Abstract
Background
We evaluated the relationship of human papillomavirus (HPV) and Epstein–Barr virus (EBV) with race in endemic and nonendemic cohorts of patients with nasopharyngeal carcinoma (NPC), and with smoking status in the nonendemic cohort.
Methods
Tissue microarrays (TMAs) were constructed using samples from 86 patients treated in southern China and 108 patients from Stanford, California. TMAs were stained with p16, HPV in situ hybridization (ISH), and EBV ISH. Polymerase chain reaction (PCR) was used to confirm EBV(−) cases and HPV status in p16(+) cases. Survival data was available for the Stanford cohort only.
Results
No HPV(+) cases were detected in the Chinese cohort. In the Stanford cohort, 5 of 11 EBV(−) cases harbored HPV-16, 10 of 10 occurred in whites, and 8 of 11 were smokers. Patients with EBV(−) NPC also showed a trend toward worse survival.
Conclusion
EBV(−) NPC shows an association with the presence of HPV, white race, and smoking. In contrast, EBV(−) NPC shows no association with HPV in the endemic cohort.
Keywords: Nasopharyngeal carcinoma, HPV, white, endemic, EBV
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is distinct from other head and neck cancers by its epidemiologic, histopathologic, and clinical characteristics. It is geographically endemic with an incidence as low as <0.5 cases/100,000 person-years in Western countries and as high as 25/100,000 person-years in southeast Asia.1 The rate for American-born Chinese is intermediate, ranging from 2.7 to 15.2 cases/100,000 person-years.2 NPC is consistently associated with the Epstein–Barr virus (EBV) and most if not all NPC tumors from endemic regions have been shown to harbor the EBV genome with the virus present in all tumor cells.1 More recently, molecular detection and quantitation of circulating cell-free EBV DNA in the plasma of patients with NPC has been demonstrated to serve as a potential noninvasive test for NPC detection and prognostication in endemic areas.3–6
However, EBV DNA has not been consistently detected in patients with NPC from nonendemic regions. Specifically, the association between EBV and keratinizing squamous cell carcinoma of the nasopharynx has been a matter of controversy. Several studies utilizing polymerase chain reaction (PCR) or in situ hybridization (ISH) for detection of EBV DNA or EBV-encoded RNA (EBER) have found that keratinizing NPC do not consistently harbor EBV.7–13 Yet other studies have reported the detection of EBV DNA by Southern blot hybridization in all cases of keratinizing NPC, albeit at low copy numbers.14 The discrepancy may be attributed to the fact that most of the EBV(+) keratinizing types of NPC are found in endemic regions, whereas those that are EBV(−) are concentrated in nonendemic regions.
The etiology of EBV(−) NPC is unclear. Some studies have shown an association with cigarette smoking or environmental exposures (eg, formaldehyde and wood dust),11,15,16 whereas others have suggested non-EBV viral causes, including human papillomavirus (HPV).10–13,17–22 Hørding et al17 was the first to identify HPV-11 and HPV-16 DNA in NPC tumors by PCR. Since then, several studies have suggested that certain types of NPC tumors may harbor the HPV virus.10,11,13 Few of these studies have been performed on a large scale and only a couple have had comparison cohorts from endemic and nonendemic populations.13,17
In our study, we evaluated the presence of EBV and HPV DNA in 108 NPC cases from the United States (nonendemic region) and 86 cases from southern China (endemic region) using several different detection assays for EBV and HPV, including EBER ISH, EBV DNA PCR, p16 immunohistochemistry (IHC), HPV DNA ISH, and HPV DNA PCR.
MATERIALS AND METHODS
Patients
Eighty-six Chinese patients with NPC were accrued from Shantou Medical College, Shantou, China, between May 1996 and August 1997. Shantou is located in the Guangdong province of southern China. All patients had tumor biopsied at the time of diagnosis, which was available as formalin-fixed paraffin-embedded blocks. All patients were treated locally; details of treatment and follow-up are not available for these patients.
One hundred eight patients were accrued from Stanford University between 1993 and 2010. The patient and tumor characteristics for this patient group are shown in Table 1. The formalin-fixed paraffin-embedded tissue samples were obtained from 1993 to 2010 with the majority from the year 2000 or later. A subset of the cases consists of recurrent (n = 37) or metastatic tumors (n = 10). There were 4 patients with available paired biopsies of the primary and locally recurrent tumors, and 4 patients with available paired biopsies of the primary and metastatic tumors. The results of the HPV and EBV analyses were identical for each pair. In addition, treatment details and outcomes are available from the time of diagnosis for most patients. Cigarette smoking status was collected based on pack-years of cigarette use. Patients were considered nonsmokers if they had less than 1 pack-year of cigarette use in their lifetime.23 Smokers, either current or former, were further grouped by pack-years of cigarette use: <10 vs ≥10 pack-years. Current plus past smokers were coded as smokers for this analysis.
TABLE 1.
Patient and tumor characteristics in the nonendemic U.S. patients.
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Male | 78 (72.2) |
| Female | 30 (27.8) |
| Age | |
| Median (range) | 46 y (18–82) |
| Race | |
| Asian | 77 (71.3) |
| Black | 2 (1.9) |
| Hispanic | 3 (2.8) |
| White | 25 (23.1) |
| Unknown | 1 (1.0) |
| Smoking status | |
| No | 67 (62.0) |
| Yes | 28 (25.9) |
| Unknown | 13 (12.0) |
| Tumor source | |
| Primary* | 61 (56.5) |
| Recurrent | 37 (34.3) |
| Metastatic | 10 (9.3) |
| WHO type | |
| Keratinizing | 4 (3.7) |
| Nonkeratinizing-differentiated | 20 (18.5) |
| Nonkeratinizing-undifferentiated | 80 (74.1) |
| Basaloid | 4 (3.7) |
| EBV ISH and PCR | |
| Positive | 93 (86.1) |
| Negative | 11 (10.1) |
| N/A | 4 (3.8) |
| p16 IHC | |
| Positive | 8 (7.4) |
| Negative | 87 (80.6) |
| N/A | 13 (12.0) |
| HPV ISH and PCR | |
| Positive | 5 (4.6) |
| Negative | 90 (83.4) |
| N/A | 13 (12.0) |
Abbreviations: WHO, World Health Organization; EBV, Epstein–Barr virus; ISH, in situ hybridization; PCR, polymerase chain reaction; N/A, not available; IHC, immunohistochemistry; HPV, human papillomavirus.
There were 4 pairs of primary/recurrent tumors and 4 pairs of primary/metastatic tumors, all yielding identical EBV and HPV results.
Institutional review board approval was obtained from Stanford University and Shantou Medical College. Study participants signed written informed consents.
World Health Organization grading
Hematoxylin-eosin stained whole sections of tumor from both cohorts were graded by 1 pathologist (C.S.K.) in accord with the 2005 World Health Organization (WHO) histologic classification system for NPCs: keratinizing squamous cell carcinoma (formerly WHO I according to the 1978 WHO classification system), nonkeratinizing-differentiated carcinoma (formerly WHO II), nonkeratinizing-undifferentiated carcinoma (formerly WHO III), and basaloid squamous cell carcinoma.24 The basaloid subtype is a new addition in the 2005 WHO system.
Tissue microarray construction
Tissue microarrays (TMAs) were constructed by using a tissue arrayer (Beecher Instruments, Silver Spring, MD) to create 3 new paraffin blocks from representative 0.6 mm cores taken in duplicate from formalin-fixed paraffin-embedded blocks of tumors.25 Two TMAs were created from 152 blocks of tumor from the U.S. cohort (TA308, TA340) and 1 TMA from 86 blocks from the southern China cohort (TA356). The TMAs also included control cores of normal tonsil and placenta.
p16 Immunohistochemistry
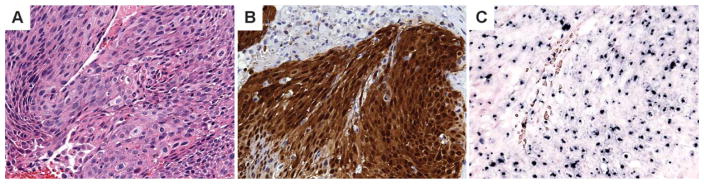
Immunoperoxidase stain for p16INK4a (clone E6H4, pre-dilute, Tris pH 9.0; Roche Diagnostics, Tucson, AZ) was performed on 4-μM thick TMA sections. The p16 staining was interpreted by a pathologist (C.S.K.), who was blinded to the clinical data, and scored based on strong nuclear or nuclear and cytoplasmic staining as follows: negative (0% to <5%), focal strong (5% to <50%), or diffuse strong (>50%).26 Only tumors that showed diffuse strong p16 staining were recorded as positive (Figure 1).
FIGURE 1.
Keratinizing type of nasopharyngeal carcinoma (A) with diffuse strong p16 reactivity (B) and positive punctate nuclear staining (arrow) with human papillomavirus (HPV) in situ hybridization (ISH) (C). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In situ hybridization
In situ hybridization for EBER ISH was performed on the Ventana Benchmark XT using a predilute EBV probe from Ventana Medical Systems (Tucson, AZ) with their proprietary ISH I’View Blue Plus Detection Kit. Four-μM thick sections of the TMAs were stained. Nuclear staining was scored as positive. Cases that were EBV(−) on TMA cores were further evaluated by EBER ISH on whole sections of tumor and by EBV DNA PCR.
The INFORM HPV DNA ISH assay (Ventana) was performed on 4-μM thick sections of the TMAs and whole sections with the Benchmark Automated Slide Stainer, utilizing the HPV III probe that demonstrates positive hybridization to HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. Punctate or diffuse nuclear staining was scored as positive (Figure 1).
DNA extraction, human papillomavirus, and Epstein–Barr virus polymerase chain reaction assays
HPV DNA PCR was performed on all p16(+) cases, regardless of HPV ISH or EBV ISH status. EBV DNA PCR was performed on all EBER ISH negative cases to confirm negative EBV status with 2 different methodologies. Tumor rich areas (>70%) were marked on hematoxylin-eosin stained sections and punch biopsies were taken from the paraffin blocks. The cores were deparaffinized with xylene and DNA using the QIAGEN (Valencia, CA) DNeasy blood and tissue kit.
Real-time PCR reactions were set up using the TaqMan PCR Master Mix (Roche, IN). HPV-16/18 E6/7 and EBV BAMHI region primers and probes were used, as previously described.27 PCR primers and probes were synthesized by Biosearch Technologies (Novato, CA). β-globin was used as an internal positive control. All PCR reactions were carried out in triplicate. A null template negative control was included in each analysis. DNA amplifications were carried out on ABI 9700 Sequence Detector (Applied Biosystems), which can detect multiple fluorescent dyes. Fluorescence data were analyzed with Sequence Detection System Software.
Statistical analysis
Statistical analysis was performed using the Statview (Computing Resource Center, Santa Monica, CA) statistical software. Chi-square tests were used to evaluate the relationship between different groups. Overall survival was measured from the first date of treatment to the date of last follow-up or death. Kaplan–Meier survival curves were generated for overall survival and compared using the log-rank test.28
RESULTS
Table 2 shows the distribution of EBV status by WHO histology for the southern Chinese patients. Three of 86 cases were EBV(−) by EBER ISH and confirmed as negative by PCR. These cases were also negative for p16 and none had keratinizing or basaloid histology. p16 was positive in 2 cases, both of which were also EBV(+). None of the evaluated cases, including the 2 p16 positive cases, was HPV DNA positive by either ISH or PCR.
TABLE 2.
Relationship between Epstein–Barr virus status, p16 status, and World Health Organization histology in the endemic southern China cohort.
| Parameter | Total (%) | No. of EBV(+) (%) | No. of EBV(−) (%) | p value |
|---|---|---|---|---|
| WHO type | ||||
| Keratinizing | 1 (1.2) | 1 (1.2) | 0 | |
| Nonkeratinizing-differentiated | 10 (11.6) | 8 (9.3) | 2 (2.3) | .027 |
| Nonkeratinizing-undifferentiated | 74 (86.0) | 73 (84.9) | 1 (1.2) | |
| Basaloid | 1 (1.2) | 1 (1.2) | 0 | |
| p16 IHC | ||||
| Positive | 2 (2.3) | 2 (2.3) | 0 | |
| Negative | 80 (93.0) | 77 (89.5) | 3 (3.5) | .78 |
| N/A | 4 (46.5) | N/A | N/A | |
Abbreviations: EBV, Epstein–Barr virus; WHO, World Health Organization; IHC, immunohisto-chemistry; N/A, not available.
Of the Stanford cases, 104 tumors were evaluable for EBV and 95 for all 3 markers: EBER ISH, p16, and HPV ISH. Eleven cases were EBV(−), of which 8 were p16(+) and 5 were HPV ISH and PCR-positive. All HPV ISH-positive tumors were PCR positive for HPV-16. All the HPV(+) tumors were p16(+), whereas the other 3 of 8 p16(+) cases were both HPV ISH (broad spectrum high-risk HPV) and HPV PCR (HPV-16 and 18) negative.
Table 3 shows the relationship between the 3 markers as well as between EBV and HPV by WHO type, race, and cigarette smoking status. As shown, EBV(−) tumors were found almost exclusively in white patients (10 of 11 patients; 1 of 11 unknown race) and in either current or past smokers (8 of 11 patients), 4 of which had ≥10 pack-year of cigarette use; the nonkeratinizing-differentiated subtype was most common (6 of 11 patients) but all types were seen. Only 18 of 84 patients with EBV(+) NPC had a history of smoking. HPV(+) tumors were found only in whites; 4 of 5 patients were either current or past cigarette smokers and 2 had more than 10 pack-years (range, 20–45 years). They consisted of keratinizing (1 of 5) and nonkeratinizing-differentiated (4 of 5) tumors only. The proportion of HPV(+) NPC tumors in whites also showed that they trended up over time with 4 of 13 white patients with HPV(+) tumors occurring from 2000 to 2010 and 1 of 12 from 1990 to 2000. For all 5 of the patients with HPV(+) tumors, we re-reviewed the pre-treatment imaging studies (CT and/or MRI). None of the studies showed tumor involvement of the oropharynx. This was confirmed by the tumor description in the recorded history and physical examinations.
TABLE 3.
Relationship between Epstein–Barr virus and human papillomavirus status by race, World Health Organization histology, and smoking status in the nonendemic U.S. cohort.
| Characteristic | No. of EBV(+) | No. of EBV(−) | p value | No. of HPV(+) | No. of HPV(−) | p value |
|---|---|---|---|---|---|---|
| p16 | ||||||
| Positive | 1 | 7 | < .0001 | 5 | 3 | < .0001 |
| Negative | 83 | 4 | 0 | 87 | ||
| HPV | ||||||
| Positive | 0 | 5 | < .0001 | |||
| Negative | 84 | 6 | ||||
| WHO | ||||||
| Keratinizing | 1 | 2 | < .0001 | 1 | 2 | .004 |
| Nonkeratinizing-differentiated | 14 | 6 | 4 | 15 | ||
| Nonkeratinizing-undifferentiated | 76 | 1 | 0 | 70 | ||
| Basaloid | 2 | 1 | 0 | 3 | ||
| Race | ||||||
| Asian | 74 | 0 | < .0001 | 0 | 66 | .001 |
| Black | 2 | 0 | 0 | 2 | ||
| Hispanic | 3 | 0 | 0 | 2 | ||
| White | 14 | 10 | 5 | 19 | ||
| Unknown | 0 | 1 | 0 | 1 | ||
| Smoking | ||||||
| No | 64 | 2 | .002 | 1 | 9 | .01 |
| Yes | 17 | 8 | 4 | 19 | ||
| (<10 vs ≥10 pack-years) | (4 vs 13) | (4 vs 4) | (2 vs 2) | (6 vs 13) | ||
| Unknown | 12 | 12 | ||||
Abbreviations: EBV, Epstein–Barr virus; HPV, human papillomavirus; WHO, World Health Organization.
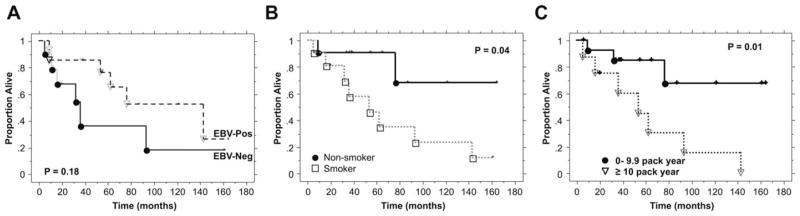
Of the 24 white patients with assessable HPV and EBV status, we evaluated treatment outcome based on marker status. The median follow-up for living patients was 48 months (range, 7–165 months). At the latest follow-up, 12 patients have died. As shown in Figure 2A, there was a trend for worse survival in patients with EBV(−) tumors as compared to those with EBV(+) tumors, but the difference was not statistically significant (p = .18). The median survival for EBV(−) tumors was 34 months as compared to 122 months for EBV(+) tumors. This difference is likely driven by cigarette use but it is unknown if race also plays a role because almost all the EBV(−) tumors were in white patients. As shown in Figure 2B, the survival for nonsmokers was significantly better than that for current or past-smokers (p = .04 when the 2 curves are compared, excluding the 2 patients with unknown smoking status). The survival difference was even greater when patients with ≥10 pack-years were compared to nonsmokers or those with <10 pack-year of cigarette use (Figure 2C; p = .01). There was no association between p16 or HPV status and overall survival in this small patient group (data not shown). A multivariate analysis was not performed because of the small number of patients and events.
FIGURE 2.
Overall survival by Epstein–Barr virus (EBV) (A), smoking status (B), and pack-years of cigarette use (C) among white patients
DISCUSSION
To our knowledge, this is one of the largest studies evaluating HPV status in NPCs from an endemic and nonendemic cohort. Where race data were available, all EBV(−) cases were from white patients and there was an association with the presence of high-risk HPV (5 of 10 cases) and smoking (8 of 9 with known smoking status). In contrast, no HPV(+) cases were found in the southern China cohort or among Chinese American patients in the U.S. cohort; instead all but 3 cases in the endemic population were associated with EBV.
Although we found that EBV(−) NPC were more likely to be p16(+) and HPV(+), the majority of the EBV(−) tumors did not have detectable HPV types 16 or 18. However, infection with other high-risk HPV subtypes cannot be excluded because the broad-spectrum high-risk HPV ISH probe is less sensitive than PCR. A more comprehensive evaluation for all oncogenic HPV subtypes with PCR may be necessary to determine the relationship between HPV and EBV(−) NPC.
EBV(−) NPC is typically found in nonendemic populations in which other risk factors such as cigarette smoking and environmental exposures are involved. In the oropharynx, HPV is now recognized to play a significant role in carcinogenesis, and risk factors for HPV-related squamous cell carcinomas include history of sexually transmitted disease, increased number of vaginal or oral sex partners, and immune suppression.29,30 Although HPV is less commonly found in NPC, various studies have reported the presence of HPV, predominantly type 16, in a subset of NPC cases. We found HPV type 16 in 5 cases that represent 6% of the nonendemic cohort and 21% of the white subgroup. Table 4 shows a summary of the literature addressing HPV and EBV status in NPC. In non-endemic populations, the reported rate of HPV(+) NPC ranges from 9%22 to 80%.19 Because these all represent limited studies of select patient populations, the true incidence of HPV(+) NPC is unknown. In the U.S. studies, HPV(+) NPC were more commonly found among white patients19 and among the keratinizing subtype.13,18 Although all of our HPV(+) cases were in white patients, they were more likely to be nonkeratinizing rather than keratinizing. We also noted a trend toward an increasing proportion of HPV(+) cases occurring over time, which parallels the well-documented increase in incidence of HPV-related oropharyngeal squamous cell carcinomas from 1973 to 2001.31,32 However, the number of HPV(+) NPC in our series was too small to draw any definitive conclusions. Unlike a prior study in which all patients with HPV(+) NPC were found to have tumors extending into the oropharynx,22 none of our patients had tumor involvement of the oropharynx at either the time of initial diagnosis or recurrence. Our data do not support the hypothesis that HPV(+) NPC merely represents superior extension of an oropharyngeal carcinoma.
TABLE 4.
Summary of literature addressing human papillomavirus and Epstein–Barr virus status in nasopharyngeal carcinomas.
| Author, year | Study population | % EBV(+) (#) | % HPV(+) (#) | % Coinfection (#) | HPV type |
|---|---|---|---|---|---|
| Tyan et al, 199320 | Taiwan | 100% (30/30) | 47% (14/30) | 17% (5/30) | HPV-16 |
| Hørding et al, 199417 | Denmark | 43% (10/23) | 27% (4/15) | 0 | HPV-16, 11 |
| Inuit (Eskimo) | 100% (15/15) | 0 | 0 | N/A | |
| Giannoudis et al, 199510 | Greece | 32% (20/63) | 19% (12/63) | 0 | Not typed |
| Punwaney et al, 199913 | United States | 86% (6/7) | 43% (3/7) | 29% (2/7) | Not typed |
| Korea and China | 71% (5/7) | 17% (4/23) | 17% (4/23) | Not typed | |
| Tung et al, 199933 | Taiwan | 83% (73/88) | 51% (45/88) | 42% (37/88) | HPV-16, 18 |
| Mirzamani et al, 200621 | Iran | 95% (19/20) | 20% (4/20) | 15% (3/20) | HPV-6/11, 16/18 |
| Maxwell et al, 201023 | United States | 20% (1/5) | 80% (4/5) | 0 | HPV-16, 18, 59 |
| Lo et al, 201011 | United States | 50% (14/28) | 15% (4/26) | 0 | HPV-16 |
| Laantri et al, 201112 | Morocco | 100% (70/70) | 34% (24/70) | 34% (24/70) | HPV-31, 16, 18, 33, 35, 45, 59 |
| Huang et al, 201118 | Taiwan - NPC | 100% (43/43) | 35% (15/43) | 35% (15/43) | HPV-16, 18, 33, 58, 66, 69, 72, 84 |
| Nasopharynx control | 15% (6/40) | 42.2% (17/40) | N/A | HPV-16, 18, 26, 54, 58, 70, 84 | |
| Singhi et al, 201222 | United States | 97% (34/35) | 9% (4/45) | 0 | 0 |
| Current study | United States | 92% (91/99) | 6% (5/88) | 0 | HPV-16 |
| China | 96% (82/85) | 0 | 0 | N/A |
Abbreviations: EBV, Epstein–Barr virus; HPV, human papillomavirus; N/A, not available; NPC, nasopharyngeal carcinoma.
Only 2 prior studies have directly compared endemic and nonendemic cohorts. Hørding et al17 detected HPV in 4 cases of keratinizing NPC in a Danish (nonendemic) cohort and no HPV-associated cases in an Inuit (endemic) cohort. Punwaney et al13 reported the detection of HPV in 3 cases of NPC in white Americans and 4 cases in Korean and Chinese patients who were also coinfected with EBV. We found no evidence of coinfection with HPV and EBV, but some studies have reported coinfection in up to 42% of cases in an endemic Taiwanese population,12,13,18,20,21,33 whereas others found no evidence of coinfection.10,17,19,22,34 Further studies are required to determine if EBV and HPV work together in carcinogenesis or if one of the viruses is a bystander.
In our study, patients with EBV(−) tumors showed a trend toward worse overall survival and all these cases occurred in white patients. These observations are similar to those reported in previous studies that have shown worse survival for white patients as compared with Chinese patients, although the latter tend to present at a higher stage. Based on Surveillance, Epidemiology, and End Results data from 1973 to 2002, 5-year and 10-year overall survival rates for Chinese patients in the United States are reported as 59.8% and 45.7%, respectively, which are comparable to rates for patients in Hong Kong and Taiwan.35 Prior studies have also shown an association of NPC subtypes with prognosis with a better outcome for nonkeratinizing NPC.36,37 Given the potential for interobserver variability in classifying NPC, sampling errors in small biopsies, and the challenge of classifying mixed types, it seems that viral status would be better as a prognostic indicator. Although we did not find an association between HPV status and overall survival, the numbers evaluated were small and future studies with larger cohorts of HPV(+) NPC would be beneficial for addressing this question. Given the improved overall survival of patients with HPV-related oropharyngeal squamous cell carcinoma, it raises the question of whether patients with HPV(+) tumors in the nasopharynx will also have better overall survival than those with EBV(−)/HPV(−) tumors. In a study of 46 Taiwanese patients with NPC, 35% of whom had HPV(+) tumors, Huang et al18 found no correlation of HPV status with survival.
Among the EBV(−) cases in our nonendemic cohort, 80% of the patients had a history of smoking, of which half had ≥ 10 pack-years of cigarettes use. Patients who smoked (especially those with ≥10 pack-years) showed significantly worse overall survival as compared with nonsmokers. For the endemic cohort, clinical data was not available so it cannot be determined if the 3.5% of patients with EBV(−) NPC had a history of cigarette smoking or environmental exposures. With the high rate of cigarette use in China, future studies will be required to determine if smoking leads to a change in the profile of NPC cases in this endemic population.
In summary, we found EBV(−) tumors are rare in patients from the southern China endemic cohort and did not occur in Asian American patients from our nonendemic cohort. In contrast, all the EBV(−) cases occurred in white patients and half were associated with HPV and smoking. Further studies will be required to determine the interplay between EBV, HPV, smoking, genetics, and environmental exposures.
Acknowledgments
Contract grant sponsor: Supported by The Li Ka Shing Foundation Research Grant.
References
- 1.Niedobitek G. Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Mol Pathol. 2000;53:248–254. doi: 10.1136/mp.53.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun LM, Epplein M, Li CI, Vaughan TL, Weiss NS. Trends in the incidence rates of nasopharyngeal carcinoma among Chinese Americans living in Los Angeles County and the San Francisco metropolitan area, 1992–2002. Am J Epidemiol. 2005;162:1174–1178. doi: 10.1093/aje/kwi345. [DOI] [PubMed] [Google Scholar]
- 3.Lo YM, Chan LY, Chan AT, et al. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res. 1999;59:5452–5455. [PubMed] [Google Scholar]
- 4.Lo YM, Chan LY, Lo KW, et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59:1188–1191. [PubMed] [Google Scholar]
- 5.Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614–1619. doi: 10.1093/jnci/94.21.1614. [DOI] [PubMed] [Google Scholar]
- 6.Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461–2470. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- 7.Niedobitek G, Hansmann ML, Herbst H, et al. Epstein-Barr virus and carcinomas: undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. J Pathol. 1991;165:17–24. doi: 10.1002/path.1711650105. [DOI] [PubMed] [Google Scholar]
- 8.Klein G, Giovanella BC, Lindahl T, Fialkow PJ, Singh S, Stehlin JS. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974;71:4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hørding U, Nielsen HW, Albeck H, Daugaard S. Nasopharyngeal carcinoma: histopathological types and association with Epstein-Barr virus. Eur J Cancer B Oral Oncol. 1993;29B:137–139. doi: 10.1016/0964-1955(93)90036-e. [DOI] [PubMed] [Google Scholar]
- 10.Giannoudis A, Ergazaki M, Segas J, et al. Detection of Epstein-Barr virus and human papillomavirus in nasopharyngeal carcinoma by the polymerase chain reaction technique. Cancer Lett. 1995;89:177–181. doi: 10.1016/0304-3835(94)03667-8. [DOI] [PubMed] [Google Scholar]
- 11.Lo EJ, Bell D, Woo JS, et al. Human papillomavirus and WHO type I naso-pharyngeal carcinoma. Laryngoscope. 2010;120:1990–1997. doi: 10.1002/lary.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laantri N, Attaleb M, Kandil M, et al. Human papillomavirus detection in Moroccan patients with nasopharyngeal carcinoma. Infect Agent Cancer. 2011;6:3. doi: 10.1186/1750-9378-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of white Americans: investigation of Epstein-Barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. doi: 10.1002/(sici)1097-0347(199901)21:1<21::aid-hed3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Raab–Traub N, Flynn K, Pearson G, et al. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987;39:25–29. doi: 10.1002/ijc.2910390106. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan TL, Shapiro JA, Burt RD, et al. Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer Epidemiol Biomarkers Prev. 1996;5:587–593. [PubMed] [Google Scholar]
- 16.Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 17.Hørding U, Nielsen HW, Daugaard S, Albeck H. Human papillomavirus types 11 and 16 detected in nasopharyngeal carcinomas by the polymerase chain reaction. Laryngoscope. 1994;104(1 Pt 1):99–102. doi: 10.1288/00005537-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Huang CC, Hsiao JR, Yang MW, et al. Human papilloma virus detection in neoplastic and non-neoplastic nasopharyngeal tissues in Taiwan. J Clin Pathol. 2011;64:571–577. doi: 10.1136/jcp.2010.087742. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32:562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyan YS, Liu ST, Ong WR, Chen ML, Shu CH, Chang YS. Detection of Epstein-Barr virus and human papillomavirus in head and neck tumors. J Clin Microbiol. 1993;31:53–56. doi: 10.1128/jcm.31.1.53-56.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirzamani N, Salehian P, Farhadi M, Tehran EA. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp Mol Pathol. 2006;81:231–234. doi: 10.1016/j.yexmp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JKC, Bray F, McCarron P, et al. Nasopharynx. In: Leon B, Eveson JW, Reichart P, Sidransky D, editors. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. pp. 81–97. [Google Scholar]
- 25.Liu CL, Prapong W, Natkunam Y, et al. Software tools for high-throughput analysis and archiving of immunohistochemistry staining data obtained with tissue microarrays. Am J Pathol. 2002;161:1557–1565. doi: 10.1016/S0002-9440(10)64434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills AM, Beck AH, Pourmand N, Le QT, Kong CS. Evaluation of ProExC as a prognostic marker in oropharyngeal squamous cell carcinomas. Am J Surg Pathol. 2012;36:1158–1164. doi: 10.1097/PAS.0b013e3182600eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H, Banh A, Kwok S, et al. Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2012;82:e351–e358. doi: 10.1016/j.ijrobp.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life-tables. J Royal Stat Soc. 1972;34:187–229. [Google Scholar]
- 29.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 30.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 32.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 33.Tung YC, Lin KH, Chu PY, Hsu CC, Kuo WR. Detection of human papilloma virus and Epstein-Barr virus DNA in nasopharyngeal carcinoma by polymerase chain reaction. Kaohsiung J Med Sci. 1999;15:256–262. [PubMed] [Google Scholar]
- 34.Huang ES, Gutsch D, Tzung KW, Lin CT. Detection of low level of human papilloma virus type 16 DNA sequences in cancer cell lines derived from two well-differentiated nasopharyngeal cancers. J Med Virol. 1993;40:244–250. doi: 10.1002/jmv.1890400314. [DOI] [PubMed] [Google Scholar]
- 35.Sun LM, Li CI, Huang EY, Vaughan TL. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiol. 2007;165:271–278. doi: 10.1093/aje/kwk008. [DOI] [PubMed] [Google Scholar]
- 36.Marcus KJ, Tishler RB. Head and neck carcinomas across the age spectrum: epidemiology, therapy, and late effects. Semin Radiat Oncol. 2010;20:52–57. doi: 10.1016/j.semradonc.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Hoppe RT, Williams J, Warnke R, Goffinet DR, Bagshaw MA. Carcinoma of the nasopharynx–the significance of histology. Int J Radiat Oncol Biol Phys. 1978;4:199–205. doi: 10.1016/0360-3016(78)90138-4. [DOI] [PubMed] [Google Scholar]