Abstract
Serious psychiatric disorders such as schizophrenia, bipolar disorder, and major depression are important causes of mortality and morbidity worldwide. While these are primarily diseases involving altered brain functioning, numerous studies have documented increased rates of gastrointestinal inflammation and dysfunction in many individuals with these disorders.
Toxoplasma gondii is an apicomplexan protozoan intracellular parasite with a widespread distribution in both developed and developing countries. Toxoplasma organisms enter the ecosystem through the shedding of oocysts by Toxoplasma infected felines. In almost all cases of post-natal human infection, Toxoplasma enters its hosts through the intestinal tract either by the ingestion of oocysts or by the consumption of meat from food animals which themselves were infected by Toxoplasma oocysts.
It had previously been thought that most cases of Toxoplasma infection in immune competent children and adults were in-apparent and asymptomatic. However, recent studies cast doubt on this concept as exposure to Toxoplasma has been associated with a range of acute and chronic symptoms. Of particular note has been the finding of an increased rate of a range of neurological and psychiatric disorders associated with serological evidence of Toxoplasma exposure. A role of Toxoplasma infection in brain diseases is also supported by the consistent finding of altered cognition and behavior in animal models of infections. Much of the attention relating to the role of Toxoplasma infection in neuropsychiatric disorders has focused on the brain, where Toxoplasma tissue cysts can persist for extended periods of time. However, recent discoveries relating to the role of the gastrointestinal tract in cognition and behavior suggests that Toxoplasma may also increase susceptibility to human brain diseases through immune activation, particularly involving the gastrointestinal mucosa.
The study of the pathways relating to the pathobiology and immunology of Toxoplasma infection may provide insights into the pathogenesis of a range of human neuropsychiatric disorders as well as into cognitive functioning in otherwise healthy individuals.
The Biology of Toxoplasma Infection
Toxoplasma gondii is an apicomplexan protozoan with a worldwide distribution. Toxoplasma organisms can undergo a complete cycle of replication in feline species, which thus serve as complete hosts for this organism. However, Toxoplasma can also undergo incomplete replication in virtually any warm-blooded animal, with those animals constituting intermediate hosts (Figure 1). In order to adapt to these multiple environments, Toxoplasma has developed an intricate and complex genome and system of gene expression capable of encoding enzymes and other proteins required for intracellular replication and maintenance in different host species. It has also developed a complex set of regulatory molecules and pathways allowing for persistence in tissues, particularly within the central nervous system (White et al, 2014).
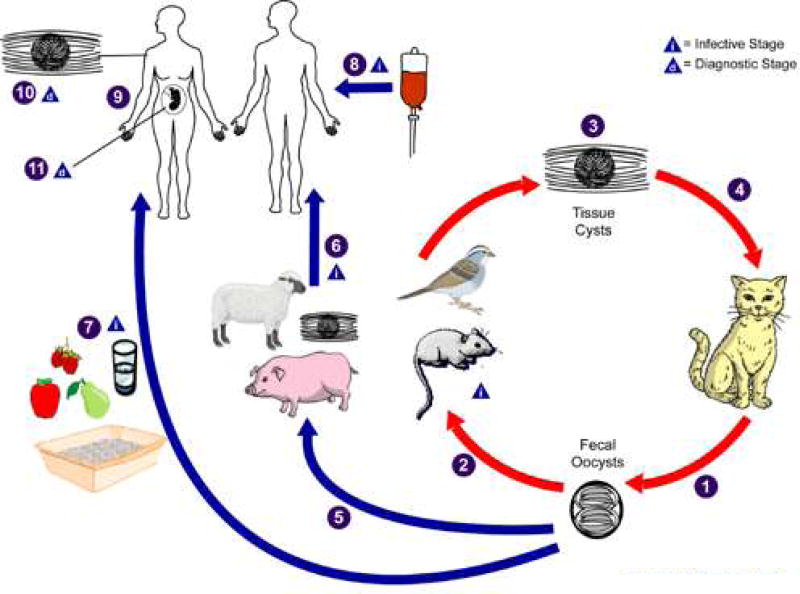
Figure 1. Life Cycle of Toxoplasma gondii.
The only known definitive hosts for Toxoplasma gondii are members of family Felidae (domestic cats and their relatives). Unsporulated oocysts are shed in the cat’s feces
 . Although oocysts are usually only shed for 1–2 weeks, large numbers may be shed especially in kittens of your cats undergoing their first infection. Oocysts take 1–5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds, rodents, and farm animals) become infected after ingesting soil, water or plant material contaminated with oocysts
. Although oocysts are usually only shed for 1–2 weeks, large numbers may be shed especially in kittens of your cats undergoing their first infection. Oocysts take 1–5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds, rodents, and farm animals) become infected after ingesting soil, water or plant material contaminated with oocysts
 . Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites
. Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites
 . Cats become infected after consuming intermediate hosts harboring tissue cysts
. Cats become infected after consuming intermediate hosts harboring tissue cysts
 . Cats may also become infected directly by ingestion of sporulated oocysts although this is a less common form of infection. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment
. Cats may also become infected directly by ingestion of sporulated oocysts although this is a less common form of infection. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment
 . Humans can become infected by any of several routes:eating undercooked meat of animals harboring tissue cysts
. Humans can become infected by any of several routes:eating undercooked meat of animals harboring tissue cysts
 consuming food or water contaminated with cat feces or by contaminated environmental samples such as fecal-contaminated soil or changing the litter box of a pet cat
consuming food or water contaminated with cat feces or by contaminated environmental samples such as fecal-contaminated soil or changing the litter box of a pet cat
 . More rarely humans can become infected through blood transfusion or organ transplantation
. More rarely humans can become infected through blood transfusion or organ transplantation
 transplacentally from mother to fetus
transplacentally from mother to fetus
 .
.
In the human host, the parasites form tissue cysts, most commonly in skeletal muscle, myocardium, brain, and eyes; these cysts may remain throughout the life of the host. Diagnosis is usually achieved by serology, although tissue cysts may be observed in stained biopsy specimens, particularly in immune compromised individuals
 . Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR
. Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR

(Adapted From http://www.cdc.gov/parasites/toxoplasmosis/biology.html)
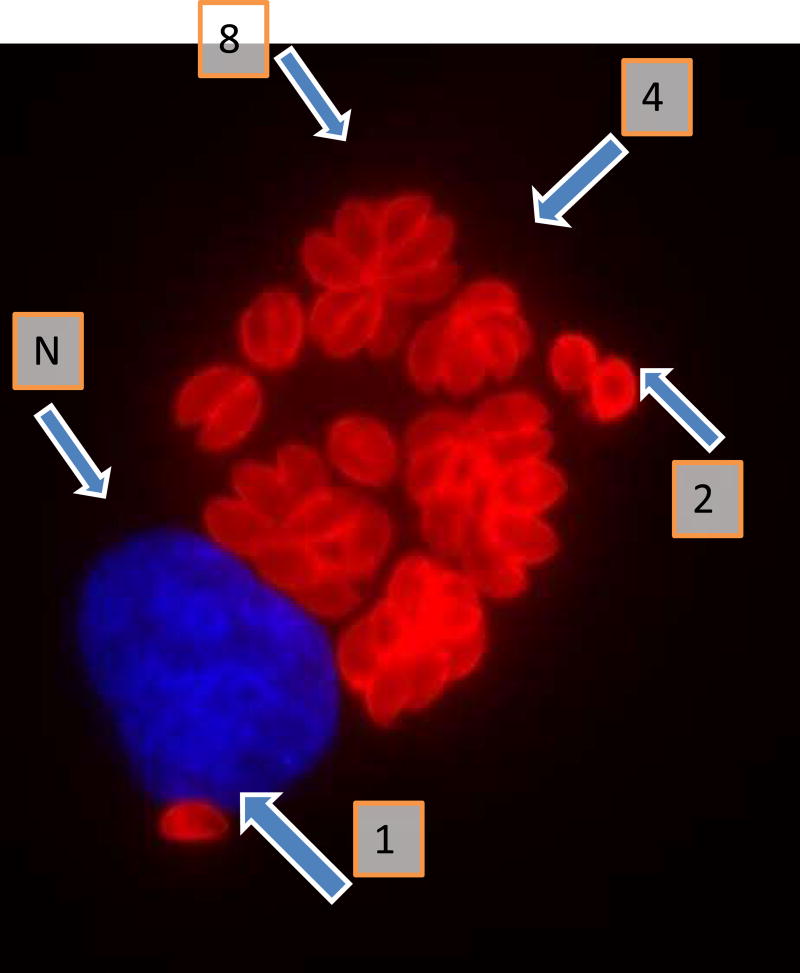
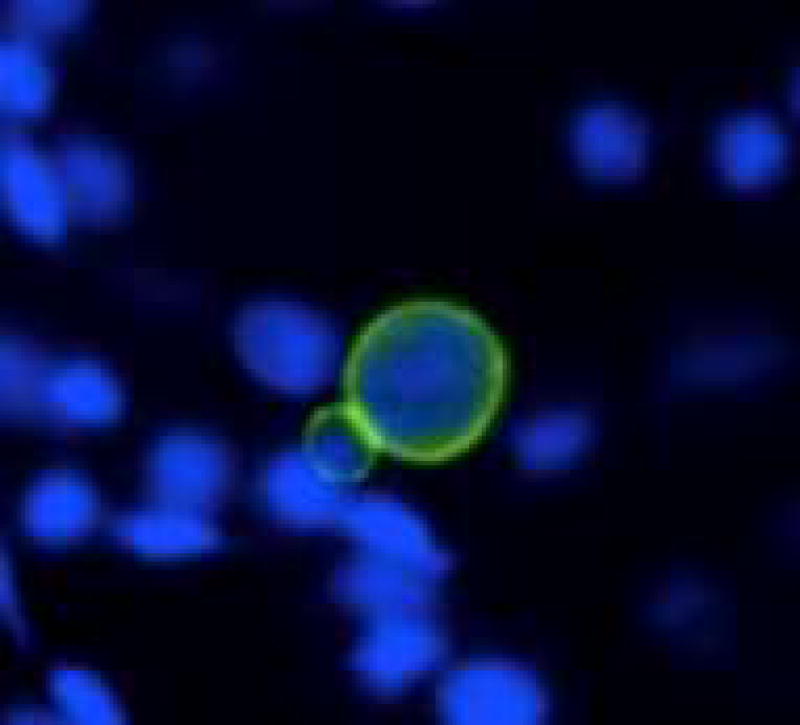
Initial infection of a host is usually accomplished by the rapidly replicating form of the organism called tachyzoites (Figure 2). However, in an immune competent host following activation of the immune system, these tachyzoites undergo a conversion to the slowly replicating forms of the organism called bradyzoites (Figure 3) which cluster to form tissue cysts. These tissue cysts can persist in the brain and other organs for extended periods of time without the generation of an apparent immune response. At various times, a portion of these tissue cysts can reactivate into tachyzoites and, if the immune response is adequate, with subsequent reconversion into bradyzoite containing tissue cysts. Through these inter-conversions Toxoplasma can establish life-long persistence in immune competent hosts.
Figure 2.
Immunofluorescent visualization of intracellular tachyzoites of T.gondii strain RH shown 26 hours after infection of human fibroblasts. The tachyzoites are reacted with rabbit antibody to the Toxoplasma p30 (SAG1) protein and then with anti-rabbit antibodies labelled with Alexa Fluor 594. Host cell nuclei are visualized using 4',6-diamidino-2-phenylindole (DAPI). The tachyzoites thus are stained red and the fibroblast nucleus (N) is stained blue. Tachyzoites typically replicate by endodyogeny. Thus a cluster of 2 tachyzoites( )is indicative of one cycle of replication, 4 tachyzoites of two cycles (4) and cluster of 8 tachyzoites (8) representing 3 cycles of replication. A single tachyzoite (1) represents one that has not yet undergone a cycle of replication (Figure courtesy of Claudia Bordón. Johns Hopkins School of Medicine)
Figure 3.
A section of brain from chronically infected mice showing two T. gondii bradyzoite tissue cysts (green), at 400x magnification. Note that the tissue cyst on the left is younger than the one on the right because of the difference in cyst size.
Epidemiology of Toxoplasma Infection
This complex lifecycle has facilitated the widespread prevalence of Toxoplasma in animal populations, as the organism have been found to infect a wide range of warm blooded animals living in different environments (Pittman and Knoll 2015). In the case of humans, Toxoplasma infection exists in essentially every human population (Pappas et al, 2009). As in the case of most other protozoa, the prevalence of Toxoplasma is higher in developing areas of the world. However, Toxoplasma is one of the few protozoans which has maintained a significant prevalence in developed countries such as the United States (Jones et al, 2014). This high level of prevalence in human populations is likely due to the fact that individuals can become infected with Toxoplasma by a number of routes. For example, humans can become infected following the ingestion of oocysts shed in the feces of infected cats which reside in soil and other environmental niches. In many areas of the world, water contaminated with oocysts is a major source of environmental infections (Jones et al, 2010). Since water systems which employ filtration and chlorination destroy most oocysts, this type of infection is less common in countries with well-functioning water purification infrastructure. Differential exposure to oocyst contaminated water is thus likely to be the environmental factor which is largely responsible for the increased rates of prevalence of Toxoplasma infection in many areas of the developing world (Flegr et al, 2014).
Humans can also become infected through ingestion of meat from animals harboring tissue cysts. Since the level of heat used in cooking disrupts tissue cysts, most infections by this route occur through the ingestion of uncooked or undercooked meat. Also food animals vary in terms of resistance to tissue cysts and thus the ability to infect humans. This is the reason why the consumption of meat from some species, such as bovines, which are relatively resistant to Toxoplasma, is a less common source of transmission as compared to the ingestion of meat from other animal food sources such as ovine or porcine species (Dubey et al, 2005; Jones & Dubey 2012).
Humans and other mammals can also become infected vertically by the passage of tachyzoites from mother to fetus. While the effects of fetal infection can be devastating, active fetal infection is a relatively rare event (Yamada et al, 2015 and Evangård et al, 2001. This fact, in addition to the fact that Toxoplasma prevalence increased with age (Wilking et al, 2016) suggests that most cases of Toxoplasma in humans are acquired after birth. Risk factors associated with the prevalence of schizophrenia in adults are largely based on age, geographic location and household and occupational environmental exposures. Household environmental exposures include lack of access to clean water, eating vegetables washed with contaminated water, eating undercooked meat, and soil floors (Alvarado-Esquivel et al, 2006). Occupational risks associated with increased Toxoplasma exposure include gardening, working with and raising farm animals (Alvarado-Esquivel et al, 2013). Several studies have also found household cats to be a risk factor (Chiang et al, 2014 ) particularly if the exposure is to multiple kittens, although some studies have not found increased risks of Toxoplasma in households with only 1 adult cat (Esch and Petersen 2013). Nonetheless, careful handling of cat feces and strategies to minimize Toxoplasma infection in cats are recommended in terms of the prevention of household transmission of infection (Opsteegh et al, 2015).
It is of note that most available data relating to Toxoplasma exposure have been collected in adults. Data relating to the age of acquisition of Toxoplasma in children living under differing environmental conditions are currently lacking and are needed to better understand the epidemiology of Toxoplasma infection in childhood and adolescence. Also, the proportion of individuals infected from the ingestion of oocysts shed from infected cats as compared to tissue cysts from consumed meat is difficult to determine since, as discussed above, both forms of the organism are often generated in the host following infection despite source of the initial infection. Recent studies suggest that antibodies to sporozoites are present only when humans or other animals have been infected with T. gondii oocysts (Munoz-Zanzi et al, 2010). While sporozoite antibodies have the potential to differentiate oocyst- versus tissue cyst-induced infection, such antibodies are only detectable in humans within 6–8 month of initial oocyst-acquired infection. This time window considerably limits the utility of the sporozoite antibody, given the majority of human infections are chronic and thus would exceed the detectable period. The availability of assays capable of distinguishing the lifecycle form of the initial infecting organisms in a large population over an extended period of time would be an important step in terms of developing efficient methods for the control of infection within a population (Hill et al, 2011).
Chronic Toxoplasma Infection of Humans and Experimental Animals
Toxoplasma is well recognized as a cause of serious central nervous system infections in neonates and in individuals with depressed T-cell immunity as can occur in HIV infection, congenital immunodeficiency diseases, hematological malignancies, and during the course of immunosuppressive chemotherapy (Robert-Gangneux and Dardé 2009). Considerably less attention has been given to the consequences of Toxoplasma infection in immune competent individuals. It has been previously thought that Toxoplasma infection in immune competent individuals was in-apparent and not associated with measurable health consequences. However, there are a number of recent observations which seriously challenge this supposition. For example, there have been a number of water-borne outbreaks which have been described to be associated with acute fever, lymphadenopathy, retinopathy and altered mental state (Bowie et al, 1997). The acute symptoms generally resolve but are sometimes followed by long term sequellae such as retinal infection and decreased visual acuity (Burnett et al, 1998).
In addition, a number of animal models of chronic Toxoplasma infection have been developed, particularly in rodents such as mice and rats. While chronically infected animals gain weight normally and appear healthy, they often have measurable changes in behavior and cognition (Xiao et al, 2012). One of the interesting findings is that chronic T. gondii infection triggers abnormal response to dopamine, as evidenced by T. gondii-infected mice displayed a striking behavioral deficit in amphetamine-trigged locomotor response (Xiao et al, 2016). These models indicate that Toxoplasma infection can have lifelong effects on the brain functioning of intermediate hosts.
Toxoplasma Exposure and Neuropsychiatric Disorders
An increased understanding of the role of persistent Toxoplasma infection in humans and animals has led to a re-consideration of the pathogenic effect of acquired Toxoplasma infections in immune competent individuals. Of particular importance are studies investigating the potential role of Toxoplasma infection in human neuropsychiatric disorders.
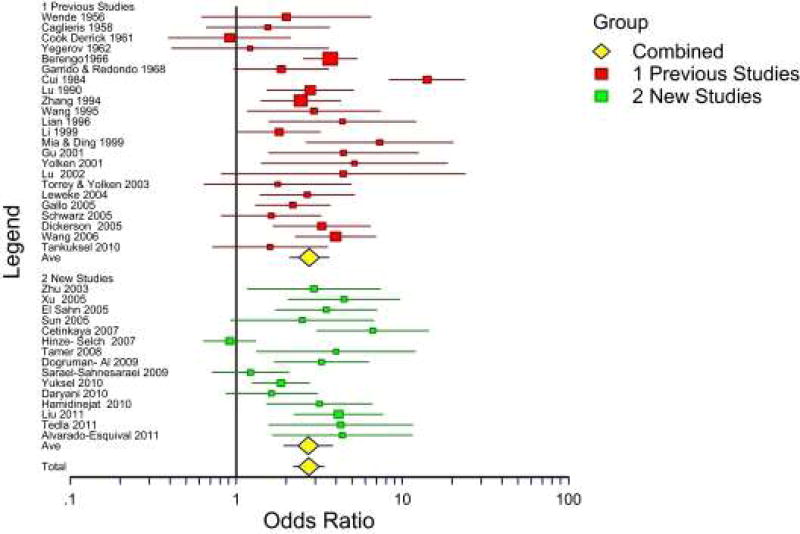
The neuropsychiatric disorder which has been studied in most detail is schizophrenia. Schizophrenia is a severe brain disorder involving altered perception and cognition. The etiology of schizophrenia is uncertain but is likely to involve both genetic and environmental factors (Børglum et al, 2014) A possible role of infectious agents in some cases of schizophrenia has been suspected since the disease was first characterized in the early part of the 20th century (Torrey et al, 2007). The first published association between Toxoplasma exposure and schizophrenia was in 1952 and there have been many subsequent studies. A meta-analysis published in 2012 calculated a pooled odds ratio relating seropositivity to Toxoplasma and risk of schizophrenia of 2.71 (95% CI 1.93–3.80) based on 38 published studies (Figure 4, Torrey et al, 2012). Several additional studies reporting an association between exposure to Toxoplasma and either increased risk of schizophrenia or increased symptoms have been published since 2012. It is of note that some studies report an association between risk of schizophrenia and Toxoplasma prevalence as defined by detectable levels of antibodies, while other studies report a quantitative association between risk of schizophrenia and the level of antibodies (Hinze-Selch et al, 2007). It should also be noted that several studies have been published which have failed to find a statistically significant association between exposure to Toxoplasma and risk of schizophrenia (Sugden et al, 2016; Avramopoulos et al, 2015). Reasons for this variation are not known with certainty but might include differences in the clinical status of the participants (recent onset vs chronic), methodological differences in the antibody measurement systems, differences in the prevalence of Toxoplasma infection, the timing of infection, the form of the infecting organism (tissue cysts from infected meat vs. oocysts from cat feces) and the genetic background of the host. It is also possible that differences in the strain of Toxoplasma contribute to differences in psychiatric manifestations of infection (Xiao and Yolken 2015). In animal models, behavioral abnormalities are associated with increased levels of antibodies to the Toxoplasma cyst protein MAG1, which is a serological marker of parasite burden (Xiao et al, 2016). It will be of great interest to determine if the measurement of specific antibodies to cyst proteins will correlate better with psychiatric outcomes than the measurement of antibodies to whole organisms or tachyzoite proteins as provided by currently available assay systems.
Figure 4.
Meta-Analysis of published studies describing associations between Toxoplasma exposure and Schizophrenia or Related Disorders. Red boxes indicate studies published before 2007 and summarized in Torrey et al, 2007. Blue boxes indicate studies published between 2007 and 2012 and summarized in Torrey et al, 2012. Yellow diamonds indicate pooled odds ratios. (Reprinted from Torrey et al (2012))
The mechanisms by which Toxoplasma exposure might be related to the risk of schizophrenia in humans are not known with certainty. However, the facts that Toxoplasma infection alters dopamine levels in the brain of some experimentally infected animals and the levels of dopamine are abnormal in schizophrenia suggest that alterations in this neurotransmitter may be a common link between Toxoplasma infection and schizophrenia (Flegr 2015). A possible association between Toxoplasma infection, dopamine metabolism and schizophrenia is also suggested by the finding that a number of pharmacological inhibitors of dopamine receptor binding, which are used for the treatment of schizophrenia, are also inhibitors of Toxoplasma replication in cell culture and animals (Dittmar et al, 2016). It is of interest that valproic acid, a medication used for the treatment of schizophrenia, the mechanisms of action of which are unknown, can also inhibit the in vitro replication of Toxoplasma (Jones-Brando et al, 2003). The source of increased dopamine is not known with certainty but may be related to both the generation of dopamine by the Toxoplasma organisms or by the host immune response (Martin et al, 2015). It is of note, however, that not all investigators have found increased levels of dopamine in Toxoplasma infected mice (Wang et al, 2015) suggesting that other neurophysiological mechanisms may be involved as well. Additional mechanisms which might be operant in Toxoplasma and which might be relevant to human psychiatric disorders include the generation of microRNAS (Li et al, 2015) and alterations in the metabolism of kynurenine. It is also of note that Toxoplasma encodes a protein which is annotated as an NMDA receptor (Toxoplasma gondii GT1 protein coding gene on TGGT1_chrXI), since the NMDA receptor is also an important component of human psychiatric disorders (Balu 2016). In particular, experimental infection of mice with Toxoplasma results in the generation of antibodies to the NMDA receptor (Kannan et al, 2016). Antibodies to the NMDA receptor have been noted to be associated with a number of human brain diseases including schizophrenia, mania (Dickerson et al, 2013) and other forms of acute psychosis.
Exposure to Toxoplasma has also been associated with other psychiatric conditions, albeit less consistently than with schizophrenia. Psychiatric conditions which have been associated either with increased prevalence of Toxoplasma infection or increased levels of antibodies include bipolar disorder (Pearce et al, 2012), general anxiety disorder (Markovitz et al, 2015), mixed anxiety and depressive disorder (Alvarado-Esquivel et al, 2016), aggressive behavior (Cook et al, 2015), and acute convulsive epilepsy (Ae-Ngibise et al, 2015). Exposure to Toxoplasma has also been associated with increased rates of suicidality as measured by the number of actual suicide attempts (Alvarado-Esquivel et al, 2013) or episodes of self-directed violence (Zhang et al, 2012). The association with suicide attempts is of interest in light of experimental models in which infected rodents lose their natural fear of feline predators, a process which has been termed “fatal attraction” (Berdoy et al, 2000). This alteration in behavior is believed to be evolutionarily favorable to the Toxoplasma organism by facilitating its transmission from intermediate hosts to the complete feline host where it can undergo sexual reproduction and complete its life cycle. Some studies indicate that the Toxoplasma organism achieves the manipulation of host behavior through alterations in brain dopamine levels as described above. Suicidality and Toxoplasma infection have also been postulated to be linked by other pathways such as ones which metabolize kynurenine (Okusaga et al, 2016). Regardless of mechanism, it remains an intriguing possibility that suicidal behavior in humans represents a vestigial effect of this behavior, despite the fact that humans have not been common prey for carnivorous felines for many thousands of years. Consistent with this hypothesis are studies indicating an association between the exposure to Toxoplasma and other risk taking behaviors such as automobile accidents (Alvarado-Esquivel et al, 2012) and impulsivity (Cook et al, 2015). Additional studies are needed to confirm the extent of these associations and mechanisms by which Toxoplasma infection might elicit these behavioral changes. In addition, it will be of interest to determine if the effects of Toxoplasma infection require the continued presence of organisms or if it can persist following organism clearance, as has been documented in animal models (Ingram et al, 2013).
In addition to being associated with altered behavior, Toxoplasma infections have shown consistent effects on cognitive functioning in experimental animals, particularly in the domains measuring memory (Wang et al, 2013). Similar alterations have also been noted in in some populations of humans. For examples, exposure to Toxoplasma has been associated with lower levels of cognitive functioning in children 12–16 years of age (Mendy et al, 2015). Exposure to Toxoplasma has also been associated with decreased memory functioning in individuals who are more than 64 years (Gajewski et al, 2014) of age as well as a decline in memory and other cognitive functioning after that age (Nimgaonkar et al, 2015 ). On the other hand, exposure to Toxoplasma was not associated with altered levels of memory in unselected adults of unknown age (Gale et al, 2015) or adults with schizophrenia of unknown age (Yolken et al, 2011). Results of studies examining the possible association between exposure to Toxoplasma and Alzheimer’s disease have shown mixed results (Perry et al, 2016. It is of note that Toxoplasma infection of cells can actually decrease the formation of beta-amyloid plaques (Möhle et al, 2016), suggesting that Toxoplasma may be involved in other forms of cognitive impairment and dementia in the elderly through an alternative mechanism.
Toxoplasma and Intestinal Inflammation
While most of the interest in mechanisms relating Toxoplasma infection and altered cognition or behavior has focused on the brain, the fact that Toxoplasma enters most hosts through the intestinal tract has also led to investigation of the possibility that Toxoplasma might also affect behavior through its effects on the intestinal tract. Numerous studies have documented alterations in intestinal functioning and inflammation in individuals with psychiatric disorders (reviewed in Severance et al 2014; Severance et al, 2015a). In addition, antibodies to Toxoplasma have been associated with markers of intestinal inflammation in individuals with psychiatric disorders (Severance et al, 2012; Severance et al, 2014). Recent studies linking changes in the intestinal microbiome to altered behavior in humans and experimental animals (Borre et al, 2014) suggest that some of the effect of Toxoplasma on behavior and cognition may be related to changes at the level of the gastrointestinal tract.
Following oral infection of experimental animals, T. gondii parasites can be found within hours in the surface epithelium and lamina propria of the small intestine, and particularly in the ileum (Dubey 1997). Within days of entry to the intestinal tract, parasites can migrate into systemic circulation from the lamina propria where they then have access to host organs (Liesenfeld 2002). Presumably this translocation of T. gondii into the blood stream is a consequence of localized intestinal inflammation and enteropathy generated by the parasite that collectively results in impaired integrity of the intestinal mucosa and gut-blood barrier. Indeed, the gut targeted inflammatory state elicited by T. gondii has been adapted for experimental animal models of inflammatory bowel diseases and of ileitis in particular, although evidence in support of cellular pathologies in the duodenum and jejunum is surfacing as well (Araujo et al, 2015; Trevizan et al, 2016). The intra- and para-cellular mechanisms for parasite invasion of gut epithelial or Peyer’s patch-associated cells are not known with certainty but are actively investigated topics (Briceno et al, 2016; Gregg et al, 2013).
Loss of cellular barrier integrity at the gut-vasculature interface has implications for the blood brain barrier in psychiatric disorders. In light of the similarities of the of gut-blood and brain-blood barriers, this cellular permeability offers a route by which products of gut-based processes may impact the brain. A permeabilized gut blood barrier in psychiatric disorders has been inferred from studies of microbial translocation and microbiome sequencing which indicate an actively dysbiotic environment in subsets of individuals with psychiatric disorders (Severance et al 2013; Severance, et al, 2016; Yolken et al, 2015; Castro-Nallar et al, 2015). A functional pathological outcome of barrier permeability is the translocation of gut-dwelling microbes including bacteria and yeast into systemic circulation and these translocation rates are increased in people with psychiatric disorders (Severance et al, 2013). Studies of gut-derived markers in the CNS also point toward barrier permeability issues of the CSF-blood interface including the choroid plexus in individuals with psychiatric disorders (Severance et al, 2015b). In the context of the present review, microbial dysbiosis is an effective perpetrator of intestinal inflammation and subsequent permeability of the gut barrier and importantly has distal consequences on the blood brain barrier (Braniste et al, 2014). Thus, a parasite-mediated endothelial barrier compromise in psychiatric disorders could be a function of intestinal inflammation produced directly by invasion or indirectly by the parasite effects on the gut microbiome. Oral infection has been demonstrated to perpetrate changes in the dynamics of the gut microbiome that are sometimes immunopathogenic and sometimes immunoprotective (Bereswill et al, 2014; Craven et al, 2012; Haag et al, 2012; Egan et al, 2012).
Thus the immune system and its response to T. gondii both systemically and locally in the gut mediate the degree of eventual neuropathy of the parasite. The gut derived immune response which even when operating functionally is complicated and dependent on a stable and homeostatically balanced gut microbiome. Toxoplasma infection in experimental animals has been shown to alter several aspects of intestinal immunity (Cohen and Denkers 2015a,b). Conversely, the intestinal microflora may be one factor controlling the immune response to Toxoplasma in the intestinal tract and hence host resistance to Toxoplasma infection (Ribeiro et al, 2016). Acute Toxoplasma infection has also been shown to change the microbiome of experimentally infected mice. In these studies, the relative abundance of Gram negative bacteria such as Enterobacteria and Prevotella increases while relative abundances of Gram positive bacteria such as Clostridia and other Firmicutes are decreased. Despite the increase in total eubacteria load, acute T. gondii infection was accompanied by loss of microbial diversity (Craven et al, 2012; Molloy et al, 2013). Initial studies also indicate that chronic Toxoplasma infection of mice is also associated with changes in the intestinal microbiome, suggesting that alterations in the microbiome may play a role in the altered behavior noted in such animals (Prandovszky et al, unpublished). Studies of the effect of Toxoplasma infection on the microbiome of humans are currently lacking.
Current Status of Anti-Toxoplasma Medications
One limitation in terms of the study and management of Toxoplasma infections relates to the paucity of effective medications. The ideal treatment against Toxoplasma would be effective at inhibiting the different life stages of the parasite. However, current treatments are only capable of suppressing the rapidly dividing tachyzoyte stage of the organism. The folate synthesis pathway is the best-known therapeutic target against Toxoplasma. Compared to mammalian cells that make extensive use of exogenous folate transport pathways to obtain folate, Toxoplasma mainly relies on folate synthesis pathways and produces most of the folate it metabolize (Allegra et al, 1987; Kovacs et al, 1989; Massimine et al, 2005). The main enzymes targeted by pharmaceuticals in the folate synthesis pathway are the dihydropteroate synthase and dihydrofolate reductase. Since the dihydropteroate enzyme is not found in mammalian cells, it provides a specific target against Toxoplasma. Prymethamine and trimethoprim are the most widely used dihydrofolate reductase inhibitors. Prymethamine is a known tetratogen but is more effective then trimethophrim, which is usually administered in conjunction with a dihydropteroate synthase inhibitor (sulfamethoxzale). The sulfonamide compounds sulfadiazine and sulfamethoxazole, which inhibit the dihydropteroate synthase, are highly effective against Toxoplasma. However, the cessation of the use of these compounds is frequently accompanied by relapse. It is also worth noting that congenital Toxoplasma infections in the first trimeseter of pregnancy are commonly treated with spiramycin, a macrolide antibiotic that has low toxicity but limited potency against the parasite.
In immune competent individuals the most widely accepted regimen involves the use of the folic acid antagonist pyrimethamine with or without added sulfadizine with the main application being the treatment of Toxoplasma associated eye disease (Pradhan et al 2016). In addition to having toxicities related to its mechanism of action as noted above, this mechanism is generally ineffective against the bradyzoite form of the organism and thus cannot be used to treat tissue cysts, the form of the organism resident in the central nervous system of immune competent individuals. Additional medications, largely antibacterials and anti-parasitics, have been employed to treat Toxoplasma infections in immune compromised individuals who do not respond to, or cannot tolerate, treatment with folate antagonists (Wei et al, 2015). However the efficacy of these regimens are difficult to evaluate and, as in the case of the folate antagonists, these medications are not effective against tissue cysts. Recently a number of pharmacological approaches have been developed for the treatment of Toxoplasma tissue cysts in the brains of experimentally infected mice (Doggett et al, 2012). The development of these compounds as human medications would represent an important step in the ability to control Toxoplasma infections and to evaluate the role of Toxoplasma brain cysts in human diseases. Similarly, the application to feline and human populations of immunization regimens for the prevention of Toxoplasma infections, which are currently in the experimental stage (Opsteegh et al, 2015) would represent another important tool both the prevention of human Toxoplasma infections and the study of the role of these infections in human pathobiology.
Ongoing Research Needs
During the past decade a great deal has been learned regarding the biology of chronic Toxoplasma infection in experimental animals, particularly in rodents. However, an understanding of the role of Toxoplasma in human biology and pathology has proceeded more slowly. The main limitation of human studies is that, due to the encysted nature of the organism, it is very difficult to obtain Toxoplasma organisms or DNA from accessible body fluids such as blood or urine. Hence most studies have relied on serological methods to define exposure and the immune response to infection. While accurate and reproducible in terms of differentiating exposed from unexposed populations, currently available serological methods have a number of limitations. Of particular importance, assays which are currently in widespread usage cannot easily determine the timing of infection earlier in life, the source of infection (tissue cysts vs oocysts) and the biotype of the infecting organism. The more widespread development of assays which have been reported to accomplish some of these goals would represent a major step forward in terms of the ability to study Toxoplasma infection in humans. Similarly, infection and inflammation within the body organs infected with Toxoplasma, such as the brain and the gastrointestinal tract, can be difficult to study in a non-invasive manner. The further development of diffusion-weighted imaging (Maschke et al, 2014) and other modalities capable of measuring infection and within these and other body organs would also represent an important step in terms of identifying individuals with clinically significant Toxoplasma infections and guiding therapeutic interventions. Finally, the role of Toxoplasma in cognition and behavior suggests that population with higher rates of Toxoplasma infections based on differential exposures may suffer societal consequences based on this exposure. The study of Toxoplasma exposure as a health disparity which can potentially be corrected through preventative measures such as improved water purification remains an important goal of Public Health research.
Conclusions
Toxoplasma gondii is an organism associated with infection of the gastrointestinal tract, local and systemic inflammation, and alterations in brain functioning. The study of the pathways relating to the gastrointestinal biology and immunology of Toxoplasma infection may provide novel insights into the pathogenesis of a range of human neuropsychiatric disorders. The development of effective means for the prevention of Toxoplasma infections and for the control of immune activation may lead to new methods for the prevention and treatment of these devastating disorders as well as an overall improvement in the physical and mental health of exposed individuals.
References
- Ae-Ngibise KA, Akpalu B, Ngugi A, Akpalu A, Agbokey F, Adjei P, et al. Prevalence and risk factors for active convulsive epilepsy in Kintampo, Ghana. The Pan African Medical Journal. 2015;21:29. doi: 10.11604/pamj.2015.21.29.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra CJ, Kovacs JA, Drake JC, Swan JC, Chabner BA, Masur H. Potent in vitro and in vivo antitoxoplasma activity of the lipid-soluble antifolate trimetrexate. Journal of Clinical Investigation. 1987;79:478–482. doi: 10.1172/JCI112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Sifuentes-Alvarez A, Narro-Duarte SG, Estrada-Martinez S, Diaz-Garcia JH, Liesenfeld O, et al. Seroepidemiology of Toxoplasma gondii infection in pregnant women in a public hospital in northern Mexico. BMC Infectious Diseases. 2006;6:113. doi: 10.1186/1471-2334-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Torres-Castorena A, Liesenfeld O, Estrada-Martinez S, Urbina-Ålvarez JD. High seroprevalence of Toxoplasma gondii infection in a subset of Mexican patients with work accidents and low socioeconomic status. Parasites & Vectors. 2012;5:13. doi: 10.1186/1756-3305-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Campillo-Ruiz F, Liesenfeld O. Seroepidemiology of infection with Toxoplasma gondii in migrant agricultural workers living in poverty in Durango, Mexico. Parasitest & Vectors. 2013;6:113. doi: 10.1186/1756-3305-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Sánchez-Anguiano LF, Arnaud-Gil CA, López-Longoria JC, Molina-Espinoza LF, Estrada-Martinez S, et al. Toxoplasma gondii infection and suicide attempts: a case-control study in psychiatric outpatients. Journal of Nervous and Mental Diseases. 2013;201(11):948–52. doi: 10.1097/NMD.0000000000000037. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Sanchez-Anguiano LF, Hernandez-Tinoco J, Berumen-Segovia LO, Torres-Prieto YE, Estrada-Martinez S, et al. Toxoplasma gondii infection and mixed anxiety and depressive disorder: a case-control seroprevalence study in Durango, Mexico. Journal of Clinical Medicine Research. 2016;8(7):519–23. doi: 10.14740/jocmr2576w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo EJ, Zaniolo LM, Vincentino SL, Góis MB, Zanoni JN, da Silva AV, et al. Toxoplasma gondii causes death and plastic alteration in the jejunal myenteric plexus. World Journal of Gastroenterology. 2015;21(16):4829–39. doi: 10.3748/wjg.v21.i16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramopoulos D, Pearce BD, McGrath J, Wolyniec P, Wang R, Eckart N, et al. Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS One. 2015;10(3):e016696. doi: 10.1371/journal.pone.0116696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT. The NMDA receptor and schizophrenia: from pathophysiology to treatment. Advanced Pharmacology. 2016;76:351–82. doi: 10.1016/bs.apha.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proceedings of the Royal Society B: Biological Sciences Journal. 2000;267(1452):1591–4. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S, Kühl AA, Alutis M, Fischer A, Möhle Struck D, et al. The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequellae in experimental Toxoplasma gondii induced ileitis. Gut Pathogens. 2014;6:19. doi: 10.1186/1757-4749-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børglum AD, Demontis D, Grove J, Pallesen J, Hollegaard MV, Pedersen CB, et al. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Molecular Psychiatry. 2014;19(3):325–33. doi: 10.1038/mp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Advances in Experimental Medicine and Biology. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, et al. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;19(9072):173–7. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6(263) doi: 10.1126/scitranslmed.3009759. 263ral58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceño MP, Nascimento LA, Nogueira NP, Barenco PV, Ferro EA, Rezende-Oliveira K, et al. Toxoplasma gondii infection promotes epithelial barrier dysfunction of Caco-2 cells. Journal of Histochemistry & Cytochemistry. 2016 Jul 1; doi: 10.1369/0022155416656349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AJ, Shortt SG, Isaac-Renton J, King A, Werker D, Bowie WR. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology. 1998;105(6):1032–7. doi: 10.1016/S0161-6420(98)96004-3. [DOI] [PubMed] [Google Scholar]
- Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Chron’s disease. PLoS One. 2012;7(7):e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabunciyan S, Severance EG, Dickerson F, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeersJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang TY, Kuo MC, Chen CH, Yang JY, Kao CF, Ji DD, Fang CT. Risk factors for acute Toxoplasma gondii diseases in Taiwan: a population-based case-control study. PLoS One. 2014;9(3):e90880. doi: 10.1371/journal.pone.0090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Denkers EY. The gut mucosal immune response to Toxoplasma gondii. Parasite Immunology. 2015;37(3):108–17. doi: 10.1111/pim.12164. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Denkers EY. Impact of Toxoplasma gondii on dendritic cell subset function in the intestinal mucosa. Journal of Immunology. 2015;195(6):2754–62. doi: 10.4049/jimmunol.1501137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook TB, Brenner LA, Cloninger CR, Langenberg P, Igbide A, Giegling I, et al. “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. Journal of Psychiatric Research. 2015;60:87–94. doi: 10.1016/j.jpsychires.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS One. 2012;7(7):e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Katsafanas E, Khushalani S, et al. A combined marker of inflammation in individuals with mania. PLoS One. 2013;8(9):e73520. doi: 10.1371/journal.pone.0073520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar AJ, Drozda AA, Blader IJ. Drug repurposing screening identifies novel compounds that effectively inhibit Toxoplasma gondii growth. mSphere. 2016;1(2):e00042–15. doi: 10.1128/mSphere.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett JS, Nilsen A, Forquer I, Wegman KW, Jones-Brando L, Yolken RH, et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proceedings of the National Academy of Science of the United States of America. 2012;109(39):15936–41. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue formation in mice fed bradyzoites of different strains of Toxoplasma gondii. Journal of Eukaryotic Microbiology. 1997;44:592–602. doi: 10.1111/j.1550-7408.1997.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Hill DE, Jones JL, Hightower AW, Kirkland E, Roberts JM, et al. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. Journal of Parasitology. 2005;91(5):1082–93. doi: 10.1645/GE-683.1. [DOI] [PubMed] [Google Scholar]
- Egan CE, Cohen SB, Denkers EY. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunity and Cell Biology. 2012;90(7):668–75. doi: 10.1038/icb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch K, Peterson CA. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clinical Microbiology Review. 2013;26(1):58–85. doi: 10.1128/CMR.00067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evengård B, Petersson K, Engman ML, Wiklund S, Ivarsson SA, Teär-Fahnehjelm K, et al. Low incidence of toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiology Infection. 2001;127(1):121–7. doi: 10.1017/s0950268801005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J. Schizophrenia and Toxoplasma gondii: an undervalued association? Expert Review of Anti-Infective Therapy. 2015;13(7):817–20. doi: 10.1586/14787210.2015.1051033. [DOI] [PubMed] [Google Scholar]
- Flegr J, Prandota J, Sovickova M, Israili ZH. Toxoplasmosis- A global treat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88. PLoS One. 2014;9(3):e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M, Hengstler JP, Golka K. Toxoplasma gondii impairs memory in infected seniors. Brain, Behavior and Immunology. 2014;36:193–9. doi: 10.1016/j.bbi.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Gale SD, Brown BL, Erickson LD, Berret A, Hedges DW. Association between latent toxoplasmosis and cognition in adults: a cross-sectional study. Parasitology. 2015;142(4):557–65. doi: 10.1017/S0031182014001577. [DOI] [PubMed] [Google Scholar]
- Gregg B, Taylor BC, John B, Tait-Wojno ED, Girgis NM, Miller N, et al. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infection and Immunity. 2013;81(5):1635–43. doi: 10.1128/IAI.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, et al. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7(5):e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D, Coss C, Dubey JP, Wroblewski K, Sautter M, Hosen T, et al. Identification of a sporozoite-specific antigen from Toxoplasma gondii. Journal of Parasitology. 2011;97(2):328–37. doi: 10.1645/GE-2782.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze-Selch D, Däubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophrenia Bulletin. 2007;33(3):782–8. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram WM, Goodrich LM, Robey EA, Eisen MB. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS One. 2013;8(9):e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Dubey JP. Wateborne toxoplasmosis—recent developments. Experimental Parasitology. 2010;124(1):10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Jones JL, Dubey JP. Foodborne toxoplasmosis. Clinical Infectious Diseases. 2012;55(6):845–51. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Rivera HN, Price C, Wilkins PP. Toxoplasma gondii seroprevalence in the United states 2009–2010 and comparison with the past two decades. American Journal of Tropical Medicine and Hygiene. 2014;90(6):1135–9. doi: 10.4269/ajtmh.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophrenia Research. 2003;62(3):237–44. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- Kannan G, Crawford JA, Yang C, Gressitt KL, Ihenatu C, Krasnova IN, et al. Anti-NMDA receptor autoantibodies and associated neurobehavioral pathology in mice are dependent on age of first exposure to Toxoplasma gondii. Neurobiology of Disease. 2016;91:307–14. doi: 10.1016/j.nbd.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JA, Allegra CJ, Beaver J, Boarman D, Lewis M, Parrillo JE, et al. Characterization of de novo folate synthesis in Pneumocystis carinii and Toxoplasma gondii: potential for screening therapeutic agents. Journal of Infectious Diseases. 1989;160:312–320. doi: 10.1093/infdis/160.2.312. [DOI] [PubMed] [Google Scholar]
- Li Y, Kannan G, Pletnikov MV, Yolken RH, Xiao J. Chronic infection of Toxoplasma gondii downregulates miR-132 expression in multiple brain regions in a sex-dependent manner. Parasitology. 2015;142(4):623–32. doi: 10.1017/S003118201400167X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? Journal of Infectious Diseases. 2002;(185 Suppl):S96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Markovitz AA, Simanek AM, Yolken RH, Galea S, Koenen KC, Chen S, et al. Toxoplasma gondii and anxiety disorders in a community-based sample. Brain Behavior and Immunity. 2015;43:192–7. doi: 10.1016/j.bbi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Martin HL, Alsasdy I, Howell G, Prandovszky E, Peers C, Robinson P, et al. Effects of parasitic infection on dopamine biosynthesis in dopaminergic cells. Neuroscience. 2015;306:50–62. doi: 10.1016/j.neuroscience.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Forsting M, Diener HC. Update on neuroimaging in infectious central nervous system disease. Current Opinion in Neurobiology. 2004;17(4):475–80. doi: 10.1097/01.wco.0000137540.29857.bf. [DOI] [PubMed] [Google Scholar]
- Massimine KM, Doan LT, Atreya CA, Stedman TT, Anderson KS, Joiner KA, et al. Toxoplasma gondii is capable of exogenous folate transport. A likely expansion of the BT1 family of transmembrane proteins. Molecular and Biochemical Parasitology. 2005;144:44–54. doi: 10.1016/j.molbiopara.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mendy A, Vieira ER, Albatineh AN, Gasana J. Toxoplasma gondii seropositivity and cognitive functions in school-aged children. Parasitology. 2015;142(9):1221–7. doi: 10.1017/S0031182015000505. [DOI] [PubMed] [Google Scholar]
- Möhle L, Israel N, Paarmann K, Krohn M, Pietkiewicz S, Müller A, et al. Chronic Toxoplasma gondii infection enhances β-amyloid phagocytosis and clearance by recruited monocytes. Acta Neuropatholica Communications. 2016;4:25. doi: 10.1186/s40478-016-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MJ, Grainger JR, Bouladoux N, Hand TW, Koo LY, Naik S, et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe. 2013;14(3):318–28. doi: 10.1016/j.chom.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Zanzi CA, Fry P, Lesina B, Hill D. Toxoplasma gondii oocyst-specific antibodies and source of infection. Emerging Infectious Diseases. 2010;16(10):1591–3. doi: 10.3201/eid1610.091674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgaonkar VL, Yolken RH, Wang T, Chung-Chou HC, McClain L, McDade E, et al. Temporal cognitive decline associated with exposure to infectious agents in a population-based, aging cohort. Alzheimer Disease and Associated Disorders. 2015 doi: 10.1097/WAD.0000000000000133. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaga O, Duncan E, Langenberg P, Brundin L, Fuchs D, Groer MW, et al. Combined Toxoplasma gondii seropositivity and high blood kynurenine—Linked with nonfatal suicidal self-directed violence in patients with schizophrenia. Journal of Psychiatric Research. 2016;72:74–81. doi: 10.1016/j.jpsychires.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Opsteegh M, Kortbeek TM, Havelaar AH, van der Giessen JW. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clinical Infectious Diseases. 2015;60(1):101–7. doi: 10.1093/cid/ciu721. [DOI] [PubMed] [Google Scholar]
- Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. International Journal for Parasitology. 2009;39(12):1385–94. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Kruszon-Moran D, Jones JL. The relationship between Toxoplasma gondii infection and mood disorders in the third National Health and Nutrition Survey. Biological Psychiatry. 2012;72(4):290–5. doi: 10.1016/j.biopsych.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CE, Gale SD, Erickson L, Wilson E, Nielsen B, et al. Seroprevalence and serointensity of latent Toxoplasma gondii in a sample of elderly adults with and without Alzheimer Disease. Alzheimer Diseases and Associated Disorders. 2016;30(2):123–6. doi: 10.1097/WAD.0000000000000108. [DOI] [PubMed] [Google Scholar]
- Pittman KJ, Knoll LJ. Long-term relationships: the complicated interplay between the host and the developmental stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiology and Molecular Biology Reviews. 2015;79(4):387–401. doi: 10.1128/MMBR.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan E, Bhandari S, Gilbert RE, Stanford M. Antibiotics versus no treatment for toxoplasma retinochoroiditis. Cochraine Database System Review. 2016;(5) doi: 10.1002/14651858.CD002218.pub2. CD002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Cde M, Zorgi NE, Meireles LR, Garcia JL, de Andrade Junior HF. CD19 lymphocyte proliferation induced by Bifidobacterium animalis subsp. Lactis in C57Bl/6 mice experimentally infected with Toxoplasma gondii. Rev Ins Med Trop Sao Paulo. 2016;58:26. doi: 10.1590/S1678-9946201658026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Review. 2012;25(2):264–96. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophrenia Research. 2012;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia Research. 2013;148(1–3):130–7. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Yang S, Stallings CR, Origoni AE, Vaughn C, et al. Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disorders. 2014;16(3):230–40. doi: 10.1111/bdi.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: More than a gut feeling. Schizophrenia Research. 2014 Jul 14; doi: 10.1016/j.schres.2014.06.027. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Alaedini A, Rohleder C, Enning F, Bumb JM, et al. IgG dynamics of dietary antigens point to cerebrospinal fluid barrier or flow dysfunction in first-episode schizophrenia. Brain, Behavior and Immunity. 2015;44:148–158. doi: 10.1016/j.bbi.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Current Psychiatry Reports. 2015;17(5):574. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Yang S, Katsafanas E, Schweinfurth L, Savage C, et al. Candida albicans exposures, sex-specificity, and cognitive deficits in schizophrenia and bipolar disorder. npj Schizophrenia. 2016;2:16018. doi: 10.1038/npjschz.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. Is Toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLoS One. 2016;11(2):e0148435. doi: 10.1371/journal.pone.0148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophrenia Bulletin. 2007;33(3):729–36. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophrenia Bulletin. 2012;38(3):642–7. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevizan AR, Vicentino-Vieira SL, da Silva Watanabe P, Góis MB, de Melo Gde a, Garcia JL, et al. Kinetics of acute infection with Toxoplasma gondii and histopathological changes in the duodenum of rats. Experimental Parasitology. 2016;165:22–9. doi: 10.1016/j.exppara.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Wang T, Tang ZH, Li JF, Li XN, Wang X, Xhao ZJ. A potential association between Toxoplasma gondii infection and schizophrenia in mouse models. Experimental Parasitology. 2013;135(3):497–502. doi: 10.1016/j.exppara.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Harmon S, O’Malley KL, Sibley LD. Reassessment of the ole of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infection and Immunology. 2015;83(3):1039–47. doi: 10.1128/IAI.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HX, Wei SS, Lindsay DS, Peng HJ. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0128204. doi: 10.1371/journal.pone.0138204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MW, Radke JR, Radke JB. Toxoplasma development - turn the switch on or off? Cell Microbiology. 2014;16(4):466–72. doi: 10.1111/cmi.12267. [DOI] [PubMed] [Google Scholar]
- Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Scientific Reports. 2016;6:22551. doi: 10.1038/srep22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Kannan G, Jones-Brando L, Brannock C, Krasnova IN, Cadet JL, et al. Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience. 2012;206:39–48. doi: 10.1016/j.neuroscience.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Xiao J, Yolken RH. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiologica (Oxford) 2015;213(4):828–45. doi: 10.1111/apha.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Li Y, Prandovszky E, Kannan G, Viscidi RP, Pletnikov MV, et al. Behavioral abnormalities in a mouse model of chronic toxoplasmosis are associated with MAG1 antibody levels and cyst burden. PLoS Neglected Tropical Diseases. 2016;10(4):e0004674. doi: 10.1371/journal.pntd.0004674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Tairaku S, Morioka I, Sonoyama A, Tanimura K, Deguchi M, et al. Nationwide survey of mother-to-child infections in Japan. Journal of Infectious Chemotherapy. 2015;21(3):161–4. doi: 10.1016/j.jiac.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Molecular Psychiatry. 2008;13(5):470–9. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophrenia Research. 2011;128(1–3):61–5. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Severance EG, Sabunciyan S, Gressitt K, Chen O, Origoni A, et al. Metagenomic sequencing indicates that the oropharyngeal virome of individuals with schizophrenia differs from that of controls. Schizophrenia Bulletin. 2015;41(5):1153–61. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Träskman-Bendz L, Janelidze S, Langenberg P, Saleh A, Constantine N, et al. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. Journal of Clinical Psychiatry. 2012;73(8):1069–76. doi: 10.4088/JCP.11m07532. [DOI] [PubMed] [Google Scholar]