Abstract
Birth cohort studies have produced significant advances toward understanding how genetic and environmental factors contribute to the inception of asthma and related allergic diseases. Histopathologic, physiologic, microbiologic, and immunologic findings indicate that critical developmental windows exist in which these various factors either alone or in concert induce the expression of the childhood asthma phenotype. The microbiome in early life clearly influences immune development and asthma. Early environmental exposures, including those related to the home, parents, siblings, pets, and pests, are all likely to influence patterns of microbial colonization and also might shape immune responses to allergens and viruses. As with other contributing factors, there appear to be windows of opportunity in early life when microbial exposures have the greatest effect on these processes. Recent studies of bacterial metabolic products and their effects might suggest new strategies for immune modulation in early life.
Keywords: Asthma, birth cohorts, microbiome, early environment
The development and ultimate clinical expression of various childhood asthma phenotypes depend on genetic and environmental determinants that interact at various stages in the development of airway structural elements and the immune system, beginning in utero. Thus, for many patients with asthma, the disease has its roots during infancy and early life, with the initial clinical presentation most commonly being wheezing caused by viral respiratory tract infections, bacterial infections, or both. These wheezing illnesses in preschool children have been classified phenotypically based on their acute temporal pattern (episodic and multitrigger)1 or their time of onset and duration chronically (transient, persistent, and late onset).2 Eventually, when these wheezing illnesses persist and respond appropriately to therapy, clinicians usually concur that the disease/syndrome of asthma can then be properly diagnosed and treated.
Based on investigations by multiple clinicians and scientists, a number of genetically regulated histopathologic, physiologic, and immunologic events have been uncovered that characterize the inception and natural history of asthma phenotypes. Some of these observations have been initially made, studying smaller cross-sectional populations beginning at or near conception and also at various ages in the first 5 years of life. Other data have been generated by using diverse birth cohorts followed longitudinally from birth (or during pregnancy) out to adulthood.3 This review will highlight factors uncovered from birth cohorts3,4 and related observations that have provided us with novel and insightful information on childhood asthma development.
PERINATAL RISK FACTORS
Early-life immune development
Birth cohort studies have consistently identified overall immune hyporesponsiveness and type 2 inflammatory skewing in mononuclear cells at birth and during infancy with the risk of lower respiratory tract illnesses (LRTIs) and allergic sensitization, although the relationship of these early-life immune responses to longer-term asthma outcomes has been less clear.3 A number of factors affect early-life immune responses, including season of birth,5–7 sex,8 maternal stress,9 and allergen and microbial exposures (farms, dog, cockroach, and endotoxin) during pregnancy and early life.7,10 Reduced IFN-γ responses in cord blood and early infancy have been linked to the risk of LRTIs and recurrent wheezing in multiple cohorts.11–14 Interestingly, early-life LRTIs are associated with a significant increase in mononuclear cell TH1 and TH2 responses in early life.7,14 An exaggerated response of PBMCs to bacterial pathogens in infancy has also been associated with an increased risk of subsequent wheezing and asthma in the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) study.15 Furthermore, a diminished regulatory T-cell response at birth has been identified as a risk factor for allergic sensitization and wheezing.16
Microbial contact and colonization
It is becoming increasingly apparent that the composition of the intestinal microbiome beginning in utero has long-term consequences on disease expression, including inflammatory bowel disease, obesity, allergic diseases, and asthma, among others.17 A number of factors influence intestinal colonization and subsequent risk of disease, including mode of delivery, prematurity, perinatal antibiotic exposure, and breast-feeding.18 These initial observations leveraged studies to determine whether treatment both during pregnancy and in the neonatal period with a number of agents (probiotics, prebiotics, dietary fiber, among others17) could alter disease expression over time. Thus far, in terms of allergic diseases, most of these interventions have had some success in attenuating the temporal onset and disease severity for atopic dermatitis but have been disappointing for asthma.
Regarding asthma specifically, accumulating evidence indicates that the environmental microbiome plays a significant role in asthma development. The very low prevalence of asthma in populations highly exposed to microbial environments (European farm children10 and Amish populations19) indicates its potential for disease prevention. These protective effects might be related to differences in gene expression related to environmental components that are capable of differentially regulating innate immune system pathways that in turn influence the development of more long-term adaptive immunity, which regulates responses to respiratory microbial pathogens and inhaled allergens.19
Vitamin D
The observation that vitamin D had a number of effects on immunoinflammatory pathways relevant to asthma made it an intriguing target for further scientific exploration, regarding its potential contributions to allergic respiratory tract disease pathogenesis.20 In an early observation higher maternal vitamin D intake during pregnancy was shown to be associated with a lower risk of wheezing illnesses by age 3 and 5 years.21 Two subsequent studies examining 25-hydroxyvitamin D (25[OH]D) in cord blood found lower levels of cord blood 25(OH)D to be related to allergic sensitization22 and wheeze23 but not asthma.22,23 Ten additional studies using both dietary histories to estimate vitamin D status and actual serum measurements found increased, decreased, or no effect on future asthma or atopy risk.24 In recent analyses in 2 high-risk birth cohorts, one of urban, largely minority, and low-income children (Urban Environment and Childhood Asthma [URECA])25 and one of largely suburban white children (Childhood Origins of Asthma [COAST]),26 cord blood concentrations of 25(OH)D were not associated with risk of asthma, wheeze, or atopic outcomes in the first 6 years of life.27
Early-life allergic sensitization and asthma risk
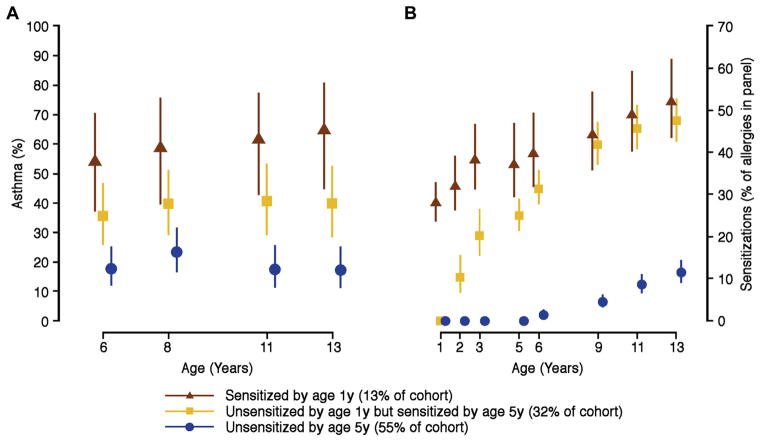
Multiple studies have identified allergic sensitization in early life as a pivotal risk factor for the subsequent development of asthma.28 Furthermore, the type, timing, and degree of sensitization to aeroallergens have all been shown to be relevant to asthma risk. The German Multicenter Allergy Study reported that children sensitized and exposed to aeroallergens by the age of 3 years had a significantly increased risk for subsequent asthma and low lung function.29 The Manchester Asthma and Allergy Study used machine learning to identify a phenotype of children with early sensitization to multiple aeroallergens who were at dramatically increased risk of wheezing, asthma, and exacerbations, leading to hospitalization.30 These relationships have been corroborated in additional birth cohort studies.31,32 A recent analysis in the COAST study has substantiated the importance of these findings to 13 years of age. Cluster analyses identified a group sensitized to aeroallergens by 1 year of age to be at highest risk of asthma, followed by those sensitized by age 5 years. Interestingly, sensitization after age 5 years did not increase asthma risk at age 13 years (Fig 1).33
FIG 1.
The contribution of age of sensitization (A) and number of allergens producing sensitization at various ages (B) on future asthma risk at various ages.
Perennial and indoor allergen sensitization is most strongly linked to the development of asthma. However, the specific allergens responsible for this increased risk vary by environment. For example, Alternaria species sensitization was strongly linked to asthma in the Tucson Children’s Respiratory Study (TCRS),34 dog sensitization was particularly important in the suburban COAST population,31 and dust mite sensitization has been identified as a critical risk factor for wheeze in climates with high humidity, such as the Dunedin Multidisciplinary Health and Development Study.35
The mechanisms by which early-life aeroallergen sensitization affect subsequent asthma risk are incompletely understood. However, sensitization leads to viral wheeze, particularly wheezing associated with rhinovirus infection,36 a critical risk factor discussed further below. Additional potential mechanisms have been reviewed previously.37
AIRWAY PHYSIOLOGIC ALTERATIONS
Airflow obstruction/limitation
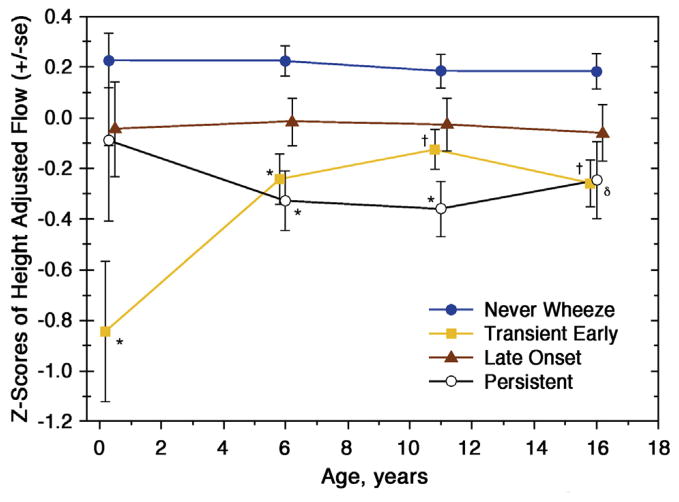
The development of infant/early childhood pulmonary function testing has enabled investigators to study the 2 major airway physiologic features of asthma: airflow obstruction/limitation and airway hyperresponsiveness at an early time point in asthma inception. One of the first prospective evaluations of lung function in a birth cohort was from the TCRS, which followed more than 800 normal-risk children out to 16 years of age.2,38 In the process they were able to define 3 wheezing phenotypes: transient (wheezed in the first 3 years of life but not after age 3 years), late onset (did not wheeze in the first 3 years of life but did so beyond age 3 years), and persistent (wheezed both before and after 3 years of age). Measurements of maximal flow at functional residual capacity were made before 6 months of age and again at ages 6, 9, and 16 years. One of the striking findings was the loss of lung function in the first 6 years of life in those children who were persistent wheezers (Fig 2).38
FIG 2.
Lung function measurements over time in children with various wheezing phenotypes.38
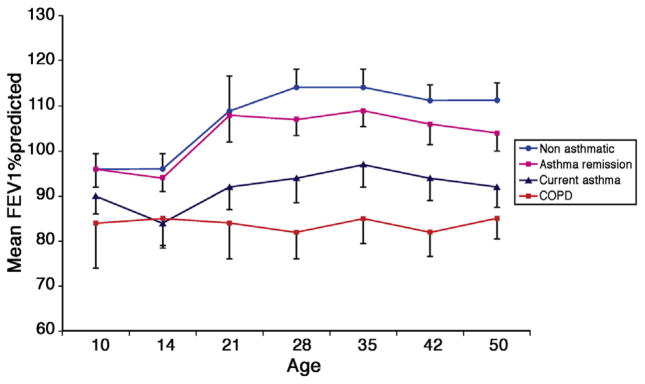
A long-term prospective study evaluating the natural history of lung function in various wheezing phenotypes has been conducted by investigators in Melbourne, Australia.35,39 Although the participants began being observed at age 9 years, follow-up has been out to age 26 years.35 Interestingly, the data indicate that once a lung function trajectory has been determined in childhood, the predicted loss of lung function with increasing age tends to follow this established pathway (Fig 3).39 Recently, this same cohort was evaluated out to age 50 years and separated into groups based on the participants’ current diagnoses: control, asthma remission, current asthma, and chronic obstructive pulmonary disease.39 All of these observations indicate that if a subject’s loss of lung function can be attenuated or even prevented, the intervention likely needs to be made at an early critical time in lung development.
FIG 3.
Percent predicted FEV1 in participants enrolled in the Melbourne Asthma Study. COPD, Chronic obstructive pulmonary disease. Adapted with permission from Tai et al.39
A number of factors could potentially influence these developments. One of the most important is exposure to passive smoke both in utero and in early life. Genetic studies have linked the risk of loss in lung function to various genotypes that might act either alone or due to interactions with environmental exposures.40 Moreover, large genome-wide association studies have indicated that 26 loci and more than 100 variants could collectively explain 7.5% of the polygenic variance in the FEV1/forced vital capacity ratio and 3.4% of the variance for the FEV1 values independent of smoking history.41,42 In addition to indoor environmental influences, outdoor pollutants have also been demonstrated to influence preschool wheezing and ultimate asthma expression as well.43
Viral respiratory illnesses in early life have been documented in a number of cohorts to be associated with subsequent asthma development and loss of lung function. Findings in both the COAST44 and Childhood Allergy Study (CAS)45 birth cohorts have documented the novel findings that preschool rhinovirus-related wheezing illnesses significantly increase future asthma risk. Furthermore, data from the COAST study have demonstrated that wheezing illnesses in the first 3 years of life related to rhinovirus-induced illnesses are significantly associated with loss of lung function during early school age; this could not be demonstrated with wheezing illnesses caused by respiratory syncytial virus (RSV).46 Conversely, low lung function during early infancy might predispose to wheezing with either RSV2 or rhinovirus.47,48 In the COPSAC cohort low lung function in neonates was a risk factor for asthma at age 7 years.49
These findings indicate that the strong relationship between rhinovirus-related wheezing in infancy and childhood asthma is likely partially due to pre-existing low lung function, at least in some children. However, it seems likely that acute episodes of viral wheeze in early life cause airway damage and promote airway remodeling50,51 and that preventing viral wheeze might preserve lung function and reduce the risk of asthma. Indeed, the frequency and severity (defined by corticosteroid intervention) of respiratory illnesses has been shown to be associated with diminished lung function during school age.52
Finally, interventional studies with palivizumab, which decreases the risk for acute RSV-related bronchiolitis, also reduces the risk for recurrent wheeze and nonatopic asthma in some populations53 and thus provide a proof of concept for this hypothesis. Interventional studies are needed to determine whether preventing rhinovirus-related wheezing illnesses would preclude loss of lung function.
Airway hyperresponsiveness
Airway hyperresponsiveness, a major physiologic feature of asthma, appears to predict and track with many outcome measures considered important in the development of asthma and the potential for loss of lung function over time. In a prospective cohort from Western Australia,54 increased airway responsiveness to histamine at 1 month of age was associated with the following at 6 years of age: decreased FEV1, decreased forced vital capacity, physician-diagnosed asthma, and lower respiratory tract symptoms. All reported significant associations of histamine responsiveness with outcomes of interest derived from multivariate modeling were independent of sex, family history of asthma, cord blood IgE levels, maternal smoking status at 1 month or 6 years, skin reactivity or length at 1 month of age, and drug therapy for asthma or height at 6 years of age. Of all the variables evaluated during the study, the only one to show such consistent associations with clinical and physiologic outcomes was airway response to histamine bronchoprovocation.
These participants had histamine bronchoprovocation performed again at ages 6, 11, and 18 years of age.55 Interestingly, asthma at age 18 years was associated with increased airway responsiveness at 6, 12, and 18 years but not during infancy. Based on these results, the investigators concluded that increased airway responsiveness and its association with asthma at age 18 years is established between infancy and age 6 years. They proposed that airway responsiveness in early life is a reflection of the initial airway geometry and that airway responsiveness later in childhood increasingly reflects immunologic responses to environmental influences.55
Airway inflammation and remodeling
Data regarding the emergence of airway histopathologic alterations consistent with the clinical expression of asthma have not been possible in birth cohort studies for obvious reasons related to the need for invasive procedures in large numbers of children. However, data are available from smaller studies in preschool children that have been informative related to the timing and nature of airway structural changes consistent with those seen in the setting of established childhood asthma (Table I).56 Saglani et al57 were one of the first groups to demonstrate that in infants with respiratory symptoms and reversible airflow obstruction 2 histopathologic features of asthma, eosinophilic airway inflammation, and reticular basement membrane thickening were not present despite the presence of physiologic alterations. However, both were present by age 3 years in some children with severe recurrent wheeze, and evidence for angiogenesis and epithelial shedding have been reported at this developmental stage as well.58 Airway smooth muscle thickening has been reported to be present only in atopic versus nonatopic wheezers and is the only histopathologic finding thus far that tracks with an increased risk for the subsequent development of childhood asthma.59 Taken together, these findings indicate that airway histopathologic changes and alterations consistent with airway remodeling occur by 3 years of age in children who are destined to have persistent asthma by school age.60
TABLE I.
Pathologic features in preschool wheezing children
|
VIRUSES AND ASTHMA
Bronchiolitis, wheezing, and asthma
Children who have wheezing illnesses with respiratory tract viruses in early life are at increased risk for going on to have childhood asthma. This statement is supported by incontrovertible evidence, but the nature of this association is less certain. Do viral infections or illnesses in early life cause asthma, or is virus-related wheezing the first sign of abnormal lung physiology and pre-existing asthma? The root of the question is whether viral lower respiratory tract infections cause asthma. The answer to this question could be yes, no, or a qualified yes (under some circumstances).
Studies from birth cohorts have shed light on this important question, but the evidence is discordant, which has engendered some controversy. The TCRS and a number of other cohort studies have demonstrated that viral wheezing illnesses and RSV-related bronchiolitis in particular are risk factors for asthma.61,62 Many viruses can cause wheezing in young children, and next to RSV, rhinovirus is the virus most often implicated. The virology of acute illnesses can vary by population and geographic location; in urban infants adenovirus infections are increased in frequency, whereas infections with rhinovirus and RSVare not as common as in suburban children.26
In most studies the cause of the wheezing illness is related to the subsequent risk for asthma. For example, in the COAST study RSV-related wheezing was associated with an odds ratio (OR) of 2.6 for having asthma, whereas rhinovirus-related wheezing was associated with an OR of 9.8.44 In the Australian CAS study a similar relationship was noted, except that rhinovirus-related wheezing was a risk factor for asthma in children who also had allergic sensitization.45 Recently, COPSAC investigators reported that the number of troublesome lung symptoms (cough, noisy breathing, or shortness of breath) in the first 3 years of life was related to asthma risk; however, the cause of the acute episodes did not add additional predictive value.63 In this analysis detection of rhinoviruses was lower than in other studies (23% of episodes),63 suggesting that reduced detection of rhinovirus Cs, which are closely related to wheezing and asthma and have considerable sequence diversity in areas targeted for molecular detection,64 might account for the difference in outcomes.
Subsequently, data from the COAST study was examined through age 13 years to evaluate the effects of viral wheezing and cause on risk of childhood asthma. Early wheezing episodes with rhinovirus (OR, 3.3; 95% CI, 1.5–7.1) but not RSV (OR, 1.0; 95% CI, 0.4–2.3) were associated with asthma at age 13 years.33 Furthermore, statistical models that included viral cause were most informative for predicting the risk for asthma at 6-, 8-, 11-, or 13-year assessments.65 Rhinoviruses were detected in 49% of wheezing episodes in the first 3 years, and the number of rhinovirus-related wheezing episodes were most predictive for asthma risk.65
Although viral wheezing episodes are closely associated with the risk for asthma, whether this relationship is causal remains unresolved. Some findings support a causal relationship. For example, RSV-related wheeze is seasonal, and follow-up of children in the Tennessee Medicaid database indicates that date of birth is related to the risk of RSV-related wheeze: there is a similar relationship between birth date and the risk of asthma.66 In addition, more severe wheezing illnesses confer a greater risk for asthma, suggesting that virus-related inflammation or lung damage promotes long-term airway narrowing.62
On the other hand, data from some cohorts suggest there are common risk factors for wheezing in infancy and childhood asthma. For example, analyses of twins in a Danish twin registry study concluded that genetic determinants for RSV-related hospitalization and asthma had complete overlap, indicating that there are genetic risk factors that promote both clinical outcomes.67 However, longitudinal observations in cohort studies suggest that viral infections can increase asthma risk in children with certain genotypes. For example, polymorphisms in the 17q21 region factor are the most replicated asthma risk alleles. In the COAST and COPSAC studies there was an interaction between 17q21 polymorphisms (TT genotype at rs7216389), rhinovirus-related wheezing illnesses, and the development of asthma. Asthma risk was greatly increased in children with the TT genotype who also had a rhinovirus-related wheezing illness.68 This finding suggests that viral infections promote the development of asthma in children who are genetically predisposed.
Cadherin-related family member 3 (CDHR3) is another gene that can interact with viral infection to modify the risk of asthma. rs6967330 is a functional polymorphism in the CDHR3 coding region (C529→Y) that puts more CDHR3 on the surfaces of epithelial cells.69 This polymorphism is associated with early childhood asthma with frequent wheezing episodes.69 CDHR3 was also recently identified as the receptor for rhinovirus C, and the asthma risk variant of this gene enables greater binding and replication of rhinovirus C compared with the more prevalent G allele.70 This suggests the theory that children with the CDHR3-Y529 variant are more likely to experience rhinovirus C illnesses and that these illnesses in early life can cause airway damage, inflammation, and remodeling to promote the development of asthma.
Bacteria, acute wheezing, and asthma
Birth cohort studies have added considerably to our understanding of the role of bacteria and fungi on asthma risk. These studies have been conducted in a variety of different environments and have provided new information about associations between asthma and bacteria in the environment, the gut, and the airways.
Environmental microbes
Studies in Western Europe provided hints that exposure to a wide variety of microbes on traditional farms could protect against asthma, and these concepts have been validated and expanded by studies in other locations. In the Prevention of Allergy-Risk Factors for Sensitization in Children Related to Farming and Anthroposophic Lifestyle (PARSIFAL) and the Multidisciplinary Study to Identify the Genetic and Environmental Causes of Asthma in the European Community (GABRIEL) studies, exposure to numbers of environmental bacteria (assessed by using single-strand conformation polymorphism) and fungi (assessed by using culture) were inversely associated with development of atopic asthma.71 More recently, animal shed exposures were found to be inversely related to transient wheezing illnesses,72 which are usually associated with viral infections.
Exposure to dogs in early life has been associated with protection against wheezing illnesses, asthma, or both in several birth cohort studies, including the TCRS, the Wayne County Health Environment Allergy and Asthma Longitudinal Study (WHEALS), and COAST.73–75 Having a dog alters the home microbiome, and mice exposed to dust from homes with dogs are protected against airway inflammatory responses to allergens and have less airway pathology after inoculation with RSV.76
Home dust exposure has also been assessed in US inner-city homes from neighborhoods with high rates of poverty in the URECA study. Surprisingly, exposure to the most common indoor allergens (cockroach, mouse, and cat) in the first year of life was inversely associated with recurrent wheeze by age 3 years.77 In a subset of homes stratified by atopy (allergic sensitization) and wheeze, the house dust microbiome was assessed by means of array (PhyloChip; Affymetrix, Santa Clara, Calif). The microbial richness (number of species) in the dust samples was inversely related to the risk of atopy or atopy together with wheeze. In fact, children with atopy and wheeze were most likely to have been exposed to the combination of low allergen exposure and low microbial richness.77 Conversely, children with neither atopy nor wheeze were most likely to have been raised in homes with high exposure to allergens and microbes in house dust.
Airway bacteria
The linkage between airway bacteria and acute wheezing and asthma is strong. In the COPSAC study bacteria cultured from pharyngeal aspirates obtained at 1 month of age predicted the risk for troublesome lung symptoms in infancy and asthma at age 4 to 5 years.78 Infants who had either Streptococcus pneumoniae, Haemophilus influenza, or Moraxella catarrhalis cultured from upper airway secretions were at increased risk for wheezing compared with cultures that were positive for Staphylococcus aureus or no pathogens. Nasal secretions in neonates were also analyzed for cytokines, and bacterial detection was associated with increased levels of inflammatory cytokines (eg, IL-1 and TNF).15 Interestingly, bacteria detected in similar specimens at 12 months of age predicted neither wheezing nor asthma.78 At ages 2 to 5 years, the same bacterial pathogens were detected more often during acute wheezing illnesses, and in fact, detection of either bacterial or viral respiratory pathogens had the same OR for wheezing illnesses.78
Children in the COAST study together with additional suburban children were monitored for both viral and bacterial pathogens by using PCR during September, which is the peak season for rhinovirus infections and exacerbations of asthma in Wisconsin.79 Analyses of weekly nasal secretions revealed evidence of an order effect; viral infections either preceded or were concurrent with increased detection of bacterial pathogens. Although rhinovirus infections were associated with colds and exacerbations of asthma, detection of the combination of rhinovirus and a bacterial pathogen was associated with the greatest risk for illness or exacerbation of asthma.
Investigators in the CAS study used 16S ribosomal sequencing to identify airway bacteria in nasal secretions of more than 200 children in the first year of life.80 The airway microbiome could be classified into 6 groups according to dominant bacterial families. Similar to findings in COAST, viral infections were associated with increased detection of pathogen-dominated microbial communities. Those children with an early-life microbiome dominated by Streptococcus species were at increased risk for subsequent asthma by age 6 years.
Gastrointestinal microbes
Two major studies have identified strong relationships between gastrointestinal microbes and the risk of atopy and wheeze. In the Canadian Healthy Infant Longitudinal Development study stool samples obtained at age 3 months from a subset of children were analyzed for microbial composition and compared with atopy and wheeze at age 1 year.81 The diversity of the stool microbiome did not differ between groups, but microbial composition was remarkable for reduction in certain bacterial genera (Lachnospira, Veillonella, Faecalibacterium, and Rothia) in children with wheeze and atopy. Predicted metabolic functions of these ‘‘protective’’ bacteria included LPS biosynthesis and short-chain fatty acid production, and some of the predicted differences in metabolites were verified by means of analysis of stool specimens.
The WHEALS recently published analyses of gastrointestinal microflora, and this study found 3 different states of neonatal gut microbiota (NGM) based on dominant bacteria and fungi.82 One of these states (NGM3) was strongly associated with multiple early allergic sensitization at age 2 years and asthma at age 4 years. Within the stool microbiome, there were inverse relationships between some bacteria and fungi. For example, NGM3 had lower relative abundance of Bifidobacterium and Faecalibacterium species and higher relative abundance of Candida and Rhodotorula species. Predicted metabolic functions of NGM3 were evaluated, and one metabolite (12,13-DiHOME) was enriched in stool samples from NGM3 and had proinflammatory immunomodulatory effects (increased IL-4–producing and reduced regulatory T-cell counts) when studied in vitro.
SUMMARY
Birth cohort studies have produced significant advances toward understanding how genetic and environmental factors contribute to the inception of asthma and related allergic diseases. Histopathologic, physiologic, microbiologic, and immunologic findings indicate that critical developmental windows exist in which these various factors either alone or in concert induce the expression of the childhood asthmatic phenotype. The microbiome in early life clearly influences immune development and asthma. Early environmental exposures, including those related to the home, parents, siblings, pets, and pests, are all likely to influence patterns of microbial colonization and also might shape immune responses to allergens and viruses. As with other contributing factors, there appear to be windows of opportunity in early life when microbial exposures have the greatest effect on these processes. Recent studies of bacterial metabolic products and their effects might suggest new strategies for immune modulation in early life.
Acknowledgments
Supported by the National Institutes Health (grants P01 HL070831 and UM1 AI114271).
Abbreviations used
- CAS
Childhood Allergy Study
- CDHR3
Cadherin-related family member 3
- COAST
Childhood Origins of Asthma
- COPSAC
Copenhagen Prospective Studies on Asthma in Childhood
- LRTI
Lower respiratory tract illness
- NGM
Neonatal gut microbiota
- 25(OH)D
25-Hydroxyvitamin D
- OR
Odds ratio
- RSV
Respiratory syncytial virus
- TCRS
Tucson Children’s Respiratory Study
- URECA
Urban Environment and Childhood Asthma
- WHEALS
Wayne County Health Environment Allergy and Asthma Longitudinal Study
References
- 1.Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, Custovic A, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008;32:1096–110. doi: 10.1183/09031936.00002108. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Gern JE, Martinez FD, Anto JM, Johnson CC, Holt PG, et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol. 2014;133:1535–46. doi: 10.1016/j.jaci.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet J, Anto J, Sunyer J, Nieuwenhuijsen M, Vrijheid M, Keil T, et al. Pooling birth cohorts in allergy and asthma: European Union-funded initiatives—a MeDALL, CHICOS, ENRIECO, and GA2LEN joint paper. Int Arch Allergy Immunol. 2013;161:1–10. doi: 10.1159/000343018. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan Dillie KT, Tisler CJ, Dasilva DF, Pappas TE, Roberg KA, Carlson-Dakes KT, et al. The influence of processing factors and non-atopy-related maternal and neonate characteristics on yield and cytokine responses of cord blood mononuclear cells. Clin Exp Allergy. 2008;38:298–304. doi: 10.1111/j.1365-2222.2007.02891.x. [DOI] [PubMed] [Google Scholar]
- 6.Thysen AH, Rasmussen MA, Kreiner-Moller E, Larsen JM, Folsgaard NV, Bonnelykke K, et al. Season of birth shapes neonatal immune function. J Allergy Clin Immunol. 2016;137:1238–46. e1–13. doi: 10.1016/j.jaci.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Gern JECA, Jaffee KF, Lynn H, Dresen A, Cruikshank WW, Lederman HM, et al. Patterns of immune development in urban preschoolers with recurrent wheeze and/or atopy. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.052. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uekert SJ, Akan G, Evans MD, Li Z, Roberg K, Tisler C, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006;118:1375–81. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Ramratnam SK, Visness CM, Jaffee KFM, Bloomberg GR, Kattan M, Sandel MTM, et al. Relationships among maternal stress and depression, type 2 responses, and recurrent wheezing at age 3 years in low income urban families. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201602-0272OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Mutius E. The microbial environment and its influence on asthma prevention in early life. J Allergy Clin Immunol. 2016;137:680–9. doi: 10.1016/j.jaci.2015.12.1301. [DOI] [PubMed] [Google Scholar]
- 11.Sumino K, Tucker J, Shahab M, Jaffee KF, Visness CM, Gern JE, et al. Antiviral IFN-gamma responses of monocytes at birth predict respiratory tract illness in the first year of life. J Allergy Clin Immunol. 2012;129:1267–73. e1. doi: 10.1016/j.jaci.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–80. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 14.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–8. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Folsgaard NV, Schjorring S, Chawes BL, Rasmussen MA, Krogfelt KA, Brix S, et al. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med. 2013;187:589–95. doi: 10.1164/rccm.201207-1297OC. [DOI] [PubMed] [Google Scholar]
- 16.Holt PG. The mechanism or mechanisms driving atopic asthma initiation: the infant respiratory microbiome moves to center stage. J Allergy Clin Immunol. 2015;136:15–22. doi: 10.1016/j.jaci.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 17.West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, et al. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015;135:3–13. doi: 10.1016/j.jaci.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–76. doi: 10.1038/nrgastro.2012.144. [DOI] [PubMed] [Google Scholar]
- 19.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375:411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 22.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128:1093–9. e1–5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 24.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18:1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gern JE. The Urban Environment and Childhood Asthma study. J Allergy Clin Immunol. 2010;125:545–9. doi: 10.1016/j.jaci.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gern JE, Pappas T, Visness CM, Jaffee KF, Lemanske RF, Togias A, et al. Comparison of the etiology of viral respiratory illnesses in inner-city and suburban infants. J Infect Dis. 2012;206:1342–9. doi: 10.1093/infdis/jis504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visness CM, Sandel MT, O’Connor G, Gern JE, Jaffee KF, Wood RA, et al. Cord blood vitamin D concentrations are unrelated to atopy and wheeze in 2 diverse birth cohort studies. J Allergy Clin Immunol. 2015;136:1108–10. e2. doi: 10.1016/j.jaci.2015.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–6. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 30.Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–6. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 31.Stoltz DJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy. 2013;43:233–41. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havstad S, Johnson CC, Kim H, Levin AM, Zoratti EM, Joseph CL, et al. Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol. 2014;134:722–77. e2. doi: 10.1016/j.jaci.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.03.049. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. 1997;155:1356–61. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 35.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 36.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–5. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson DJ, Gern JE, Lemanske RF., Jr The contributions of allergic sensitization and respiratory pathogens to asthma inception. J Allergy Clin Immunol. 2016;137:659–65. doi: 10.1016/j.jaci.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai A, Tran H, Roberts M, Clarke N, Wilson J, Robertson CF. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69:805–10. doi: 10.1136/thoraxjnl-2013-204815. [DOI] [PubMed] [Google Scholar]
- 40.Scholtens S, Postma DS, Moffatt MF, Panasevich S, Granell R, Henderson AJ, et al. Novel childhood asthma genes interact with in utero and early-life tobacco smoke exposure. J Allergy Clin Immunol. 2014;133:885–8. doi: 10.1016/j.jaci.2013.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med. 2016;375:871–8. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 42.Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082–90. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz ES, Hallberg J, Gustafsson PM, Bottai M, Bellander T, Bergstrom A, et al. Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. J Allergy Clin Immunol. 2016;138:930–2. e5. doi: 10.1016/j.jaci.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. J Allergy Clin Immunol. 2011;128:532–8. e1–10. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Gugten AC, van der Zalm MM, Uiterwaal CS, Wilbrink B, Rossen JW, van der Ent CK. Human rhinovirus and wheezing: short and long-term associations in children. Pediatr Infect Dis J. 2013;32:827–33. doi: 10.1097/INF.0b013e318290620e. [DOI] [PubMed] [Google Scholar]
- 48.van der Zalm MM, Uiterwaal CS, Wilbrink B, Koopman M, Verheij TJ, van der Ent CK. The influence of neonatal lung function on rhinovirus-associated wheeze. Am J Respir Crit Care Med. 2011;183:262–7. doi: 10.1164/rccm.200905-0716OC. [DOI] [PubMed] [Google Scholar]
- 49.Bisgaard H, Jensen SM, Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–9. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 50.Leigh R, Oyelusi W, Wiehler S, Koetzler R, Zaheer RS, Newton R, et al. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol. 2008;121:1238–45. doi: 10.1016/j.jaci.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 51.Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134:429–39. doi: 10.1016/j.jaci.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Brian AL, Lemanske RF, Jr, Evans MD, Gangnon RE, Gern JE, Jackson DJ. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol. 2012;129:1162–4. doi: 10.1016/j.jaci.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simoes EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126:256–62. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, LeSouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- 55.Cox DW, Mullane D, Zhang GC, Turner SW, Hayden CM, Goldblatt J, et al. Longitudinal assessment of airway responsiveness from 1 month to 18 years in the PIAF birth cohort. Eur Respir J. 2015;46:1654–61. doi: 10.1183/13993003.00397-2015. [DOI] [PubMed] [Google Scholar]
- 56.Saglani S, Bush A. Onset of structural airway changes in preschool wheezers. A window and target for secondary asthma prevention? Am J Respir Crit Care Med. 2015;192:121–2. doi: 10.1164/rccm.201505-0989ED. [DOI] [PubMed] [Google Scholar]
- 57.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–7. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 58.Turato G, Barbato A, Baraldo S, Zanin ME, Bazzan E, Lokar-Oliani K, et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am J Respir Crit Care Med. 2008;178:476–82. doi: 10.1164/rccm.200712-1818OC. [DOI] [PubMed] [Google Scholar]
- 59.Regamey N, Ochs M, Hilliard TN, Muhlfeld C, Cornish N, Fleming L, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177:837–43. doi: 10.1164/rccm.200707-977OC. [DOI] [PubMed] [Google Scholar]
- 60.Lezmi G, Gosset P, Deschildre A, Abou-Taam R, Mahut B, Beydon N, et al. Airway Remodeling in Preschool Children with Severe Recurrent Wheeze. Am J Respir Crit Care Med. 2015;192:164–71. doi: 10.1164/rccm.201411-1958OC. [DOI] [PubMed] [Google Scholar]
- 61.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 62.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–61. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vissing NH, Larsen JM, Rasmussen MA, Chawes BL, Thysen AH, Bonnelykke K, et al. Susceptibility to lower respiratory infections in childhood is associated with perturbation of the cytokine response to pathogenic airway bacteria. Pediatr Infect Dis J. 2016;35:561–6. doi: 10.1097/INF.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 64.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–71. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson HM, Lemanske RF, Jr, Evans MD, Gangnon RE, Pappas T, Grindle K, et al. Assessment of wheezing frequency and viral etiology on childhood and adolescent asthma risk. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.07.031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–6. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomsen SF, van der SS, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–7. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 68.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–5. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 70.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–90. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ege MJ, Mayer M, Normand A-C, Genuneit J, Cookson WOCM, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 72.Loss GJ, Depner M, Hose AJ, Genuneit J, Karvonen AM, Hyvarinen A, et al. The early development of wheeze. Environmental determinants and genetic susceptibility at 17q21. Am J Respir Crit Care Med. 2016;193:889–97. doi: 10.1164/rccm.201507-1493OC. [DOI] [PubMed] [Google Scholar]
- 73.Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol. 2001;108:509–15. doi: 10.1067/mai.2001.117797. [DOI] [PubMed] [Google Scholar]
- 74.Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38:1635–43. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 75.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 76.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111:805–10. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134:593–601. e12. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–7. e3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant naso-pharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 82.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–91. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]