Abstract
OBJECTIVE
To investigate the relationship between prostate-specific antigen (PSA) level and Gleason sum, and its impact on biochemical failure (persistent PSA level of >0.2 ng/mL) after radical prostatectomy (RP), as the PSA, Gleason sum and clinical stage are commonly used preoperative predictors of outcome in men with localized prostate cancer.
PATIENTS AND METHODS
The Columbia Urologic Oncology Database was reviewed (1988–2006); 3460 had undergone RP. Patients who received neoadjuvant/adjuvant therapy or had incomplete data were excluded, yielding 1932 in the analysed sample. Analysis of variance (ANOVA) methods were used to assess differences in PSA level (on a log scale) among three different groups of patients, categorized by their Gleason sum scores, as <7, 7 and >7. To account for full penetrance of PSA screening, surgery before 1998 was considered as a potential confounder. ANOVA was used to determine whether the association of Gleason score and PSA levels differed before and after 1998. The effect of PSA level on biochemical failure was examined for variance among the three Gleason score groups using a Cox proportional hazards model with time to biochemical failure as the outcome, logPSA, Gleason sum (<7, 7 and >7), their interaction, and clinical stage as the predictors. Concordance indices (c-index) were calculated for the model with and without the interaction term between PSA and Gleason sum to determine its significance.
RESULTS
Of 1932 patients, 1190 (61.6%) had a Gleason sum of <7, 595 (30.8%) of 7 and 146 (7.6%) of >7. The median PSA level was 5.9, 6.1 and 7.8 ng/mL, respectively (P < 0.001). After adjusting for clinical stage, there was no significant interaction effect (P = 0.34) between Gleason sum and time of surgery on PSA level, implying that the relationship between Gleason sum and PSA levels has not changed over these two periods, despite changes in PSA screening. Results from the Cox model showed that PSA level, Gleason sum, their interaction term and clinical stage were significant predictors of biochemical failure. The c-index for the model without the interaction term was 0.70 and increased to 0.72 when including it, indicating an increase in the predictive ability of the model when including the interaction term.
CONCLUSION
PSA level and Gleason sum are highly interrelated variables, although they each carry additional information that significantly contributes to the prediction of biochemical failure. This study shows that, for an individual patient, the higher the initial PSA level the higher the risk of having poorly differentiated prostate cancer. Also, predictive models of biochemical failure can be improved by considering the interaction between PSA and Gleason sum.
Keywords: prostate-specific antigen, Gleason score, interaction term, prostate cancer, outcome
INTRODUCTION
Preoperative PSA level, Gleason sum and clinical stage are the traditional preoperative predictors of outcome after radical prostatectomy (RP) for prostate cancer. The predictive strength of these factors is manifest in many reports of the risk stratification and prediction outcome before RP. The most notable examples are perhaps the nomograms of Partin et al. [1] and Kattan et al. [2], considered by most urologists to be the most well-established and respected predictive models of outcome after RP. These models were built on the premise that PSA level, Gleason sum and clinical stage individually and independently predict the outcome after RP. In combination, the three predictive values allow physicians to accurately predict failure rates at specific periods after surgery. Despite the increased predictive strength of PSA level, Gleason sum and clinical stage when combined, we explored exactly how PSA level and Gleason sum best predicts a patient’s prognosis, and the interaction each of these variables has on the other.
The well-documented changes in the demographics of PSA levels [3,4] and Gleason sum [5], and their subsequent predictive value over the past 20 years, adds an additional degree of complexity. To thoroughly assess the relationship between PSA level and Gleason sum, time must be considered as an important and influential variable. Therefore, it was a secondary objective of the present analysis to examine changes in the relationship between PSA level and Gleason sum as clinical experience has progressed throughout the PSA era.
PATIENTS AND METHODS
The Columbia Urologic Oncology Database was reviewed; 3460 patients were identified who had RP between 1988 and 2006. In all, 1932 patients were included with complete preoperative data; patients who received neoadjuvant/adjuvant therapy or had incomplete data were excluded.
Patients were stratified into groups with a Gleason sum of <7, 7 or >7, to approximate low-, intermediate- and high-risk prostate cancer features (according to the modified criteria of D’Amico et al. [6]), respectively. ANOVA techniques were used to assess differences in PSA level on the log scale among the Gleason groups. We were able to verify differences in the PSA distribution on the raw scale using a Kruskal-Wallis test. As a Gleason score of 7 might have arisen via different major and minor scores (3 + 4 vs 4 + 3), and as there might be clinical differences between these patients, the analysis was repeated by categorizing patients into four groups, i.e. <7, 7 (3 + 4), 7 (4 + 3) and >7. In addition, to assess for variation in the relationship between PSA level and Gleason sum over time, the cohort was further stratified into patients operated on before and after 1998. In this database, 1998 approximates the full penetrance of PSA screening into clinical practice, and marks the contemporary era of prostate cancer detection following PSA, grade and stage migration. ANOVA techniques were again used to test for an interaction effect between Gleason sum, PSA level and time cohort.
A Cox proportional hazards model was created, regressing time from surgery to biochemical failure, defined as the presence of a detectable PSA level (>0.2 ng/mL) at ≥6 months after RP, using logPSA, Gleason sum (<7, 7, >7), their interaction, and clinical stage. The additional interaction term between PSA and Gleason sum was used to assess whether the effect of one factor varied with the levels of the other. The interaction term is used to describe the relationship between variables and, when it is significant, indicates that the predictive ability of one variable is altered by changes in the second variable. Concordance indices (c-index) were calculated for the model with and without the interaction term between PSA level and Gleason sum, to determine its significance. To more formally test the interaction term, the difference in the fraction of pairs between the model with and without the interaction term was calculated.
The Columbia University Urologic Oncology Database is Institutional Review Board approved the study. All statistical tests were performed at the 0.05 level of significance.
RESULTS
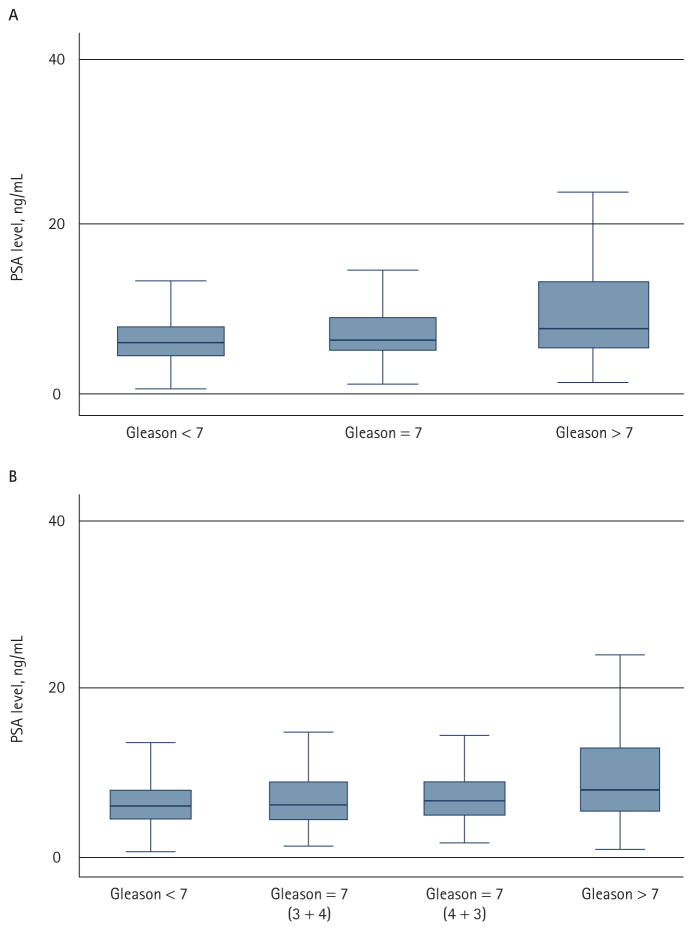
The median (range) patient age was 60.5 (36.0–75.4) years; additional information on preoperative PSA level, biopsy Gleason sum, clinical and pathological stage is detailed in Table 1. Of 1932 patients, 1190 (61.6%) had a Gleason sum of <7, 595 (30.8%) of 7 and 146 (7.6%) of >7. The median PSA level was 5.9, 6.1 and 7.8 ng/mL, respectively (P < 0.001; ANOVA; Fig. 1A). Of the 595 patients with a Gleason sum of 7, 368 (61.8%) were 3 + 4, 187 (31.4%) were 4 + 3 and 40 (6.7%) were not specified; the respective median PSA levels of the 3 + 4 and 4 + 3 groups were 6.0 and 6.5 ng/mL. PSA levels were significantly different among patients in the four-group stratification (P < 0.001), but there was no difference between patients with a Gleason sum of <7 and 7 (3 + 4) (P = 0.17), and patients with 3 + 4 and 4 + 3 (P = 0.19; Fig. 1B). Subsequent analyses therefore used the three-group stratification.
TABLE 1.
The characteristics of prostate cancer in the 1931 patients
| Variable | n (%) |
|---|---|
| PSA level, ng/mL | |
| 0–4 | 327 (16.9) |
| 4–10 | 1293 (66.9) |
| >10 | 311 (16.1) |
| Biopsy Gleason sum | |
| <7 | 1190 (61.6) |
| 7 | 595 (30.8) |
| 7 (3 + 4) | 368 (19.0) |
| 7 (4 + 3) | 187 (9.7) |
| >7 | 146 (7.6) |
| Clinical stage | |
| T1c | 1066 (55.2) |
| T2 | 857 (44.4) |
| T3 | 8 (0.4) |
FIG. 1.
PSA by Gleason sum: (A) the median PSA level for patients with a Gleason sum of <7, 7 and >7 was 5.9, 6.1 and 7.8 ng/mL, respectively (P < 0.001); (B) the median PSA level for patients with a Gleason sum of <7, 7 (3 + 4), 7 (4 + 3) and >7 was 5.9, 6.0, 6.5 and 7.8 ng/mL, respectively,
In the analysis of PSA level and Gleason sum over time, the interaction term between Gleason sum and time (before or after 1998), when controlling for PSA level and clinical stage, was not significant (P = 0.34), indicating that the effect of PSA level on Gleason sum does not vary between the time cohorts. When the analysis was repeated with an interaction term between PSA and time, while controlling for Gleason sum and clinical stage, the interaction term was significant (coefficient 0.78, 95% CI ± 0.03; P < 0.001), indicating an almost 20% reduction in the absolute PSA levels between time cohorts.
In the Cox model predicting biochemical failure, PSA level (as logPSA; hazard ratio 3.2, 95% CI 0.17), Gleason sum 7 (9.1, 0.51) and >7 (12.4, 0.64), when compared to Gleason <7, and the interaction terms between PSA and Gleason sum 7 (0.51, 0.23) and >7 (0.57, 0.26); and clinical stage T2 (1.3, 0.13) and T3 (2.7, 0.59) when compared to clinical stage T1, were significant predictors of outcome (P < 0.001 for all variables). The c-index for the model without the interaction terms was 0.70 and this increased to 0.72 for the model including the interaction terms, indicating an increase in the predictive ability of the model when the interaction term was included. The difference in the fraction of pairs between the model with and without the interaction terms was estimated as 0.19 (95% CI ± 0.07), implying a significant increase in the predictive ability of the model with the interaction term for PSA level and Gleason sum.
DISCUSSION
Attempts to predict the outcome after RP, using mathematical models, have been made since Oesterling et al. [7] used preoperative clinical variables to predict pathological stage in 1987. Since then the variables used to predict outcome have included serum markers, imaging techniques, volume calculations and various pathological findings, as well as patient demographics like age and ethnicity [8–11]. The most practical and simple means to predict outcome from preoperative variables has been shown and validated to be through the combination of clinical stage, biopsy Gleason sum and PSA level. These three variables individually predict the outcome after RP, and when used together enhance the ability of the predictive model [1]. As such, they are incorporated into many nomograms and models, including the well-known Partin and Kattan nomograms, that have been validated and updated [12–15] by several centres and researchers to date. With these three variables a practising urologist can stratify and counsel patients with prostate cancer as to the likelihood of cure and relapse after surgery.
Serum PSA level and clinical stage are relatively easily obtained; Gleason sum requires a prostate biopsy following a high level of suspicion for disease secondary to an elevated PSA level or abnormal findings from a physical examination. Unfortunately, before biopsy, physicians can only offer predictions as to whether or not patients have prostate cancer, based on high PSA levels or palpable findings on a DRE. Therefore, the Gleason sum can be considered the necessary and practical variable that describes the severity of prostate cancer, enhances the predictive value of nomograms, and allows physicians to stratify and counsel patients.
If physicians were afforded an insight as to the likely biopsy Gleason sum based solely on PSA level they would be able to make valuable statements about outcome probabilities at a much earlier stage in the process of screening and following a patient for prostate cancer. The hypothesis of the present study is not contrary to the previously established models of outcome after RP, but rather it exists in parallel. It is conceded that PSA level and Gleason sum are strong predictors of outcome, but it is our assertion that their interaction adds to the understanding of the disease and the process of predicting outcome. The results of the Prostate Cancer Prevention Trial (PCPT) hinted toward a relationship between PSA level and Gleason sum by showing a step-wise increase in the risk of high-grade disease with increasing PSA levels [16]. The PCPT study focused on the prevalence of prostate cancer in men with PSA levels of ≤ 4.0 ng/mL and noted a considerable overlap of the distributions of PSA levels over stratified Gleason sums. The present study evaluated the relationship between PSA and Gleason sum over the full range of observed PSA values.
The findings of the present analysis also mark a departure from traditional thinking about PSA level and Gleason sum. To substantiate these claims, the methods investigating the relationship between PSA and Gleason sum were necessarily complex, ensuring the validity and reproducibility of this analysis. It was important to limit Gleason sum to three categories (<7, 7 and >7) to accommodate for constriction of the Gleason sum range in the more contemporary era of prostate cancer diagnosis [5,17]. Although Gleason sum ranges have become more constricted, low-, intermediate- and high-risk disease of the more recent experience encompasses roughly the same Gleason sum ranges (<7, 7 and >7, respectively) as during the early experience with the disease.
There was a strong relationship between PSA level and Gleason sum, as an increase in the mean PSA level coincided with an increase in the Gleason sum. Using the results of the evaluation of mean serum PSA level, the conclusion is easily reached that a higher PSA level significantly correlates with a worse Gleason sum. The difficulty, and perhaps weakness, of the present study lies in assigning practical limits to this conclusion and describing the exact serum levels at which PSA becomes predictive of increasing Gleason sum. In the current analysis, a PSA level of ≥10 ng/mL correlated with a Gleason sum of 7 more than 47% of the time. For a PSA level of >15 ng/mL, 55% of patients had a Gleason sum of ≥7.
Importantly, the present data were drawn from a population that already had a RP; there are significantly many patients with elevated PSA levels who have benign biopsies and therefore cannot have a correlative biopsy Gleason sum. However, the focus and position of the present study was to designate the risk of ‘bad’ cancer associated with the risk of having cancer based on an elevated PSA level and subsequent prostate biopsy.
Part of the difficulty in assessing the PSA level at which an unfavourable Gleason sum becomes more likely is the wide range of PSA values in the present dataset. There has been a dramatic change in PSA demographics as the knowledge and utility of PSA has increased since its inception. In the present study, >95% of the PSA values were <25 ng/mL, but values up to 62 ng/mL were still encountered across all Gleason sum categories. When PSA levels were observed based on time cohorts, the more recent experience (after 1998) showed a PSA level of <15 ng/mL in 95% of the patients, as compared to a distribution of 95% of patients with PSA level of <21 ng/mL in the early experience. This observation validated the methods of assessing the relationship between PSA level and Gleason sum as affected by time. The results of the time analysis ensure that not only was there a relationship between PSA level and Gleason sum, but that this relationship persists and can be used by practising clinicians as a valuable tool for predicting the outcome.
In recent years the utility of the PSA test has been questioned by several physicians and scientists. The present study suggests a relationship between predictors of prostate cancer outcome not previously investigated in depth. It offers the possibility that physicians might be able to make predictions about a patient’s Gleason sum before biopsy and offers further arguments in favour of maintaining PSA as a valuable screening device.
In conclusion, PSA level and Gleason sum are highly interrelated variables, although each carry additional information that significantly contributes to the prediction of biochemical failure after RP. Our analysis indicates that for any individual patient, the higher the initial PSA level the higher the risk of having a poorly differentiated prostate cancer on biopsy. This relationship has persisted throughout the PSA era.
Abbreviations
- RP
radical prostatectomy
- PCPT
Prostate Cancer Prevention Trial
- c-index
concordance index.
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:110–4. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 2.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 3.ACS. Cancer Facts and Figures. Atlanta: The American Cancer Society; 2006. [Google Scholar]
- 4.Mullan RJ, Jacobsen SJ, Bergstralh EJ, et al. Decline in the overall incidence of regional-distant prostate cancer in Olmsted County, MN, 1980–2000. BJU Int. 2005;95:951–5. doi: 10.1111/j.1464-410X.2005.05445.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith EB, Frierson HF, Jr, Mills SE, Boyd JC, Theodorescu D. Gleason scores of prostate biopsy and radical prostatectomy specimens over the past 10 years: is there evidence for systematic upgrading? Cancer. 2002;94:2282–7. doi: 10.1002/cncr.10457. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 7.Oesterling JE, Brendler CB, Epstein JI, Kimball AW, Jr, Walsh PC. Correlation of clinical stage, serum prostatic acid phosphatase and preoperative Gleason grade with final pathological stage in 275 patients with clinically localized adenocarcinoma of the prostate. J Urol. 1987;138:92–8. doi: 10.1016/s0022-5347(17)43003-5. [DOI] [PubMed] [Google Scholar]
- 8.Badalament RA, Miller MC, Peller PA, et al. An algorithm for predicting nonorgan confined prostate cancer using the results obtained from sextant core biopsies with prostate specific antigen level. J Urol. 1996;156:1375–80. [PubMed] [Google Scholar]
- 9.Moul JW, Connelly RR, Lubeck DP, et al. Predicting risk of prostate specific antigen recurrence after radical prostatectomy with the Center for Prostate Disease Research and Cancer of the Prostate Strategic Urologic Research Endeavor databases. J Urol. 2001;166:1322–7. [PubMed] [Google Scholar]
- 10.Veltri RW, Miller MC, Partin AW, Poole EC, O’Dowd GJ. Prediction of prostate carcinoma stage by quantitative biopsy pathology. Cancer. 2001;91:2322–8. [PubMed] [Google Scholar]
- 11.Narayan P, Gajendran V, Taylor SP, et al. The role of transrectal ultrasound-guided biopsy-based staging, preoperative serum prostate-specific antigen, and biopsy Gleason score in prediction of final pathologic diagnosis in prostate cancer. Urology. 1995;46:205–12. doi: 10.1016/s0090-4295(99)80195-2. [DOI] [PubMed] [Google Scholar]
- 12.Blute ML, Bergstralh EJ, Partin AW, et al. Validation of Partin tables for predicting pathological stage of clinically localized prostate cancer. J Urol. 2000;164:1591–5. [PubMed] [Google Scholar]
- 13.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–51. [PubMed] [Google Scholar]
- 14.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–8. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 15.Khan MA, Partin AW, Mangold LA, Epstein JI, Walsh PC. Probability of biochemical recurrence by analysis of pathologic stage, Gleason score, and margin status for localized prostate cancer. Urology. 2003;62:866–71. doi: 10.1016/s0090-4295(03)00674-5. [DOI] [PubMed] [Google Scholar]
- 16.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350:2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 17.Ghani KR, Grigor K, Tulloch DN. Bollina Trends in reporting Gleason score 1991–2001: changes in the pathologist’s practice. Eur Urol. 2005;47:196–201. doi: 10.1016/j.eururo.2004.07.029. [DOI] [PubMed] [Google Scholar]