Abstract
Heat-killed Saccharomyces cerevisiae (HKY) used as a vaccine protects mice against systemic aspergillosis and coccidioidomycosis. Little is known about the immune response induced by HKY vaccination, consequently our goal was to do an analysis of HKY-induced immune responses involved in protection. BALB/c mice were vaccinated subcutaneously 3 times with HKY, a protective reagent, and bronchoalveolar lavage fluid, spleen, lymph nodes, and serum collected 2–5 weeks later. Cultured spleen or lymph node cells were stimulated with HKY. Proliferation of HKY-stimulated spleen or lymph node cells was tested by Alamar Blue reduction and flow cytometry. Cytokines from lymphocyte supernatants and antibody to glycans in serum collected from HKY-vaccinated mice were measured by ELISA. The results show that HKY promoted spleen cell and lymph node cell proliferation from HKY-vaccinated mice but not from PBS-vaccinated control mice (all P < 0.05). Cytokine measurement showed HKY significantly promoted IFNγ, IL-6 and IL-17A production by spleen cells and lymph node cells (all P < 0.05 and P < 0.01, respectively). Cytokine production by HKY-stimulated cells from PBS-vaccinated mice was lower than those from HKY-vaccinated (P < 0.05). Cytokines in BAL from HKY-vaccinated were higher, 1.7-fold for IFNγ and 2.1-fold for TNFγ, than in BAL from PBS-vaccinated. Flow cytometry of lymphocytes from HKY-vaccinated showed 52% of CD3+ or 56% of CD8+ cells exhibited cell division after stimulation with HKY, compared to non-stimulated controls (26 or 23%, respectively) or HKY-stimulated cells from PBS-vaccinated (31 or 34%). HKY also induced antibody against Saccharomyces glucan and mannan with titers 4- or 2-fold, respectively, above that in unvaccinated. Taken together, the results suggested that HKY vaccination induces significant and specific Th1 type cellular immune responses and antibodies to glucan and mannan.
Keywords: Saccharomyces cerevisiae, Vaccines, Fungal infection
1. Introduction
Numerous efforts have been made in developing antifungal vaccines to meet the increasing medical needs, yet no vaccine is commercially available. Polysaccharide-containing conjugates may be desirable candidates for use in vaccine development [1]. A polysaccharide–protein conjugate that uses the algal glucan, laminarin, was shown to elicit antibodies to β-glucan in fungal cell walls and to induce protection in an infection model of aspergillo-sis and candidiasis [2]. In addition, protection could be passively transferred by immune sera or anti-β-glucan antibodies. Using recombinant S. cerevisiae as a vaccine vehicle to express and deliver carcinoembryonic antigens has been shown to have the advantages of less toxicity, good stability, ease of administration along with having inherent adjuvant properties to activate murine den-dritic cells [3,4]. Vaccination resulted in increased gene expression of interleukin (IL)-12, interferon-gamma (IFNγ) and GM-CSF indicating stimulation of cellular immunity [3,4]. We have reported the use of a vaccine preparation using hemolysin expressed in a Saccharomyces cerevisiae vector and observed that the empty vector also conferred partial protection in an infection model of coccidioidomycosis [5]. These observations laid the foundation for us to test heat-killed yeast (HKY) as a vaccine candidate against aspergillosis and coccidioidomycosis. We have shown that HKY is effective in protecting CD-1 and BALB/c mice against systemic aspergillosis [6–10], coccidioidomycosis [5], candidiasis [11], and cryptococcosis (T. Majumder, K.V. Clemons, V. Chen, M. Martinez, D. Alvarado, and D.A. Stevens, unpublished data), yet the overall humoral and cellular immune responses after HKY vaccination have not been reported. The aim of this study was to analyze the underlying immune responses associated with HKY-induced protection of the host.
2. Materials and methods
2.1. Animals
Five-week-old male BALB/c mice (Charles River Laboratories, Wilmington, MA) were used in these experiments. Three mice were established per experimental group.
Vaccination
The HKY vaccine was prepared as described previously [5]. HKY (6 × 107 cells) were administered subcutaneously at two dorsal sites (split dose of 0.75 μl each site) once a week for 3 weeks. All animal experiments were done with the approval of the Institutional Animal Care and Use Committee of the California Institute for Medical Research.
2.2. Bronchoalveolar lavage fluid (BALF) collection
Two weeks after the last vaccination, mice were anesthetized with ether and bled from the brachial artery. Lungs were lavaged with 1–2 ml of PBS injected via the trachea as described previously [12]. The lavage fluid (BALF) was centrifuged, and the supernatant stored at −80 °C until analyzed for cytokines. The amount of cytokine present in BALF was expressed as pg/μg total lavage protein.
2.3. Isolation of lymphocytes from tissues
Two or five weeks after the last vaccination, mice were anesthetized and bled from the brachial artery before collection of tissue. Spleens were removed and dispersed through a fine-mesh stainless steel screen in complete tissue culture medium (CTCM; RPMI-1640, 10% fetal bovine serum, penicillin-streptomycin [100 U and 100 μg/ml, respectively]) to obtain single cell suspensions [13]. The cells were pelleted, washed with CTCM, and counted. A total of 105 cells/200 μl was added to each well of a 96-well microplate, and incubated at 37°C in 5% CO2 + 95% air overnight.
The lymph nodes were harvested from brachial, axillary, inguinal, popiliteal sites. An average of 8 nodes was dispersed through a fine-mesh stainless steel screen in CTCM [13]. Cells were treated and cultured following the same protocol used for spleen cells.
2.4. Stimulation and cytokine detection in culture supernatant
Spleen cells and lymph node cells from PBS-vaccinated and HKY-vaccinated mice were stimulated with different doses of HKY. Four μg/ml concanavalin A (ConA, Sigma–Aldrich Inc., St. Louis, MO) was used as the mitogen positive control. The optimal dose of ConA and the optimal harvest times to study lymphocyte proliferation and cytokine production were derived from preliminary experiments (not shown). For cytokine assay in spleen and lymph node, cytokine responses were assayed from mouse lymphocytes isolated at two different time points, 2 or 5 weeks after the last dose of HKY immunization. Spleen or lymph node cells were stimulated and cultured for 24 h or 5 d, respectively, at 37 °C in 5% CO2 + 95% air; the supernatant of the cell culture was used for cytokine analysis. Because the maximum response for each cytokine differed at the two time points in some assays, for ease of presentation, data from the maximum response time has been selected for presentation.
2.5. Enzyme-linked immunosorbent assay (ELISA)
Supernatant fractions of cell culture were centrifuged at 1000 × g for 5 min, divided into microtubes and stored at −80 °C until use. To simultaneously test the cytokine expression profile, we performed a fast multiplex detection of cytokines in the cultured supernatant using a multi-protein profiling ELISA kit (Superarray Biosciences, Frederick, MD). The expression level of each cytokine was measured using specific ELISA kits for INFγ, IL-6 (BD Biosciences, Franklin Lakes, NJ) and IL-17A (Superarray Biosciences, Frederick, MD). The microplates were read at 450 nm and 550 nm, the optical imperfections in the microplate were corrected by subtracting 550 nm values from 450 nm values of absorbance.
2.6. Cell proliferation assay
Lymphocyte proliferation was tested after 3 d (spleen cells) or 7 d (lymph node cells) of incubation with the ConA or HKY stimulus. Twenty μl Alamar Blue was used as an indicator in each well. After 8 h of incubation, the microplate was read at 570 and 600 nm in plate reader. Proliferation was defined by:
where R0 = A570 (absorbance of oxidized form at 570 nm)/A600 (absorbance of oxidized form at 600 nm).
2.7. Determination of mononuclear cell proliferation by flow cytometry
Spleen cells from PBS-vaccinated and HKY-vaccinated mice were isolated 4 weeks after the last vaccine dose. The mononu-clear cells were isolated using Histopaque density centrifugation (Sigma–Aldrich, Inc., St. Louis, MO). Cells were washed by centrifugation and suspended in PBS with 0.1% BSA (w/v) to 106 cells/ml. Carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR) was added to a final concentration of 0.5 μM and incubated at 37 °C for 10 min. Cells were washed by centrifugation three times with ice-cold CTCM, counted and cultured at 10 6 cells/well at 37 °C in 5% CO2 + 95% air. Cell cultures were stimulated with 4 × 103, and 4 × 104 and 4 × 105 HKY cells for 64 h. Cells were collected and washed three times by centrifugation at 200 × g with PBS. Cells were stained with Live/Dead Fixable Aqua Dead Cell Stain (Invitrogen) for 30 min for gating. Cells were collected by centrifugation and washed three times with PBS/0.1% BSA. To stain cell surface antigens, cells were incubated for 30 min with 1 μM Pacific Blue anti-mouse CD3 (eBioscience), PE-Cy5 (phycoerythrin-cyanine dye 5) labeled anti-mouse CD4 (eBioscience), and PE-Texas Red labeled rat anti-mouse CD8α (Invitrogen). After washing three times at 250 × g with PBS–0.1% BSA, cells were analyzed by flow cytometry (LSRII flow cytometer, BD Bioscience).
2.8. Antibody titration
Serum was isolated from blood collected 2 weeks after the last vaccination from PBS or HKY-vaccinated mice. Antibody titers in the serum for glucan and mannan were determined by the optimal ELISA method described previously [14], and expressed as the reciprocal of the highest positive dilution. Final titers are expressed as the mean ± SD of triplicate determinations.
2.9. Statistical analysis
Proliferation and cytokine expression were measured from three experiments; data are presented as a mean plus or minus standard error of triplicate samples. Statistical analysis was done using Student’s t-test.
3. Results
3.1. HKY stimulates proliferation of spleen and lymph node cells
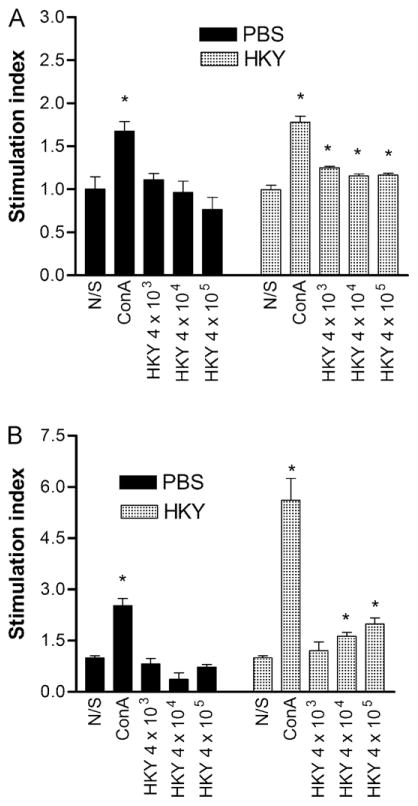
We sought to evaluate whether HKY induced lymphocyte proliferation in a specific or nonspecific manner. Mice were vaccinated with HKY or PBS and cells harvested 5 weeks after the last dose. Cell proliferation, as measured by Alamar Blue reduction, was assessed for spleen and lymph node cells in response to HKY stimulus. Only the mitogen, ConA, promoted significant proliferation of spleen cells from PBS-vaccinated mice (P = 0.02) compared to its non-stimulated control (Fig. 1A); HKY did not induce significant proliferation. For spleen cells from HKY-vaccinated mice, all doses of HKY promoted significant proliferation compared to the non-stimulated control (P = 0.002, P = 0.01, P = 0.02, respectively); stimulation with 4 × 104 cells of HKY trended toward significant induction of proliferation. Proliferation of the cells from HKY-vaccinated mice was significantly greater than that induced in cells from the PBS-vaccinated mice stimulated with 4 × 105 HKY (P = 0.049).
Fig. 1.
Proliferation of spleen and lymph node cells from PBS-vaccinated and HKY-vaccinated mice stimulated with HKY or ConA (4 μg/ml). (A) Spleen cells were stimulated for 3 d with ConA or HKY doses as indicated. (B) Lymph node cells were stimulated for 7 d with ConA or HKY doses as indicated. Alamar Blue was added to each well for additional 8 h to detect proliferation on the basis of reduction of the Alamar Blue. Each bar represents the mean ± SE. An * indicates P < 0.05 in comparison with the non-stimulated (N/S) control cells. Abbreviations: N/S, non-stimulated; ConA, concanavalin A; HKY, heat-killed yeasts.
Lymph node cells from PBS-vaccinated mice responded similarly to spleen cells in that only ConA promoted significant proliferation (P = 0.01) (Fig. 1B). Interestingly, these lymph node cells exposed to HKY stimulus showed a non-significant reduction in proliferative response. In contrast, lymph node cells from HKY-vaccinated mice showed a dose-responsive proliferative response, compared to the non-stimulated control, with 4 × 104 and 4 × 105 cells of HKY promoting significant proliferation (P = 0.01, P < 0.01) (Fig. 1B). Proliferation of the lymph node cells from HKY-vaccinated mice stimulated with doses of 4 × 104 or 4 × 105 HKY was significantly higher than that induced by the same doses of HKY for cells from the PBS-vaccinated mice (P = 0.049).
3.2. HKY stimulates IFNγ, IL-6 and IL-17A production in spleen cells
To obtain a global picture of cytokine expression profiles, a multi-cytokine array with the supernatant from cultured spleen cells or lymph node cells was performed. Array results showed that expression of IFNγ, IL-6 and IL-17A was up-regulated after stimulation of the cells with HKY (data not shown). Therefore, each of these cytokines was further quantified using single cytokine ELISA.
IFNγ
IFNγ production by spleen cells was tested 5 weeks after the last vaccination (Fig. 2A). In spleen cells from PBS-vaccinated mice, compared to the unstimulated control, all doses of HKY and ConA (4 μg/ml) significantly promoted IFNγ expression. The highest level of IFNγ was found in HKY (4 × 104 HKY per well) stimulated cell culture supernatant (640 pg/ml). In spleen cells from HKY-vaccinated mice, compared to the unstimulated control, all doses of HKY significantly stimulated IFNγ production (all P < 0.05) and the highest level was produced by cells stimulated with 4 × 105 HKY (11,403 pg/ml). IFNγ induction by all doses of HKY used to stimulate the cells from HKY-vaccinated mice was significantly higher than was induced in cells from PBS-vaccinated mice (all P < 0.01).
Fig. 2.
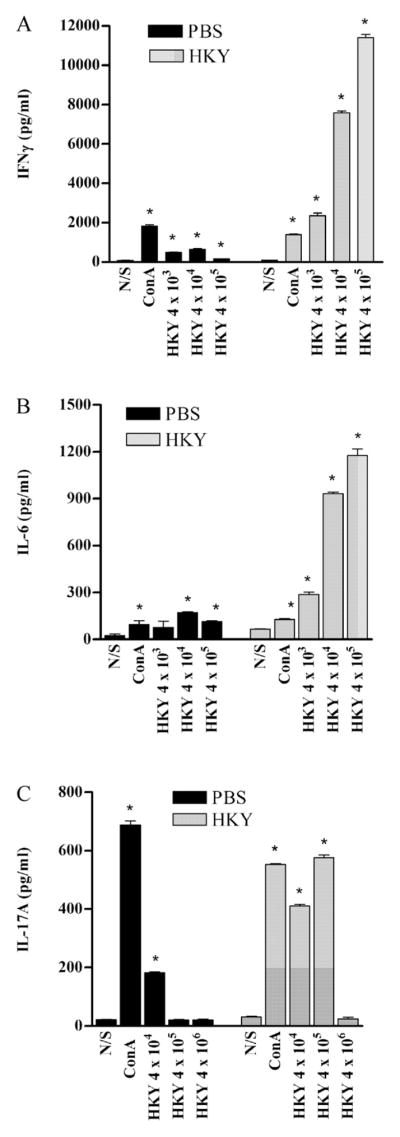
Cytokines detected in supernatants of spleen cell culture from PBS- and HKY-vaccinated mice, 5 weeks after last dose of vaccination. Cells were stimulated with different doses of HKY or ConA (4 μg/ml) for 3 d and culture supernatant was collected for ELISA. (A) IFNγ production. (B) IL-6 production. (C) IL-17A production. Each bar represents the mean ± SE. An * indicates P < 0.05 in comparison with the non-stimulated (N/S) control cells. Abbreviations: N/S, non-stimulated; ConA, concanavalin A; HKY, heat-killed yeasts.
IL-6
IL-6 production by spleen cells was assayed 5 weeks after the last dose of HKY (Fig. 2B). Compared to the non-stimulated control cells, HKY stimulation (4 × 104 or 4 × 105 HKY per well) (both P < 0.01) of spleen cells from PBS-vaccinated mice and ConA (4 μg/ml) significantly promoted IL-6 expression (P = 0.048). The highest level of IL-6 was produced by HKY (4 × 104 HKY) stimulated cells (171 pg/ml). After 24 h of stimulation of spleen cells from HKY-vaccinated mice, compared to the unstimulated control, all doses of HKY and ConA (4 μg/ml) significantly stimulated IL-6 production. The highest level of IL-6 was produced by cells stimulated with HKY (4 × 105 HKY per well) (1177 pg/ml). IL-6 induction by different doses of HKY (4 × 103, 4 × 104, or 4 × 105 HKY) by cells from HKY-vaccinated mice was significant compared to stimulation of cells from PBS-vaccinated mice (all P < 0.01).
IL-17A
IL-17A production by spleen cells was tested 5 weeks after the last dose of HKY vaccination (Fig. 2C). For spleen cells from PBS-vaccinated control mice, compared to the unstimulated control, the 4 × 104 dose of HKY or ConA (4 μg/ml) significantly promoted IL-17A production (all P < 0.05) and for 4 × 104 HKY stimulated cell culture supernatant was 182 pg/ml. In spleen cells from HKY-vaccinated mice, 4 × 104 or 4 × 105 doses of HKY or 4 μg/ml ConA significantly stimulated IL-17A production, while 4 × 106 dose of HKY did not significantly induce IL-17 A production (P > 0.05); 4 × 105 HKY stimulated cells to produce the most IL-17A (576 pg/ml). IL-17A induction by different doses of HKY (4 × 104 or 4 × 105) in the cells from HKY-vaccinated mice was significant compared to those induced by equivalent doses of HKY by cells from PBS-vaccinated mice (all P < 0.01).
3.3. HKY stimulates cytokine production in lymph node cells
IFNγ
IFNγ expression by stimulated lymph nodes cells was assayed 5 weeks after the last HKY vaccination (Fig. 3A). For lymph node cells from PBS-vaccinated mice, compared to the unstim-ulated control, 4 × 105 of HKY and ConA (4 μg/ml) significantly promoted IFNγ expression (both P < 0.01). The highest level of IFNγ was produced by cells stimulated with 4 × 105 HKY per well (296 pg/ml). For lymph node cells from HKY-vaccinated mice, 4 × 104 and 4 × 105 HKY per well significantly stimulated IFNγ production (P = 0.002, P = 0.005, respectively). The highest level of IFNγ was produced by cells stimulated with 4 × 104 HKY (540 pg/ml). IFNγ induction in the cells from HKY-vaccinated mice by different doses of HKY (4 × 103, 4 × 104, and 4 × 105 HKY per well) were significantly higher than those induced by the equivalent doses of HKY for cells from PBS-vaccinated mice (all P < 0.01).
Fig. 3.
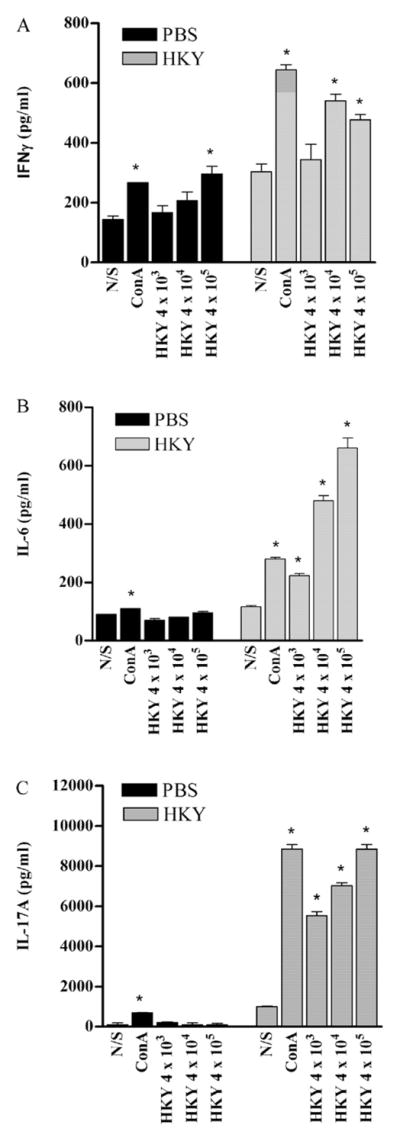
Cytokines detected in supernatants of lymph node cell culture from PBS-vaccinated mice and HKY-vaccinated mice, 2 weeks (IL-6, IL-17A) or 5 weeks (IFNγ) after the last vaccination. Cells were stimulated with different doses of HKY or ConA (4 μg/ml) for 3 d. Culture supernatant was collected for ELISA. (A) IFNγ production. (B) IL-6 production. (C) IL-17A production. Each bar represents the mean ± SE. An * indicates P < 0.05 in comparison with the non-stimulated (N/S) control cells. Abbreviations: N/S, non-stimulated; ConA, concanavalin A; HKY, heat-killed yeasts.
IL-6
IL-6 expression in lymph node cells was assayed 2 weeks after the last HKY vaccination (Fig. 3B). For lymph node cells from PBS-vaccinated mice, only ConA (4 μg/ml) significantly promoted IL-6 production. However, for lymph node cells from HKY-vaccinated mice, compared to the unstimulated control, all doses of HKY or ConA (4 μg/ml) significantly stimulated IL-6 production (all P < 0.05). The highest level of IL-6 was produced by cells stimulated with 4 × 105 HKY (660 pg/ml). IL-6 production, after different doses of HKY (4 × 103, 4 × 104, and 4 × 105 HKY per well), by cells from HKY vaccinated mice was significantly higher than those from cells from PBS-vaccinated mice stimulated with equivalent doses of HKY (all P < 0.01).
IL-17A
IL-17A production by lymph node cell was assayed 2 weeks after the last dose of HKY (Fig. 3C). No HKY dose significantly promoted IL-17A production by lymph node cells from PBS-vaccinated mice, compared to the unstimulated control, whereas ConA (4 μg/ml) significantly induced IL-17A (P < 0.01). In contrast, all doses of HKY or ConA significantly stimulated IL-17A production by lymph nodes cells from HKY-vaccinated mice (all P < 0.01). Cells stimulated with 4 × 105 HKY per well produced 8848 pg/ml of IL-17A. IL-17A from cells from HKY vaccinated mice induced by different doses of HKY (4 × 103, 4 × 104, and 4 × 105 HKY) was significantly higher than those induced by the equivalent doses of HKY for cells from PBS-vaccinated mice (all P < 0.01).
3.4. HKY stimulates IFNγ and TNFα in bronchoalveolar lavage fluid (BALF)
Expression of cytokines in BALF was tested in comparison to the PBS-vaccinated control group (Fig. 4). Significant levels of IFNγ and TNF-α were detected in BALF from HKY-vaccinated mice in comparison with PBS-vaccinated mice (P < 0.01). BALF from HKY-vaccinated mice contained 1.594 pg IFNγ/μg total lavage proteins, a 1.73-fold increase compared to BALF from PBS-vaccinated. Similarly, BALF from HKY-vaccinated mice contained 0.992 pg TNFγ/μg total lavage proteins, which was 2.11-fold higher than in BALF from PBS vaccinated mice (P = 0.007).
Fig. 4.
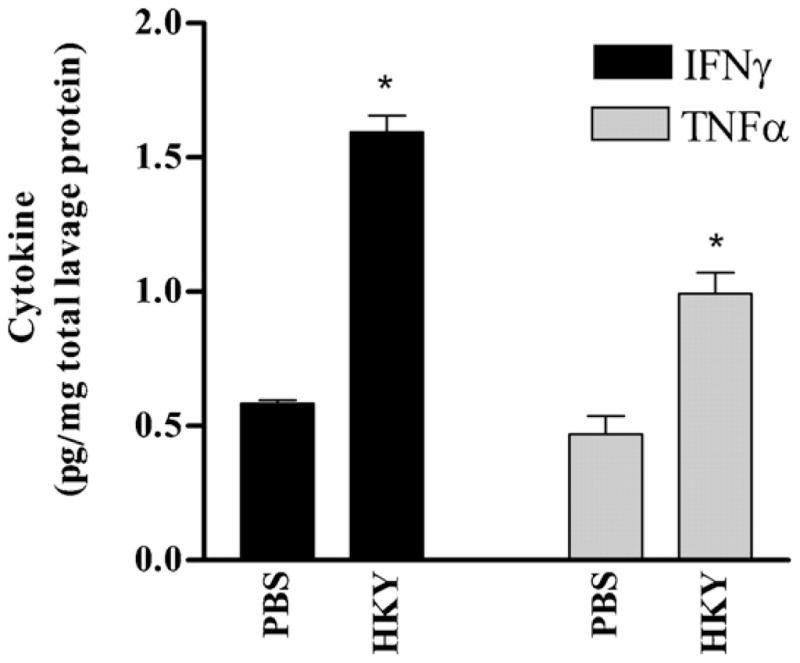
IFNγ and TNF-α detected in bronchoalveolar lavage fluid (BALF) from BALB/c mice 2 weeks after the last PBS or HKY vaccination. An * indicates P < 0.05 in comparison with PBS-vaccination.
3.5. Flow cytometry analysis of cell proliferation
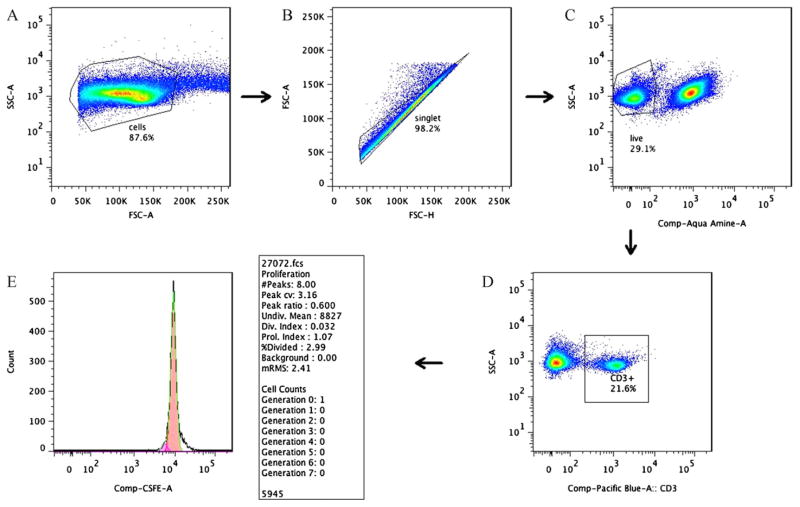
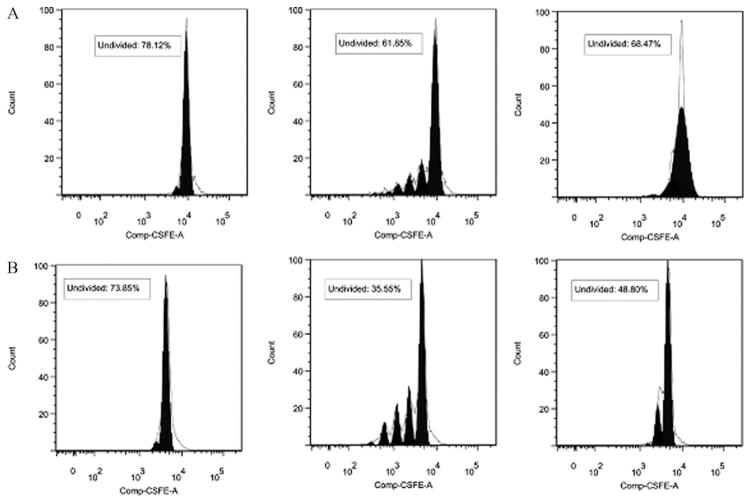
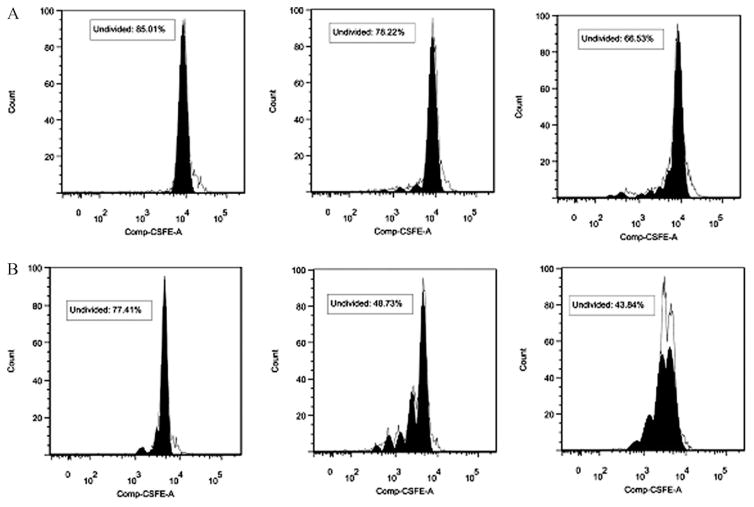
To determine the subpopulation of spleen lymphocytes proliferating in response to HKY, we used a CFSE-based method to stain individual cells that had undergone cell divisions coupled with different cell surface markers. An example of the gating sequence used for Flow cytometry analysis of cell proliferation by cultured lymphocytes is shown in Fig. 5. HKY-vaccinated mice showed that approximately 52% of CD3+ cells exhibited cell division after stimulation with 4 × 105 HKY. In comparison, 26% of the non-stimulated control cells or 31% of HKY-stimulated cells from PBS-vaccinated mice proliferated (Fig. 6). Similarly, 56% of CD8+ cells exhibited cell division after stimulation with 4 × 105 HKY, in comparison to 23% for non-stimulated control cells or 33.5% of HKY-stimulated cells from PBS-vaccinated mice (Fig. 7). We did not observe cell division of CD4+ cells after HKY stimulation.
Fig. 5.
Flow chart illustrated the gating sequence used for cell proliferation analysis by multi-color flow cytometry. (A) Gated total population of all stained mononuclear cells, (B) singlet of gated mononuclear cells, (C) gating of aqua-stained mononuclear cells for live cells (i.e., nonstained), (D) gating of live cells in panel C for CD3+ mononuclear cells, and (E) example of a proliferation histogram of CD3+ control cells showing no cell division (dashed line indicates the model generated by FlowJo 7.2.4 software).
Fig. 6.
Histograms showing proliferation of CD3+ T cells in spleen cells isolated from non-vaccinated (A) and HKY vaccinated (B) mice after in vivo stimulation: non-stimulated control cells (left), ConA (middle) and 4 × 105 cells of HKY (right) for 64 h. CFSE profile from gate illustrates fitted model sum (proliferation model according to FlowJo 7.2.4 software, shown by the clear peaks under the black lines) to determine gates for division number (shown by the filled solid peaks in black). The right black peak in each graph represents generation 0 (undivided) lymphocytes. The number of peaks located on the left side of each generation 0 lymphocytes indicates the division numbers undergone by the lymphocytes.
Fig. 7.
Histograms showing proliferation of CD8+ spleen cells isolated from non-vaccinated (A) and HKY-vaccinated (B) mice after in vivo stimulation with: non-stimulated cells (left), ConA (middle) and 4 × 105 cells of HKY (right) for 64 h. CFSE profile from gate illustrates fitted model sum (proliferation model according to FlowJo 7.2.4 software shown by the clear peaks under the black lines) to determine gates for division number (shown by the filled solid peaks in black). The right black peak in each graph represents generation 0 (undivided) lymphocytes. The number of peaks located on the left side of each generation 0 lymphocytes indicates the division numbers undergone by the lymphocytes.
3.6. Antibody response induced by HKY
To assess whether HKY induced protection might result from an antibody response, we measured specific anti-mouse IgG titers against glucan and mannan by ELISA assay using serum collected from HKY-vaccinated mice. Antibody assay against glucan and mannan from Saccharomyces showed titers 1600 ± 38 and 400 ± 36, respectively, which was 4 or 2 times above that in PBS-vaccinated. Statistical analysis indicated a significant difference (P < 0.01) between the mean antibody titers of HKY-vaccinated and PBS-vaccinated mice for both tested antigens.
4. Discussion
Our goal in this study was that of examining the immune response induced by vaccination with HKY that might in part explain its utility as a vaccine against experimental coccidioidomycosis and aspergillosis. To determine ability of HKY in triggering cell-mediated immune responses, we performed cellular proliferation assays that showed HKY were effective in stimulating cell proliferation in cells from HKY-vaccinated mice, but not from sham-vaccinated mice. These results indicated that HKY vaccination induced specific lymphocyte responses to HKY antigens and an expansion of a population of primed lymphocytes indicative of an adaptive immune response. Presumably these responses are directed against protein and/or glucan antigens shared among Saccharomyces, Aspergillus, and Coccidioides.
Our results from multiple-color flow cytometry staining of lymphocyte populations indicated that HKY-stimulation of cells from HKY-vaccinated mice resulted in proliferation of CD3+ and CD8+ T cells, but not CD4+ cells. There are reports showing that proinflammatory cytokines, such as IFN-γ, are also produced by different immune cell populations, including CD8+ and CD3+ cells in response to various fungal infections or antigens [15–18]. In addition, there is evidence from other fungi showing that CD4+ T cells are dispensable for vaccine-induced resistance against experimental pulmonary infections [16,19,20]; instead, antigens are capable of activating CD8 memory cells to produce TNFα and IFNγ for protection. These results further support our conclusion that the immune response to HKY-vaccination results in adaptive rather than innate immune response. CD8+ T cells recognize pathogen-derived antigens complexed with major histocompatibility complex (MHC) class I molecules on the surface of antigen presenting cells (APCs). Usually dendritic cells or macrophages are APCs and secrete cytokines such as TNF and IFNγ to contribute toward antimicrobial defense [21]. Priming of antigen-specific CD8+ T cells results in proliferation and contraction into stable memory populations [21]. HKY vaccination-induced protective efficacy may depend on CD8+ and CD3+ cell function to produce defensive cytokines.
IFNγ, IL-6 and IL-17A were elevated in the supernatant of lymphocyte cell cultures from HKY-vaccinated mice in response to HKY. The low level of cytokine production occasionally observed in response to HKY in PBS-vaccinated mice could represent a low level of cross-reactivity to environmental antigens. Thus, HKY-vaccination primed lymphocytes for Th-1 type of cytokine production. Heat-killing of HKY causes denaturation of proteins, which tends to shift the host response to a Th1 type [22].
IFNγ is a cytokine necessary for activation of phagocytes to kill several different fungal pathogens, potentiating the oxidative responses to cause hyphal damage, and enhances monocyte function against a variety of fungi [23] including Aspergillus [24] and Coccidioides [25]. Similarly, IL-6 is a pro-inflammatory cytokine and a potent activator of T-lymphocytes and neutrophils [26]. IL-6 was shown to potentiate host defense against coccidioidomycosis [27], and IL-6-deficient mice are more susceptible to invasive pulmonary aspergillosis [28]. IL-6 in conjunction with TGF-γ induces the differentiation of naïve T lymphocytes into IL-17-producing Th17 cells [29]. IL-17 recently has been identified as a T cell-derived cytokine that is involved in host defense response against bacteria and fungal infection [30,31]. IL-17 has been shown particularly important in resistance to Aspergillus infection in an animal model [28] and is also considered critical to a protective response against candidiasis [32,33]. In human cells, IL-17 expression was induced above baseline by stimulation with whole S. cerevisiae cells proposed to be a result of the interaction between mannan and mannan receptor (MR) triggering IL-17 production [34]. In addition, HKY-stimulation of lymphocytes might also result in elevated levels of IL-17 from exposed β-glucan stimulation of macrophages or mononuclear cells (i.e., innate immune cells), which further prime Th17 cells [35].
Macrophages are the first defensive line against fungal infection, particularly pulmonary infection, and the antimicrobial function of bronchoalveolar macrophages is regulated by proinflammatory cytokines. TNFα and IFNγ were measurable in the BALF collected from non-challenged lungs of mice 2 weeks after the last HKY vaccination and significantly higher in concentration in BALF from HKY-vaccinated mice compared to BALF from PBS or unvaccinated mice. Both IFNγ and TNFα play key roles in pulmonary macrophage activation and destroying different infectious bacterial [36] and fungal agents, including Blastomyces, Paracoccidioides, Histoplasma, and Aspergillus [23,36,37]. The expression of IFNγ and TNFα in BALF suggests that HKY-vaccination induces a specific cytokine response that would be essential for the host defense against fungal infection. Future studies using models of pulmonary aspergillosis or coccid-ioidomycosis infection will be essential in determining whether immune responses to HKY vaccination expressed through the pulmonary immune system, such as the IFNγ and TNFα in the BALF that we demonstrated exemplifies, will impart resistance to infection. In line with the results of our study, the capability of S. cerevisiae to induce proinflammatory cytokine mRNA expression and cytokine secretion also has been shown in other studies [34,38].
It remains to be determined which particular antigens evoke protective recall responses in HKY-vaccinated mice. Fungal cell wall components, glucan and mannan, are predominant antigens common to many fungi. These components have immunostimulatory properties, often associated with the innate immune response, as a result of recognition of the pathogen-associated molecular patterns by cellular receptors such as mannose receptor and dectin-1, which in turn triggers phagocytosis, respiratory activity and induction of cytokine gene expression [39]. However, glycans can also act as antigens and are usually classified as T-independent antigens evoking only a humoral response [40].
Glucans and mannans can induce antibodies that confer protection to mice against aspergillosis and candidiasis [2,41–48]. Our antibody assay against Saccharomyces glucan and mannan showed that HKY-vaccination induced significant IgG titers, which were 4 or 2 times higher, respectively, than those in PBS-vaccinated mice. B-cells, unlike T-cells, are stimulated directly by antigen and do not depend on antigen presentation by APCs. B-cells bind antigen by surface IgM resulting in signaling pathways leading to antibody producing plasma cells.
We have shown here that HKY-vaccination induces a specific adaptive and comprehensive immune response in mice. These results are supportive of those of our in vivo studies, where the protective efficacy of HKY vaccination against coccidioidomycosis, candidiasis and aspergillosis has been repeatedly observed [5–11]. However, the components of HKY responsible for the cross-protective response against these infections are not known. Several homologous proteins have been described among Saccharomyces, Aspergillus and Coccidioides. These include the chitinases, cts1, in Saccharomyces, cts2 in Coccidioides, and chitinases from Aspergillus [49]; and the alkyl hydroperoxide peroxidase, Ahp1, in Saccharomyces, with peroxisomal matrix proteins, Pmp1, in Coccidioides, and Aspf3 in Aspergillus [50,51]. Further study on homologous proteins and their association with cell wall glycans will contribute to understanding the mechanism of HKY-induced adaptive protection.
Acknowledgments
We thank Priya Asok for assistance during part of these studies. I. M. was supported by a grant from the Slovak Research and Development Agency, APVV-LPP-0194-09.
References
- 1.Robbins JB, Schneerson R. Polysaccharide–protein conjugates: a new generation of vaccines. J Infect Dis. 1990;161(5):821–32. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 2.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005;202(5):597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostbock S, et al. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26(4):509–21. doi: 10.1016/j.vaccine.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Remondo C, Cereda V, Mostbock S, Sabzevari H, Franzusoff A, Schlom J, et al. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27(7):987–94. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capilla J, Clemons KV, Liu M, Levine HB, Stevens DA. Saccharomyces cerevisiae as a vaccine against coccidioidomycosis. Vaccine. 2009;27(27):3662–8. doi: 10.1016/j.vaccine.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Capilla J, Clemons KV, Stevens DA. The friend of man a friend again? Saccharomyces as a vaccine against invasive aspergillosis. 47th intersci. conf. antimicrob. agents chemother; 2007. [Abstract # G-1708] [Google Scholar]
- 7.Johansen M, Danielle D, Liu M, Clemons K, Stevens D. Efficacy of different strains of Saccharomyces cerevisiae as a vaccine against systemic aspergillosis. 4th advances against aspergillosis; 2010. [Abstract #22] [Google Scholar]
- 8.Liu M, Clemons K, Bigos M, Stevens D. Immune responses induced by heat killed yeast, a vaccine against aspergillosis in mice. 4th advances against aspergillosis; 2010. [Abstract #20] [Google Scholar]
- 9.Liu M, Clemons K, Stevens D. Saccharomyces as a vaccine against invasive aspergillosis is enhanced by alum but is not mouse strain or Saccharomyces species specific. 4th advances against aspergillosis; 2010. [Abstract # 21] [Google Scholar]
- 10.Stevens DA, Clemons KV, Liu M. Developing a vaccine against aspergillosis. Med Mycol. 2010 doi: 10.3109/13693786.2010.497775. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Clemons K, Johasen M, Martinez M, Chen V, Stevens D. Saccharomyces as a vaccine against systemic candidiasis. 50th interscience conference on antimicrobial agents and chemotherapy; 2010. [Abstract # G1-1656] [Google Scholar]
- 12.Sugar AM, Brummer E, Stevens DA. Murine pulmonary macrophages: evaluation of lung lavage fluids, miniaturized monolayers, and candidacidal activity. Am Rev Respir Dis. 1983;127(1):110–2. doi: 10.1164/arrd.1983.127.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Brummer E, Vris TW, Lawrence HS. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 × 10−5 M mercaptoethanol. J Immunol Methods. 1977;17(3–4):319–27. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- 14.Libjakova L, Bystricky S, Lizicarova I, Paulovicova E, Machova E. Evaluation of different mannan polysaccharide usage in enzyme-linked immunosor-bent assay for specific antibodies determination. J Pharm Biomed Anal. 2007;45(3):521–5. doi: 10.1016/j.jpba.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Ampel NM, Christian L. Flow cytometric assessment of human peripheral blood mononuclear cells in response to a coccidioidal antigen. Med Mycol. 2000;38(2):127–32. doi: 10.1080/mmy.38.2.127.132. [DOI] [PubMed] [Google Scholar]
- 16.Fierer J, Waters C, Walls L. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J Infect Dis. 2006;193(9):1323–31. doi: 10.1086/502972. [DOI] [PubMed] [Google Scholar]
- 17.Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of cytotoxic T cell responses directed to human leucocyte antigen Class I restricted epitopes from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;140(1):81–91. doi: 10.1111/j.1365-2249.2005.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spellberg B, Ibrahim AS, Lin L, Avanesian V, Fu Y, Lipke P, et al. Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis. 2008;197(7):967–71. doi: 10.1086/529204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuthrich M, Filutowicz HI, Warner T, Deepe GS, Jr, Klein BS. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J Exp Med. 2003;197(11):1405–16. doi: 10.1084/jem.20030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng M, Ramsay AJ, Robichaux MB, Norris KA, Kliment C, Crowe C, et al. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest. 2005;115(12):3536–44. doi: 10.1172/JCI26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong P, Pamer EG. CD8+ T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 22.Johansen P, Senti G, Martinez Gomez JM, Wuthrich B, Bot A, Kundig TM. Heat denaturation, a simple method to improve the immunotherapeutic potential of allergens. Eur J Immunol. 2005;35(12):3591–8. doi: 10.1002/eji.200535076. [DOI] [PubMed] [Google Scholar]
- 23.Stevens DA, Brummer E, Clemons KV. Interferon-gamma as an antifungal. J Infect Dis. 2006;194(Suppl 1):S33–7. doi: 10.1086/505357. [DOI] [PubMed] [Google Scholar]
- 24.Roilides E, Katsifa H, Walsh TJ. Pulmonary host defences against Aspergillus fumigatus. Res Immunol. 1998;149(4–5):454–65. doi: 10.1016/s0923-2494(98)80769-4. [discussion 523–4] [DOI] [PubMed] [Google Scholar]
- 25.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, et al. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol. 2004;42(3):189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 26.Brummer E, Capilla J, Bythadka L, Stevens DA. Production of IL-6, in contrast to other cytokines and chemokines, in macrophage innate immune responses: effect of serum and fungal (Blastomyces) challenge. Cytokine. 2007;39(3):163–70. doi: 10.1016/j.cyto.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Cox RA, Magee DM. Production of tumor necrosis factor alpha, interleukin-1 alpha, and interleukin-6 during murine coccidioidomycosis. Infect Immun. 1995;63(10):4178–80. doi: 10.1128/iai.63.10.4178-4180.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cenci E, Mencacci A, Casagrande A, Mosci P, Bistoni F, Romani L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J Infect Dis. 2001;184(5):610–7. doi: 10.1086/322793. [DOI] [PubMed] [Google Scholar]
- 29.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170(9):4432–6. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelante T, De Luca A, D’Angelo C, Moretti S, Romani L. IL-17/Th17 in anti-fungal immunity: what’s new? Eur J Immunol. 2009;39(3):645–8. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190(3):624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 34.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5(4):329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–9. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 36.Leenen PJ, Canono BP, Drevets DA, Voerman JS, Campbell PA. TNF-alpha and IFN-gamma stimulate a macrophage precursor cell line to kill Listeria monocytogenes in a nitric oxide-independent manner. J Immunol. 1994;153(11):5141–7. [PubMed] [Google Scholar]
- 37.Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;162(3):1633–40. [PubMed] [Google Scholar]
- 38.Saegusa S, Totsuka M, Kaminogawa S, Hosoi T. Saccharomyces cerevisiae and Candida albicans stimulate cytokine secretion from human neutrophil-like HL-60 cells differentiated with retinoic acid or dimethylsulfoxide. Biosci Biotechnol Biochem. 2009;73(12):2600–8. doi: 10.1271/bbb.90410. [DOI] [PubMed] [Google Scholar]
- 39.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog. 2010;6(4):e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulovicova E, Machova E, Tulinska J, Bystricky S. Cell and antibody mediated immunity induced by vaccination with novel Candida dubliniensis mannan immunogenic conjugate. Int Immunopharmacol. 2007;7(10):1325–33. doi: 10.1016/j.intimp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Han Y, Riesselman MH, Cutler JE. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect Immun. 2000;68(3):1649–54. doi: 10.1128/iai.68.3.1649-1654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han Y, Ulrich MA, Cutler JE. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis. 1999;179(6):1477–84. doi: 10.1086/314779. [DOI] [PubMed] [Google Scholar]
- 43.Ishibashi K, Yoshida M, Nakabayashi I, Shinohara H, Miura NN, Adachi Y, et al. Role of anti-beta-glucan antibody in host defense against fungi. FEMS Immunol Med Microbiol. 2005;44(1):99–109. doi: 10.1016/j.femsim.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Casadevall A, Pirofski LA. Antibody-mediated protection through cross-reactivity introduces a fungal heresy into immunological dogma. Infect Immun. 2007;75(11):5074–8. doi: 10.1128/IAI.01001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakhtoura M, Rahal E, Halas Y, Al-Sabbagh Z, Abdelnoor AM. Preparation of anti-Aspergillus fumigatus antibodies in egg laying hens and their protective efficacy in BALB/c mice. J Appl Res. 2008;8:168–77. [Google Scholar]
- 46.Chaturvedi AK, Kavishwar A, Shiva Keshava GB, Shukla PK. Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glyco-protein protects against experimental murine aspergillosis. Clin Diagn Lab Immunol. 2005;12(9):1063–8. doi: 10.1128/CDLI.12.9.1063-1068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulovicova E, Bystricky S, Masarova J, Machova E, Mislovicova D. Immune response to Saccharomyces cerevisiae mannan conjugate in mice. Int Immunopharmacol. 2005;5(12):1693–8. doi: 10.1016/j.intimp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Young SH, Roberts JR, Antonini JM. Pulmonary exposure to 1 → 3-beta-glucan alters adaptive immune responses in rats. Inhal Toxicol. 2006;18(11):865–74. doi: 10.1080/08958370600822425. [DOI] [PubMed] [Google Scholar]
- 49.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77(8):3196–208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orsborn KI, Shubitz LF, Peng T, Kellner EM, Orbach MJ, Haynes PA, et al. Protein expression profiling of Coccidioides posadasii by two-dimensional differential in-gel electrophoresis and evaluation of a newly recognized peroxisomal matrix protein as a recombinant vaccine candidate. Infect Immun. 2006;74(3):1865–72. doi: 10.1128/IAI.74.3.1865-1872.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito JI, Lyons JM, Hong TB, Tamae D, Liu YK, Wilczynski SP, et al. Vaccinations with recombinant variants of Aspergillus fumigatus allergen Asp f 3 protect mice against invasive aspergillosis. Infect Immun. 2006;74(9):5075–84. doi: 10.1128/IAI.00815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]