Abstract
Advances in medical genetics and imaging have resulted in a significant increase in the number of diagnosed thoracic aortic aneurysms. Recent findings establish a link between diminished nicotinamide phosphoribosyltransferase (NAMPT) and compromised smooth muscle cell vitality in aortic dilatation. These findings have myriad implications given the diverse roles of NAMPT, which is central to the production of nicotinamide adenine dinucleotide (NAD+), and thus ATP production, as well the activity of multiple NAD+ consuming proteins. Given its central role in vascular cell vitality and thus matrix integrity, further attention should be directed to the NAMPT-NAD+ control system in thoracic aortopathy.
Keywords: actomyosin activity, apoptosis, ATP, mechanotransduction, inflammation
Aging is a primary risk factor for many cardiovascular diseases. The manifold effects of vascular aging result, in part, from adverse phenotypic changes to endothelial cells and vascular smooth muscle cells (VSMCs), increased inflammation, and associated changes in extracellular matrix (ECM) composition, structure, and mechanical properties1. These aging-related changes progress in different ways throughout the vasculature. Aging of the aorta manifests at the vessel level as a gradual dilatation and structural stiffening, which impact the global hemodynamics and local wall mechanics. In particular, a stiffer aorta increases the pulse wave velocity, which can augment the pulse pressure in the proximal (thoracic) aorta and increase mechanical stress. Increases in mechanical loading are typically sensed by the VSMCs and result in changes in gene expression and downstream gene products that affect the composition and structure of the aortic wall, thus establishing a feedback loop connecting mechanical loading, cell function, and structural integrity2.
In addition to genetic mutations and uncontrolled hypertension, aging is an important risk factor for the development of thoracic aortic aneurysms (TAAs). Defined as a 50% or greater dilatation of the aorta, these lesions are characterized histopathologically by damaged elastic fibers, compromised smooth muscle function, pooled mucoid material, and remodeled collagen fibers2, all of which are seen to a lesser degree in aortic aging1. It seems that vascular aging either primes the thoracic aorta for aneurysmal dilatation in response to second insults or it exacerbates dilatation once initiated via other mechanisms, including consequences of particular genetic mutations. The collection of genetic mutations that predispose to TAAs – affecting VSMC contractile proteins, transmembrane proteins, and select ECM proteins – suggests further that the loss of aortic wall integrity that characterizes these lesions may result from dysfunctional mechano-sensing or mechano-regulation of the ECM by the VSMCs2. Regardless, the VSMC serves as a central node in establishing, maintaining, or repairing the aortic media, the layer of the aortic wall most affected in TAAs. VSMC vitality is central to aortic health.
NAMPT, Genome Integrity, and Aortopathy
Nicotinamide phosphoribosyltransferase (NAMPT) is a critical rate-limiting enzyme involved in the production of nicotinamide adenine dinucleotide (NAD+), a key molecule of cellular metabolism as well as gene integrity and gene expression3,4. NAD+ plays a fundamental role in generating adenosine triphosphate (ATP), which is essential to many cellular processes including metabolism, molecular biosynthesis and biotransport, and actomyosin-mediated motions. ATP is also important in signal transduction, as, for example, in the production of the second messenger cAMP and when used by kinases to phosphorylate proteins. NAD+ is not only central to many downstream gene products, it plays additional roles when consumed by sirtuins (SIRT), poly(ADP-ribose) polymerases (PARP), and CD38 in their functions.
Watson and colleagues5 report provocative findings in human tissue that correlate the NAMPT-NAD+ control system with thoracic aortic dilatation. Among other observations, the NAMPT promoter was hypermethylated in situ and in vitro in VSMCs from patients with dilated ascending aortopathy, and lower levels of NAMPT correlated with increased DNA breaks. The authors then generated VSMC-specific Nampt deficient mice to test the hypothesis that diminished NAMPT leads to aortic dilatation. These mice exhibited an ~40% reduction in aortic NAD+ and, importantly, presented with mild dilatation, especially in the ascending aorta. When challenged with chronic infusion of angiotensin II (AngII), which increases hemodynamic loads and inflammation, the VSMC-Nampt deficient aortas exhibited regions of VSMC loss and intramural hemorrhage, not unlike lesions seen with VSMC-specific postnatal disruption of transforming growth factor-beta receptor II6. Both of these studies report localized accumulations of mucoid material, similar to that in human histopathology and a potential initiator of dissection7. Watson et al.5 conclude that aortic integrity depends on an intrinsic NAMPT-NAD+ system, which when compromised results in unrepaired DNA damage and VSMC senescence.
VSMC Vitality
A striking finding in the pathologic aortas of the AngII-infused VSMC-Nampt deficient mice was loss of VSMCs5. Although TUNEL staining could not document VSMC apoptosis after 28 days of infusion, the evident senescence and yet dramatic loss of medial cells suggest that many VSMCs could have undergone apoptosis earlier. Indeed, knockdown of Nampt in VSMCs has been shown to increase VSMC apoptosis whereas overexpression of Nampt can enhance VSMC survival8.
Why this focus on the possibility that there was apoptosis? Increased VSMC apoptosis has been observed in the ascending aorta of the most commonly studied animal model of Marfan syndrome, the Fbn1C1039G/+ mouse, and blocking caspase activity using a pan-caspase inhibitor prevents aortic enlargement9. Moreover, these apoptotic VSMCs have increased elastolytic potential when compared with viable cells, and thus can contribute further to the compromised ECM that is characteristic of aneurysmal dilatation. We have also shown that loss of the forkhead transcription factor Foxe3 increases VSMC apoptosis and rupture when the thoracic aorta is stressed mechanically by increasing blood pressure using aortic constriction; these ruptures are prevented by administering a p53 inhibitor (pifithrin-α) or crossing the Foxe3−/− mice with p53−/− mice10. Finally, loss of VSMC attachment to the ECM can drive a form of apoptosis referred to as anoikis (Greek for homeless or wandering). Anoikis-related cell drop-out would be expected following loss of ECM mechano-sensing. Thus, loss of VSMCs can contribute to dilatation and dissection/rupture of the thoracic aorta, suggesting that early VSMC apoptosis could contribute to pathology in AngII infused VSMC-specific Nampt deficienct mice.
Inflammation and SIRT1
Inflammation is a hallmark of vascular aging1 and the associated aortic stiffening that adversely affects the hemodynamics and mechanobiology. Although it is unclear whether inflammation is a consistent initiator of TAAs, inflammatory cells co-localize with medial degeneration in these lesions and appear to contribute to the progressive pathology11. Among the many adverse effects of inflammation on the aortic wall, including an excessive proteolytic activity that can compromise structural integrity, increases in oxidative stress can drive cellular damage and dysfunction. Noting that NAD+ bioavailability decreases with age, augmenting the NAMPT-NAD+ system via nicotinamide mononucleotide (NMN; see Figure) supplementation reduces oxidative stress within the aorta of aged mice and associated decreases in intramural collagen and aortic stiffness reduce the pulse wave velocity12. One possible driver of this beneficial finding is that increased NAD+ increases SIRT-1, a NAD+ consuming deacetylase (Figure). Additional findings show that, among other effects, SIRT-1 downregulates AngII type 1 receptors, which reduces oxidative stress in the aorta and its sequelae. Not surprisingly, chronic AngII infusion within VSMC-specific Sirt1 knock-out mice results in marked increases in oxidative stress and matrix metalloproteinase activity leading to increased aortic stiffness and increased dissection13.
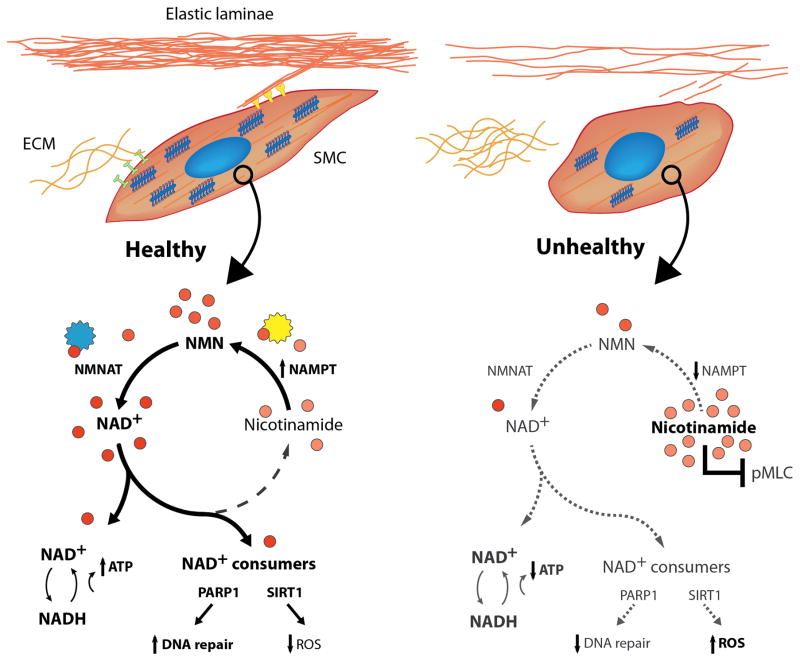
Figure 1.
Healthy (spindle-shaped; left) and unhealthy (rounded; right) vascular SMCs, the latter with few connections to ECM and lower actomyosin activity. Shown too, the salvage biosynthesis pathway with select NAD+ consuming proteins (SIRT1, PARP1) leading to important biological outcomes. Increased NAMPT-NAD+ has multiple effects, including suppression of inflammation and direct and indirect influences on contractility. NMNAT = nicotinamide mononucleotide adenylyltransferase 1, NADH = nicotinamide adenine dinucleotide hydrate, ROS = reactive oxygen species, and pMLC (phosphorylated myosin light chain). Courtesy Dr. S-I. Murtada.
ECM Integrity and VSMC Contractility
The ECM of a healthy aorta consists of myriad proteins, glycoproteins, and glycosaminoglcans organized within a multi-layered structure – the intima, media, and adventitia. Normal ECM composition and structure endows the aortic wall with considerable compliance and resilience (allowing the media to bear most of the normal pulsatile loading and to store elastic energy during diastole that can be used to augment flow during systole), but also sufficient strength (primarily within the adventitia, which serves as a protective sheath that prevents acute increases in pressure-induced mechanical stresses from damaging the media). With the exception of the elastic fibers, which are produced early on and typically have a long half-life, most ECM constituents turnover continually during mechanical homeostasis. VSMCs are fundamental to the synthesis, maintenance and, if needed, repair of medial ECM, which requires actomyosin activity both to sense (e.g., assess the stress in or stiffness of) and regulate (i.e., organize newly synthesized) the ECM2. For this reason, mutations to genes affecting actomyosin activity, including ACTA2 (which encodes smooth muscle α-actin) or MYH11 (which encodes smooth muscle myosin heavy chain), contribute to the propensity to TAAs15.
VSMC contractility results from ATP-driven interactions between actin and myosin filaments. The NAMPT-NAD+ control system can affect VSMC contractility in multiple ways, particularly via the production of ATP that fuels actomyosin interactions and the prevention of cell senescence or apoptosis, both of which necessarily reduce overall contractile capacity. Noting that nicotinamide (a key molecule in the salvage pathway for NAD+ synthesis; Figure) can attenuate VSMC contractility by blocking phosphorylation of myosin light chain, a recent study showed further that an inhibitor (rucaparib) of the NAD+-consuming DNA-repair enzyme PARP-1 also attenuates VSMC contraction14, perhaps in a similar manner. A corollary, therefore, is that NAMPT deficiency could increase nicotinamide and thereby decrease VSMC contractility. Hence, despite some controversy4,5, the NAMPT-NAD+ control system may influence actomyosin activity directly and indirectly and thereby affect VSMC contraction-mediated ECM integrity.
Conclusion
TAAs are responsible for significant morbidity and mortality. Advances in genetics, molecular and cellular biology, medical imaging, and bioengineering have advanced our understanding of this disease, yet clear pharmacotherapy remains elusive. The paper by Watson and colleagues5 further highlights the complexity of this disease process. We concur that increased attention should be directed to the NAMPT-NAD+ control system in studying thoracic aortopathy, but with broad appreciation of its myriad direct and indirect temporal effects on cell vitality and thus matrix integrity.
Acknowledgments
Sources of Funding
This work was supported, in part, by grants from the NIH (R01 HL105297, U01 HL116323, R01 HL109942, P01 HL 110869) and the John Ritter Foundation.
Footnotes
Disclosures: none
References
- 1.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: Focus on thoracic aortic aneurysms and dissections. Circ Res. 2015;116:1448–1461. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Li W-L, Liu J-M, Miao C-Y. NAMPT and NAMPT-controlled NAD metabolism in vascular repair. J Cardiovasc Pharmacol. 2016;67:359–360. doi: 10.1097/FJC.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 5.Watson A, Nong Z, Yin H, et al. Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration and is suppressed in human thoracic aneurysm disease. Circ Res. 2017;xx:xxx–xxx. doi: 10.1161/CIRCRESAHA.116.310022. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Li Q, Jian Y, et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124:755–757. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roccabianca S, Ateshian G, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol. 2014;13:13–25. doi: 10.1007/s10237-013-0482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Veer E, Nong Z, O’Neil C, et al. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 9.Emrich FC, Okamura H, Dala AR, et al. Enhanced caspase activity contributes to aortic wall remodeling and early aneurysm development in a murine model of Marfan syndrome. ATVB. 2015;35:146–154. doi: 10.1161/ATVBAHA.114.304364. [DOI] [PubMed] [Google Scholar]
- 10.Kuang S-Q, Medina-Martinez O, Guo D-C, et al. FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest. 2016;126:948–961. doi: 10.1172/JCI83778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He R, Guo D-G, Estrera AL, et al. Characterization of inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardivasc Surg. 2006;131:671–678. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 12.De Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry JL, Shiraishi Y, Turcotte R, et al. Vascular smooth muscle sirtuin-1 protects against aortic dissection during angiotensin II-induced hypertension. JAHA. 2015;4:e002384. doi: 10.1161/JAHA.115.002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrudden CM, O’Rourke MG, Cherry KE, et al. Vasoactivity of rucaparib, a PARP-1 inhibitor, is a complex process that involves myosin light chain kinase, P2 receptors, and PARP itself. PLoS One. 2015;10:e0118187. doi: 10.1371/journal.pone.0118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milewicz DM, Trybus KM, Guo DC, et al. Altered smooth muscle force generation as a driver of thoracic aortic aneurysms and dissections. ATVB. 2017;37:26–34. doi: 10.1161/ATVBAHA.116.303229. [DOI] [PMC free article] [PubMed] [Google Scholar]