrn time trial. Circulation research. 2015;116:99–107. doi: 10.1161/CIRCRESAHA.116.304710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor DA, Perin EC, Willerson JT, Zierold C, Resende M, Carlson M, Nestor B, Wise E, Orozco A, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Traverse JH, Cooke JP, Schutt RC, Bhatnagar A, Grant MB, Lai D, Johnstone BH, Sayre SL, Moye L, Ebert RF, Bolli R, Simari RD, Cogle CR. Identification of bone marrow cell subpopulations associated with improved functional outcomes in patients with chronic left ventricular dysfunction: An embedded cohort evaluation of the focus-cctrn trial. Cell Transplant. 2015 doi: 10.3727/096368915X689901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG. The immune system and cardiac repair. Pharmacological research : the official journal of the Italian Pharmacological Society. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Takagawa J, Lam VC, Haddad DJ, Tobler DL, Mok PY, Zhang Y, Clifford BT, Pinnamaneni K, Saini SA, Su R, Bartel MJ, Sievers RE, Carbone L, Kogan S, Yeghiazarians Y, Hermiston M, Springer ML. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Science translational medicine. 2011;3:100ra190. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zierold C, Carlson MA, Obodo UC, Wise E, Piazza VA, Meeks MW, Vojvodic RW, Baraniuk S, Henry TD, Gee AP, Ellis SG, Moye LA, Pepine CJ, Cogle CR, Taylor DA. Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular cell therapy research network biorepository core laboratory rationale. Am Heart J. 2011;162:973–980. doi: 10.1016/j.ahj.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Simpson LM, Penn MS, Byrne BJ, Perin EC, Gee AP, Hatzopoulos AK, McKenna DH, Forder JR, Taylor DA, Cogle CR, Baraniuk S, Olson RE, Jorgenson BC, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moye LA, Simari RD Cardiovascular Cell Therapy Research N. Latetime: A phase-ii, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Tex Heart Inst J. 2010;37:412–420. [PMC free article] [PubMed] [Google Scholar]
- 17.Dewberry RM, King AR, Crossman DC, Francis SE. Interleukin-1 receptor antagonist (il-1ra) modulates endothelial cell proliferation. FEBS letters. 2008;582:886–890. doi: 10.1016/j.febslet.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Betts BC, St Angelo ET, Kennedy M, Young JW. Anti-il6-receptor-alpha (tocilizumab) does not inhibit human monocyte-derived dendritic cell maturation or alloreactive t-cell responses. Blood. 2011;118:5340–5343. doi: 10.1182/blood-2011-06-363390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Hein MJ, Deddens JA, Hines CJ. Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using sas. The Annals of occupational hygiene. 2011;55:97–112. doi: 10.1093/annhyg/meq061. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Liu P, Min J, Zhang H. application of multiple limits regression in dealing with nondetects with multiple limits of detect. Wei sheng yan jiu = Journal of hygiene research. 2008;37:745–747. [PubMed] [Google Scholar]
- 21.Ammirati E, Cannistraci CV, Cristell NA, Vecchio V, Palini AG, Tornvall P, Paganoni AM, Miendlarzewska EA, Sangalli LM, Monello A, Pernow J, Bjornstedt Bennermo M, Marenzi G, Hu D, Uren NG, Cianflone D, Ravasi T, Manfredi AA, Maseri A. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6-interleukin-10+ cytokine patterns in st-elevation acute myocardial infarction. Circulation research. 2012;111:1336–1348. doi: 10.1161/CIRCRESAHA.111.262477. [DOI] [PubMed] [Google Scholar]
- 22.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circulation research. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 23.Puhakka M, Magga J, Hietakorpi S, Penttila I, Uusimaa P, Risteli J, Peuhkurinen K. Interleukin-6 and tumor necrosis factor alpha in relation to myocardial infarct size and collagen formation. Journal of cardiac failure. 2003;9:325–332. doi: 10.1054/jcaf.2003.38. [DOI] [PubMed] [Google Scholar]
- 24.Guillen I, Blanes M, Gomez-Lechon MJ, Castell JV. Cytokine signaling during myocardial infarction: Sequential appearance of il-1 beta and il-6. The American journal of physiology. 1995;269:R229–235. doi: 10.1152/ajpregu.1995.269.2.R229. [DOI] [PubMed] [Google Scholar]
- 25.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB, Framingham Heart S. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The framingham heart study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 26.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovascular research. 2002;55:329–340. doi: 10.1016/s0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 27.Mewton N, Opdahl A, Choi EY, Almeida AL, Kawel N, Wu CO, Burke GL, Liu S, Liu K, Bluemke DA, Lima JA. Left ventricular global function index by magnetic resonance imaging--a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: The multi-ethnic study of atherosclerosis. Hypertension. 2013;61:770–778. doi: 10.1161/HYPERTENSIONAHA.111.198028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 29.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 30.Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, Matsubara H, Nakata T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res. 2010;87:424–430. doi: 10.1093/cvr/cvq078. [DOI] [PubMed] [Google Scholar]
- 31.Hartman MH, Vreeswijk-Baudoin I, Groot HE, van de Kolk KW, de Boer RA, Mateo Leach I, Vliegenthart R, Sillje HH, van der Harst P. Inhibition of interleukin-6 receptor in a murine model of myocardial ischemia-reperfusion. PloS one. 2016;11:e0167195. doi: 10.1371/journal.pone.0167195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Espevik T, Aakhus S, Damas JK, Aukrust P, Wiseth R, Gullestad L. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin t release in patients with non-st-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. European heart journal. 2016;37:2406–2413. doi: 10.1093/eurheartj/ehw171. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 34.Voermans C, Rood PM, Hordijk PL, Gerritsen WR, van der Schoot CE. Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells. 2000;18:435–443. doi: 10.1634/stemcells.18-6-435. [DOI] [PubMed] [Google Scholar]
- 35.Yonemura Y, Ku H, Hirayama F, Souza LM, Ogawa M. Interleukin 3 or interleukin 1 abrogates the reconstituting ability of hematopoietic stem cells. Proc Natl Acad Sci U S A. 1996;93:4040–4044. doi: 10.1073/pnas.93.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the virginia commonwealth university-anakinra remodeling trial (2) (vcu-art2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW Investigators V-A. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (virginia commonwealth university anakinra remodeling trial [vcu-art] pilot study) Am J Cardiol. 2010;105:1371–1377. e1371. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 38.Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-st elevation acute coronary syndromes: The mrc-ila heart study. Eur Heart J. 2015;36:377–384. doi: 10.1093/eurheartj/ehu272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, Toldo S, Smithson L, Hoke NN, Chau VQ, Robati R, Abbate A. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55:117–122. doi: 10.1097/FJC.0b013e3181c87e53. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (cantos) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. doi: 10.1155/2013/434010. [DOI] [PMC free article] [PubMed] [Google Scholar]