Abstract
Polyols are sugar alcohols found in certain fruits, vegetables, and sugar-free sweeteners. They make up a component of the diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, which is gaining popularity in the treatment of patients with irritable bowel syndrome (IBS). We conducted a systematic review to evaluate the effects of polyols on the gastrointestinal tract in healthy men and women and in patients with IBS. Utilizing PubMed, Ovid, and Embase databases, we conducted a search on individual polyols and each of these terms: fermentation, absorption, motility, permeability, and gastrointestinal symptoms. Standard protocols for a systematic review were followed. We found a total of 1823 eligible articles, 79 of which were included in the review. Overall, available work has shown that polyol malabsorption generally occurs in a dose-dependent fashion in healthy individuals, and malabsorption increases when polyols are ingested in combination. However, studies in patients with IBS have shown conflicting results pertaining to polyol malabsorption. Polyol ingestion can lead to intestinal dysmotility in patients with IBS. Regarding the microbiome, moderate doses of polyols have been shown to shift the microbiome toward an increase in bifidobacteria in healthy individuals and may therefore be beneficial as prebiotics. However, data are limited regarding polyols and the microbiome in patients with IBS. Polyols can induce dose-dependent symptoms of flatulence, abdominal discomfort, and laxative effects when consumed by both healthy volunteers and patients with IBS. Further research is needed to better understand the effects of specific polyols on gastrointestinal function, sensation, and the microbiome in health and gastrointestinal disorders such as IBS.
Keywords: artificial sweeteners, fermentable oligosaccharides, disaccharides, monosaccharides, polyols, microbiome, motility, sugar alcohols
Introduction
Irritable bowel syndrome (IBS) is a gastrointestinal condition characterized by recurring episodes of abdominal pain, bloating, and changes in stool form or frequency. This syndrome represents one of the most commonly diagnosed gastrointestinal disorders in the United States, with the prevalence reaching ∼12% (1). The pathogenesis of IBS is likely multifactorial, secondary to abnormalities in motility, visceral sensation, brain-gut interactions, intestinal permeability, immune activation, neuroendocrine function, bile acids, and the gut microbiome (Figure 1) (2–4). Many patients report exacerbations of their symptoms after the ingestion of particular foods, resulting in an increasing interest in the role that diet plays in IBS. Specifically, diets low in short-chain, poorly absorbed carbohydrates known as fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) have been shown to help relieve gastrointestinal symptoms in patients with IBS (5, 6). FODMAPs likely promote intestinal discomfort through osmotic effects, as well as through increased gas production after rapid fermentation by bacteria in the distal small and large bowel (Figures 1 and 2) (7). Although FODMAPs are often categorized together in the discussion of carbohydrates, it is important to note that they are a diverse group of sugars that exert different effects in different parts of the gastrointestinal tract (8).
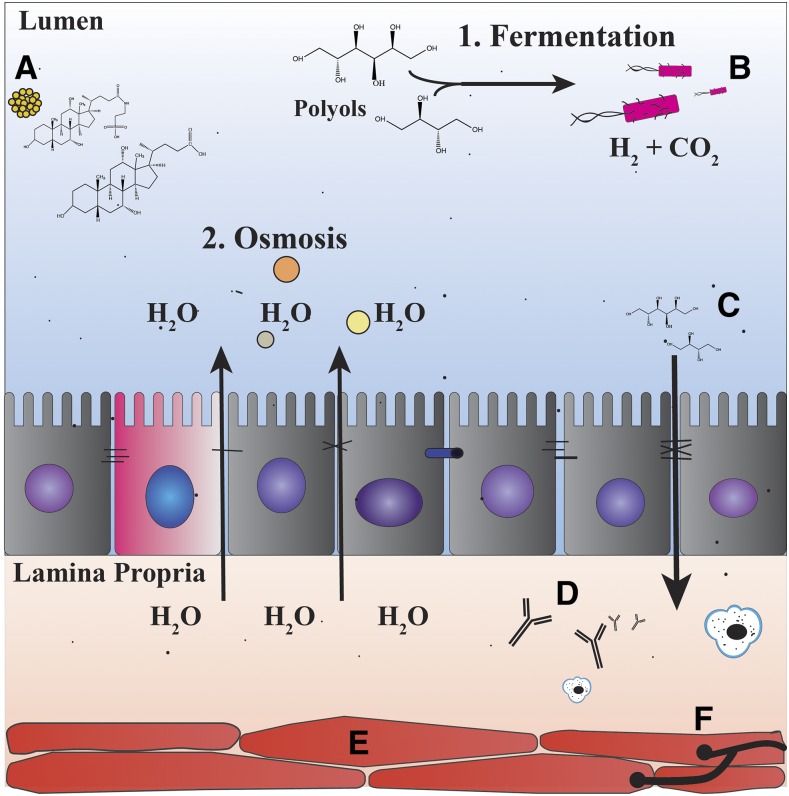
FIGURE 1.
Factors involved in the pathophysiology and symptomatology of irritable bowel syndrome. Pathophysiology: (A) changes in bile acid concentrations, (B) gut microbiome, (C) changes in intestinal permeability, (D) immune activation, (E) dysmotility, and (F) visceral hypersensitivity. Symptomatology: (1) increased gas production secondary to rapid fermentation of carbohydrates by bacteria in the intestine and (2) increased water retention in the gut secondary to the effects of osmosis.
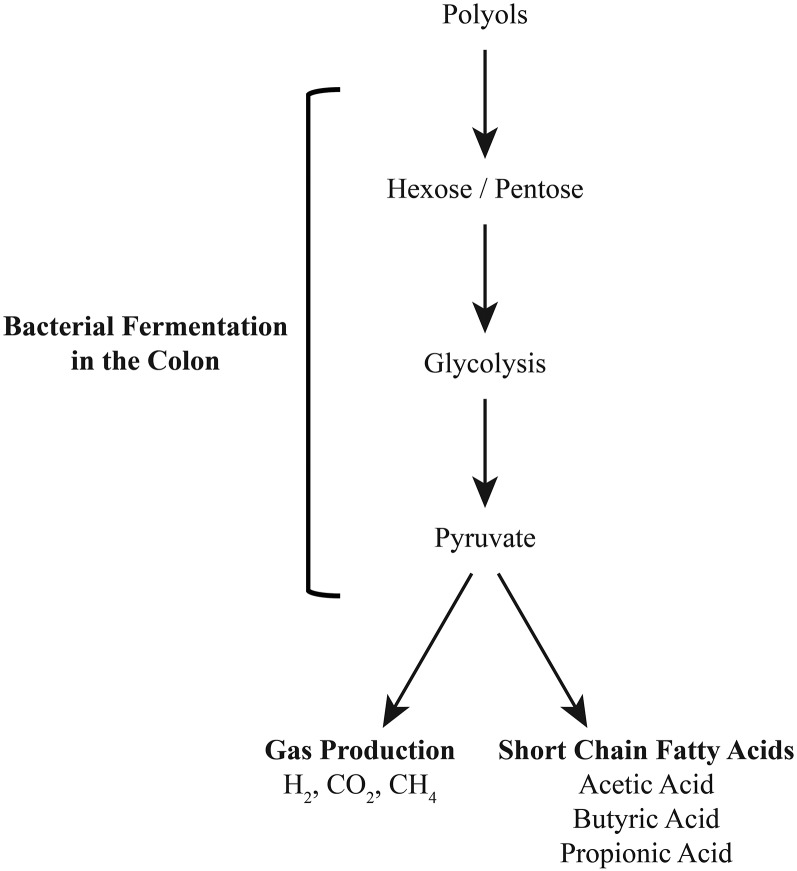
FIGURE 2.
Polyol metabolism and fermentation in the colon.
Polyols are a specific group of sugar alcohols that are formed via the catalytic hydrogenation of carbohydrates. They are found naturally in certain fruits, vegetables, and mushrooms; however, they are also commonly used as sugar-free sweeteners in products such as chewing gum, candies, and beverages. Polyols represent attractive alternatives to sucrose, because they provide fewer calories per gram, do not promote tooth decay, and are not associated with an elevated blood glucose response (9, 10). The US FDA has currently approved the use of 8 different polyols, which include erythritol, hydrogenated starch hydrolysates, isomalt, lactitol, maltitol, mannitol, sorbitol, and xylitol. Although polyols have many attractive commercial benefits, they have also been shown to trigger gastrointestinal symptoms and exert laxative effects when consumed in excess (10). The purpose of this review is to evaluate the available literature on the effects of polyols on the gastrointestinal tract in persons with and without IBS.
Methods
We first constructed a search strategy for PubMed, Ovid, and Embase using free-text terms to explore articles on individual polyols and terms including absorption, fermentation, motility, permeability, and gastrointestinal symptoms. The searches were then limited to articles in the English language. Articles involving both animal and human studies were included. All searches were completed by October 2016. We retrieved potentially relevant articles and reviewed their references to identify any missed articles from our original searches. Our search yielded a total of 1823 eligible articles, which were reviewed separately by 2 reviewers (AL and WDC). Case reports, letters to the editor, and articles irrelevant to the specific question asked were excluded. Seventy-nine of the original articles were included in our final review. Values are expressed as means ± SDs.
Results
Articles referencing the following polyols were included: erythritol (3 articles), isomalt (5 articles), lactitol (6 articles), maltitol (2 articles), mannitol (5 articles), sorbitol (13 articles), xylitol (3 articles), and hydrogenated starch hydrolysates (1 article). Background information pertaining to the chemical structure, properties, metabolism, and production of the particular polyols is presented first.
Structure, metabolism, production, and use
Erythritol, known by the chemical name of 1,2,3,4-butaneterol, is a polyol that is found naturally in fruits, vegetables, mushrooms, and fermented foods such as wine and soy sauce. It is stable in extreme temperatures as well as in acidic and alkaline environments. Isomalt is a mixture of 2 isomeric disaccharide alcohols: gluco-mannitol (α-d-gluco-pyranosyl-1–6-mannitol) and gluco-sorbitol (α-d-gluco-pyranosyl-1–6-sorbitol). It is resistant to degradation at high temperatures and is currently used in a variety of sugar-free products. Lactitol is a disaccharide polyol, made up of sorbitol and galactose. It is stable in high temperatures as well as in acidic and alkaline environments and is usually combined with other low-calorie sweeteners (10). Maltitol, also known by its chemical name 4-O-α-d-glucopyranosyl-d-glucitol, is a disaccharide polyol made up of sorbitol and glucose. It is used in commercial products under the trade names of Lesys, Maltisweet, and SweetPear.
Mannitol is a 6-carbon polyol, which is an isomer of sorbitol. It can be found in nature in the exudates of certain trees as well as in marine algae and mushrooms. It is stable at elevated temperatures and has a low hygroscopicity (the ability of a substance to attract and hold water molecules), allowing it to be used as a coating for hard candies and chocolates. Sorbitol, also known as d-glucitol, is a 6-carbon polyol that is found naturally in apples, pears, peaches, apricots, and some vegetables. It is stable at high temperatures, and unlike other polyols, it has a high hygroscopicity and protects against loss of moisture, allowing it to be used in confectionary products (10). Xylitol is a 5-carbon polyol, which is produced from d-xylose. It is found naturally in different fruits, berries, vegetables, oats, and mushrooms, and a small percentage is also produced by the human body. Xylitol is widely used in various pharmaceutical products in addition to sugar-free candies and chewing gums. Further properties of individual polyols and examples of food products containing polyols are illustrated in Figure 3 and in Tables 1 and 2.
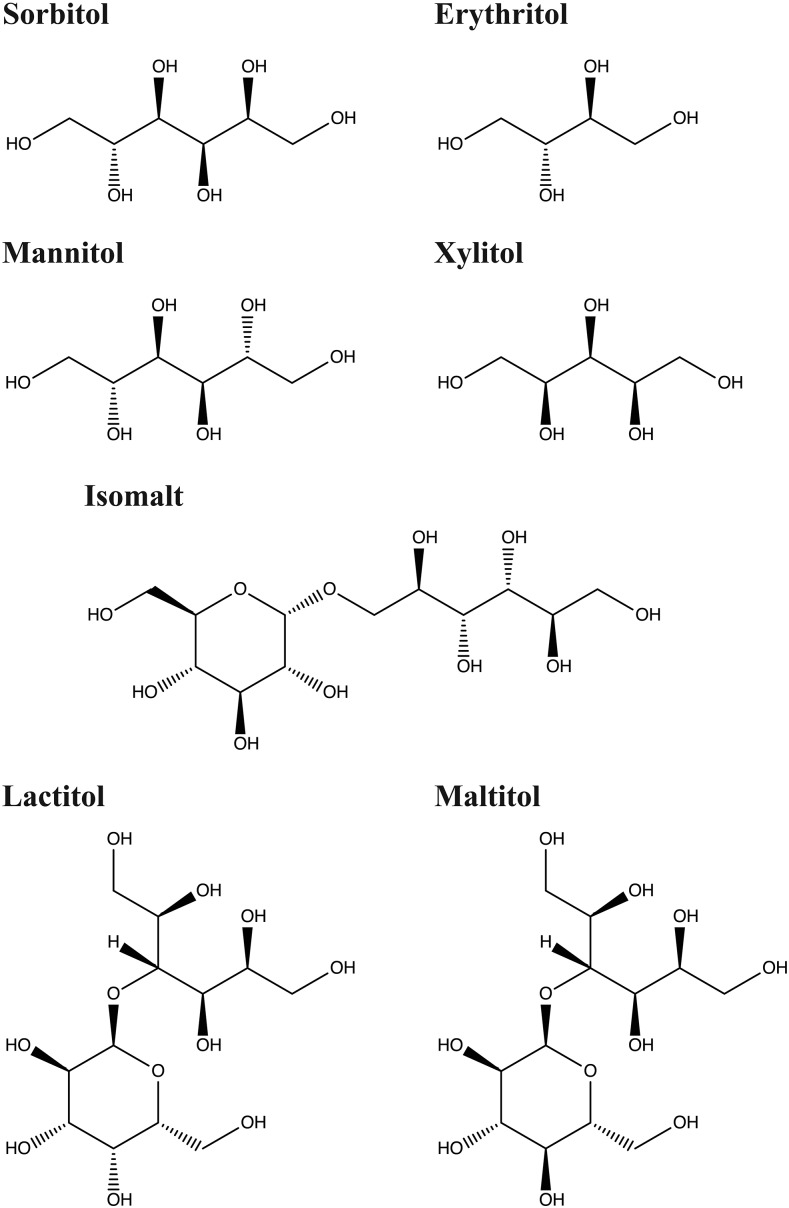
FIGURE 3.
Chemical structures of polyols.
TABLE 1.
Properties of different polyols
| Polyol | Production | Caloric value, kcal/g | Sweetness compared with sucrose, % | Typical food products |
| Erythritol | Fermentation by yeasts (Moniliella pollinis, Trichosporonides megachiliensis) and lactic acid bacteria | 0.2 | 60–80 | Found naturally in fruits, vegetables, mushrooms, and fermented foods (wine, soy sauce); bulk sweetener in low-calorie foods |
| Isomalt | Enzymatic transglucosidation of sucrose into maltose then hydrogenated into isomalt | 2 | 45–65 | Hard candies, toffee, chewing gum, chocolate, and cough drops |
| Lactitol | Catalytic hydrogenation with Raney nickel | 2–2.4 | 30–40 | Hard and soft candies, ice cream, chocolate, and some baked goods (cookies and cakes) |
| Maltitol | Obtained from starch by hydrogenating maltose | 2.1–2.4 | 75–90 | Hard candies, chewing gum, chocolates, baked goods, and ice cream |
| Mannitol | Catalytic hydrogenation of a glucose/fructose mixture (formed from starch or sucrose); second fermentation step by using lactic acid bacteria | 1.6 | 50–70 | Coating for hard candies; dusting powder for chewing gum, dried fruits, and chewing gum |
| Sorbitol | Catalytic hydrogenation of glucose/sucrose with hydrogen gas and a nickel catalyst or through electrochemical reduction of dextrose | 2.6 | 50–70 | Sugar-free hard candies, chewing gum, frozen desserts, baked goods, and confectionary items |
| Xylitol | Substrate xylan is hydrolyzed to xylose and then metal catalyzed | 2.4 | 100 | Sugar-free hard candies, chewing gum, gum drops, and pharmaceuticals (throat lozenges, cough syrup, toothpaste, and mouthwash) |
TABLE 2.
Common products containing polyols
| Products | Type of polyol |
| Gum/Sugar-free candies | |
| Altoids Mints, Sugar-Free | Sorbitol |
| Big Red Gum | Mannitol |
| Breath Savers: Wintergreen mints | Sorbitol |
| Crème Savers Sugar Free Hard Candies | Isomalt |
| Doublemint Gum | Sorbitol, mannitol, and maltitol |
| Eclipse Sugar-Free Gum | Sorbitol, mannitol, and maltitol |
| Extra Sugar-Free Gum | Sorbitol, mannitol, maltitol, hydrogenated starch hydrolysate |
| Hershey Sugar-Free York Peppermint Patties | Sorbitol, maltitol, lactitol, isomalt |
| Ice Breakers Sugar-Free Mints | Sorbitol, maltitol |
| Jelly Belly Sugar-Free Jelly Beans | Maltitol |
| Jolly Rancher Sugar-Free Hard Candies | Isomalt |
| Life Savers Sugar-Free Wint-O-Green Breath Mint | Sorbitol |
| LifeSavers Sugar-Free Hard Candies | Isomalt |
| Stride Sugar-Free Gum | Sorbitol, mannitol, maltitol, xylitol |
| Trident Sugar-Free Gum | Sorbitol, mannitol, maltitol, xylitol |
| Werther’s Original Sugar-Free Hard Candies | Isomalt |
| Wrigley’s Orbit Sugar-Fee Gum | Sorbitol, mannitol, maltitol, hydrogenated starch hydrolysate |
| 5 Gum Sugar-Free | Sorbitol, mannitol, maltitol, hydrogenated starch hydrolysate |
| Ice cream | |
| Breyers Light and No Sugar Added Ice Cream | Sorbitol, maltitol, lactitol |
| Edy’s Carb Benefit and No Sugar Added Ice Cream | Sorbtiol, mannitol, maltitol |
| Blue Bunny Reduced Fat and No Sugar Added Ice Cream | Sorbitol, mannitol |
| Klondike No Sugar Added Ice Cream Bars | Maltitol |
| Syrup | |
| Mrs. Butterworth’s Sugar-Free Syrup | Sorbitol |
| Hungry Jack Butter Sugar-Free Syrup | Sorbitol |
| Log Cabin Sugar-Free Syrup | Sorbitol |
| Jelly/Jam | |
| Smucker’s Sugar-Free Jam | Sorbitol, maltitol |
| Beverages | |
| Arizona Diet Tea Drink | Sorbitol |
| Sparkling Ice Drinks | Mannitol |
| Zevia Soda | Erythritol |
| Monster Absolute Zero | Erythritol |
| Vitamin Water Zero | Erythritol |
| Lozenges | |
| Brach’s Wintergreen Lozenges | Sorbitol |
Effects on Gastrointestinal Absorption
A substantial amount of research has focused on the slow and incomplete absorption of polyols (11–13). Approximately one-third of polyols that are consumed in the human diet are absorbed in the small intestine, and the amount of absorption varies depending on the individual polyol (14). Polyols are believed to be absorbed via the process of passive diffusion, meaning that there is no requirement for additional energy input in the absorptive process (14, 15). Monosaccharides are thought to be absorbed via the transcellular route, although larger disaccharide molecules may be absorbed paracellularly (16, 17). The rate of absorption is dependent on multiple factors, including the individual patient, the molecular size of the polyol, the intake amount of the polyol, confounding gastrointestinal diseases, and the variation in small intestinal epithelial pore size, because the distal small intestine is likely less permeable than the proximal small intestine (15, 18). The effect of molecular size becomes apparent when comparing different monosaccharide polyols, such as sorbitol and erythritol. Sorbitol has the highest molecular weight of the monosaccharide polyols at 182 g/mol, whereas erythritol has the lowest molecular weight at 122 g/mol. The larger size of sorbitol may be approaching the upper limit for diffusion across the small intestinal epithelium, resulting in poorer absorption, although the lower molecular weight of erythritol may explain its more complete absorption in comparison with the other polyols (19).
Studies in healthy volunteers
Polyol malabsorption in human subjects has been studied with the use of jejunal perfusion techniques; breath testing measuring hydrogen gas, carbon dioxide, and/or methane; ileal effluents, and ileostomy models. Patil et al. (20) evaluated the intestinal absorption of lactitol using an in vivo jejunal perfusion model, which assessed absorption via brush-border hydrolysis. This showed that galactose was not detected in the jejunal aspirate, demonstrating that lactitol was not well absorbed by the small intestine (20). Grimble et al. (21) also studied the absorption of lactitol using hydrogen breath testing. In this study, 6 healthy volunteers were given 20 g lactitol daily for 14 d, and on the seventh day, radiolabeled lactitol was given along with the unlabeled polyol, and excretion of 14C in breath tests, urine, and feces was measured. The total 14CO2 measured accounted for 62.9% ± 5% SE of the isotope given, suggesting that lactitol was poorly absorbed in the small intestine and rather extensively metabolized in the colon (21).
A dose-dependent relation for polyol malabsorption has also been observed. Corazza et al. (22) evaluated sorbitol absorption in 30 healthy volunteers using hydrogen breath testing. After ingesting 10- and 20-g solutions of sorbitol, malabsorption was present in 90% and 100% of healthy volunteers, respectively (22). Another part of this study found that after ingesting a 5-g solution of sorbitol at concentrations of 2%, 4%, 8%, and 16%, malabsorption was present in 10%, 12%, 22%, and 43% of healthy volunteers (22). Thus, dose and concentration influence the degree to which sorbitol is malabsorbed. A study by Hyams (23) also demonstrated dose-dependent malabsorption with hydrogen breath testing by administering 5, 10, and 20 g of sorbitol to 7 healthy volunteers. Although intersubject variability existed in the maximum increase in hydrogen concentration [measured in parts per million (ppm)] after administration of sorbitol, significant differences were seen between groups at 5 g (12 ± 4 ppm) and 10 g (26 ± 6 ppm) (P = 0.008) and 5 g and 20 g (68 ± 17 ppm). No significant difference was observed between groups at 10 and 20 g (23).
Langkilde et al. (13) used an ileostomy model to evaluate absorption patterns of sorbitol, maltitol, and isomalt. In the first part of this study, 9 ileostomy patients consumed 2 milk-chocolate bars/d (each containing 15 g of sorbitol, maltitol, or isomalt), and ileostomy effluents were collected. In the second part of the study, subjects consumed 5, 10, or 20 g of sorbitol or isomalt in a liquid solution, and the ileostomy effluent was again measured over a 24-h period. The excretion of sorbitol increased from 2% with a 5-g intake of sorbitol to >25% with a 20-g intake. The mean α-O-glucopyranosyl-1,6-sorbitol and mean α-O-glucopyranosyl-1,6-mannitol (breakdown products of isomalt) excreted after ingestion of 5, 10, and 20 g of isomalt represented 3.6%, 6.7%, and 15.5% and 10.2%, 16.2%, and 25.6%, respectively. Similar to previous studies, the authors therefore concluded that polyol absorption was dose dependent (13).
The absorptive properties of polyols when ingested with other carbohydrates have also been evaluated. Studies by Rumessen and Gudmand-Hoyer (24, 25) examined the absorption of sorbitol alone or in combination with fructose, because these carbohydrates are frequently found together in sugar-free products. The authors discovered that in healthy volunteers sorbitol and fructose were relatively well absorbed when ingested individually but incompletely absorbed when taken together (24, 25). The reasons for this are not clear but may be related to the 2 sugars competing for the same intestinal transporters. A study by Beaugerie et al. (15) examined the digestion and absorption of 30 g sorbitol, 57 g maltitol containing 30 g sorbitol, and 69 g Lycasin 80/55 (a hydrogenated glucose syrup containing 30 g sorbitol and maltitol) by ileal aspiration after a single meal in 6 healthy volunteers during three 11-d periods. The mean percentage of sorbitol absorbed was higher for pure sorbitol than in the solutions containing sorbitol plus maltitol and Lycasin. Stool polyols were negligible, indicating that the sugar alcohols that reached the colon were almost completely digested (15). Overall, these studies demonstrated that malabsorption increases when polyols are ingested in combination with other carbohydrates.
Unlike the other polyols, erythritol appears to be well absorbed within the small intestine, with around 90% absorbed via passive diffusion. In a study by Hiele et al. (26), the metabolism of erythritol was compared with lactitol in 6 healthy volunteers by measuring the amount of breath carbon dioxide and hydrogen excretion, as well as the amount of erythritol excreted in the urine. In contrast to the results obtained for lactitol, no increase in breath carbon dioxide or hydrogen gas was observed after ingestion of erythritol, and nearly all erythritol was recovered in the urine (26).
Prevalence of polyol malabsorption in IBS
Studies that have evaluated polyol absorption in patients with IBS have yielded conflicting results. In a controlled study with the use of hydrogen breath testing in 73 patients with IBS and 87 healthy controls, fructose-sorbitol malabsorption was frequent but not significantly different in the 2 groups (IBS: 30.1% compared with controls: 40.2%). The authors concluded that fructose-sorbitol malabsorption was unlikely to play a substantial role in the etiology or symptomatology of IBS (27). However, another breath-testing study that evaluated fructose-sorbitol malabsorption in 25 patients with IBS and 25 healthy controls given 5 g of sorbitol and a mixture of 25 g fructose plus 5 g sorbitol found increased breath hydrogen excretion in the IBS group, which was suggestive of increased malabsorption (28).
Absorption of sorbitol and mannitol was studied in patients with IBS and healthy controls in a randomized, double blind, placebo-controlled crossover study by Yao et al. (29). Somewhat surprisingly, this study found that patients with IBS absorbed polyols twice as well as healthy controls. In addition, mannitol absorption was more rapid than sorbitol absorption in patients with IBS but not in healthy controls (29). The authors performed a second study, which once again, showed a more complete absorption of polyols in patients with IBS than in healthy controls (30). It has been hypothesized that patients with IBS might have slower oral-cecal transit times than healthy individuals, allowing for additional absorption of the polyols. Alternatively, differences in polyol absorption might be secondary to intestinal barrier dysfunction in patients with IBS (29).
Several studies have used the urinary saccharide excretion test to evaluate intestinal permeability in patients with and without IBS by using mannitol and lactulose. The combined administration of a large (lactulose) and a small (mannitol) molecule yields a specific ratio in the urine, which provides a reflection of intestinal permeability (31, 32). Mannitol displays transcellular permeability, whereas lactulose is transported via a paracellular route (16, 17, 33, 34). The lactulose-mannitol test consists of the simultaneous ingestion of the sugars in water, a fasting period of 2 h, and a 24-h urine collection. The lactulose/mannitol ratio from the first 6 h of collection is then used to measure small intestinal permeability (35). Other less commonly used methods of this test include urine collections at 0–3, 3–5, and 5–24 h to assess permeability in the proximal small intestine, distal small intestine, and colon, respectively (17, 36, 37).
Effects on Gastrointestinal Motility and Transit
Gastrointestinal dysmotility is recognized as one of the pathophysiological factors that contributes to the etiology of IBS. In patients with abdominal pain and diarrhea-predominant IBS (IBS-D), colonic dysmotility may be secondary to an increased incidence of high-amplitude propagating contractions and elevated intraluminal pressures that parallel rapid colonic transit time (38). Small intestinal dysmotility, identified as clustered contractions during episodes of abdominal colic, has also been observed in patients with IBS (39, 40). Animal studies showed that duodenal motility can be stimulated by hyperosmolar solutions of mannitol, and these motility changes occur without changes in gastric inhibitory peptide or insulin (41, 42). However, to our knowledge, few studies in adult patients have specifically examined the relation between polyol consumption and gastrointestinal motility.
Studies in healthy volunteers
Although there are few data on the effects of polyols on gastrointestinal motility in healthy volunteers, several studies from the surgical literature evaluated the effects of chewing sugar-free gum, which contains various polyols, on postsurgical recovery of gastrointestinal function and motility (43, 44). The mechanism by which gum chewing enhances bowel motility is presumed to be secondary to direct stimulation of the cephalic-vagal reflex. However, little research has been conducted to determine the effects of individual polyols on gastrointestinal motility or transit. Gong et al. (45) performed a randomized controlled trial of 109 women (53 study patients and 56 controls) undergoing laparoscopic surgery to evaluate the effects of xylitol chewing gum on gastrointestinal motility, which was measured as the time to first bowel sounds, flatus, and bowel movement. The authors found that first flatus and first bowel sounds occurred significantly earlier in patients who received xylitol-containing gum than in controls [first flatus: 22.43 ± 7.19 h for study group compared with 28.12 ± 10.36 h for controls (P < 0.001); and first bowel sounds: 8.26 ± 1.04 h for study group compared with 12.07 ± 3.26 h for controls (P < 0.001)]. No significant differences in the time to first bowel movement occurred between groups. The differences in the time to first flatus and bowel sounds suggested that xylitol might affect postoperative gastrointestinal motility and transit (45). However, this study did not investigate the mechanisms behind the enhanced transit, and it is impossible to distinguish between the effects of the cephalic-vagal reflex compared with the effects from the polyol.
Madsen et al. (46) conducted a double-blind crossover study in 11 healthy volunteers who ingested 10% solutions containing 30 g glucose or 25 g fructose-sorbitol mixture to examine the effects of malabsorption and fermentation of these different sugars on small intestinal transit. A radiolabeled marker, 99mTc-diethylenetriaminepentaacetic acid, was added to each solution, and breath hydrogen and methane concentrations, as well as gastrointestinal transit, were assessed over 6 h. Malabsorption of the fructose-sorbitol mixture was observed in all subjects. In addition, the oral-cecal transit time of the radiolabeled marker was faster, and the percentage of the radiolabeled marker in the colon was higher for the fructose-sorbitol mixture than for the glucose solution. The authors believed that this accelerated transit time was secondary to the osmotic effects of the nonabsorbed fructose-sorbitol within the small bowel, leading to increased luminal volume and secondarily increasing peristalsis (46).
Effects on motility in patients with IBS
Only one study was found that addressed polyol consumption and gastrointestinal motility in patients with IBS. Evans et al. (12) evaluated 15 female patients with IBS (aged 44 ± 15 y) who underwent hydrogen breath testing after ingesting 10 g lactulose and 25 g fructose plus sorbitol. Jejunal motility was evaluated by using 24-h manometry. Although the majority of patients in this study displayed jejunal dysmotility in both the fasting and postprandial state, no association was found between dysmotility, carbohydrate malabsorption, and symptom provocation (12).
Effects on Visceral Hypersensitivity in Humans
Visceral hypersensitivity is a term used to describe heightened sensory perceptual responses to visceral stimulation and is believed to contribute to the pathogenesis of IBS (47–49). Visceral hypersensitivity is thought to arise as a consequence of complex changes in the enteric and central nervous systems and can be triggered by psychological stressors and previous intestinal injuries (48, 50–55). No studies were found that examined the effects of polyols on visceral sensation in healthy individuals. However, the previously mentioned study by Evans et al. (12) also evaluated the relation of fructose-sorbitol malabsorption and visceral sensation in patients with IBS using a balloon distention model. Although the majority of patients with IBS displayed jejunal hypersensitivity, there was no significant correlation with polyol malabsorption or provocation of gastrointestinal symptoms. Zhou et al. (56) further investigated the effects of ingesting a 5-g lactulose and 2-g mannitol solution on intestinal membrane permeability and visceral sensation in patients with IBS and healthy controls after rectal balloon distention. This study found that an increased visceral sensation correlated with increased intestinal permeability to the lactulose and mannitol solution (56). The authors hypothesized that increased permeability may allow bacteria and other inflammatory triggers to pass through the intestinal mucosal barrier, leading to sensitization of myenteric neurons and visceral hypersensitivity.
Effects on the Microbiome
In recent years, there has been a great deal of interest in the role that the gut microbiome plays in health and disease. Indigestible or poorly absorbed carbohydrates are known to be important prebiotics for the gut microbiome (57, 58). Thus, it is important to understand how polyols affect the gut microbiome in healthy persons and patients with IBS.
Studies in healthy volunteers
There are some data from animal and human studies, which have addressed the effects of polyols such as xylitol, isomalt, and lactitol on the gut microbiome. A study by Tamura et al. (59) compared the fecal microbiome of 2 groups of mice after consuming either a 0.05% isoflavone with 5% xylitol diet or a 0.05% isoflavone-containing diet (control diet). The concentration of Bacteroides was significantly greater in the control diet than in the xylitol-rich diet (P < 0.05), suggesting that xylitol might affect the metabolism of isoflavones by altering the metabolic activity of the fecal microbiota (59). An additional study by Salminen et al. (60) went on to assess the effect of xylitol on the microbiome in humans and found a shift in the intestinal flora from gram-negative to gram-positive bacteria after a single 30-g oral dose of xylitol. Another double-blind, placebo-controlled study by Gostner et al. (61) evaluated the effects of consuming diets enriched with either 30 g isomalt or 30 g sucrose on the colonic microbiome of 19 healthy volunteers. Results showed a shift in the colonic microbiome toward an increase in bifidobacteria with the isomalt diet compared with the sucrose diet (P < 0.05) (61). However, the abundance of lactobacilli, Bacteroides, Escherichia coli, and enterococci was not significantly different between the 2 diets. Given the bifidogenic effects of isomalt, the authors concluded that this polyol might be a potential prebiotic that could contribute to a healthy colonic environment (61).
A study by Ballongue et al. (62) examined the effects of lactulose and lactitol on the colonic microbiome and enzymatic activity in 36 healthy volunteers. Ten-gram solutions of lactulose and lactitol were administered to study subjects, which resulted in reduced activity of procarcinogenic enzymes (azoreductase, 7-α-dehydrogenase, β-glucuronidase, nitroreductase, and urease) and an increase in prebiotic, fecal SCFA. However, these effects were more pronounced with lactulose than with lactitol, suggesting that lactulose may have a stronger prebiotic effect (62). An additional randomized, longitudinal, double-blind study by Finney et al. (63) examined the effects of low-dose lactitol on the fecal microbiome. In this study, 75 healthy volunteers consumed 25-g tablets of milk chocolate containing 10 g sucrose and lactitol in ratios of 10:0, 5:5, or 0:10 daily for 7 d. In the group that consumed the 0:10 ratio, there was a significant increase in bifidobacteria but no significant change in fecal anaerobes, aerobes, Enterobacteriacae, or lactobacilli (63). The authors therefore concluded that low doses of lactitol might also function as a prebiotic and be beneficial in inducing a healthy colonic microbiome (63).
Studies in patients with IBS
Multiple studies have shown differences in the diversity and abundance of bacterial communities, as well as in the stability and metabolic activity of the microbiome in patients with IBS compared with healthy controls (4, 64–69). It is well recognized that dietary interventions can affect the gut microbiome (70); however, we were not able to find studies that specifically addressed the effects of polyols on the microbiome. However, there are data on the effects of the low-FODMAP diet on the fecal microbiome of patients with IBS. Because oligosaccharides have been shown to have positive effects on the microbiota in healthy individuals (57, 58), there has been some concern that a low-FODMAP diet may negatively affect colonic health. One randomized controlled trial by Staudacher et al. (71) found that, although a low-FODMAP diet lessened gastrointestinal symptoms, it also reduced the concentration and proportion of intestinal bifidobacteria, which are considered biomarkers of a well-balanced microbiome. Another randomized controlled trial from Australia found that, compared with a control diet, a low-FODMAP diet was associated with greater microbial diversity and reduced bacterial abundance of total bacteria, butyrate-producing bacteria, probiotic bacteria, Akkermansia muciniphila, Ruminococcus gnavus, and Clostridium cluster XIVa (72). On the other hand, the control diet in this study, which had a higher FODMAP intake, stimulated the growth of bacterial groups with presumed health benefits.
Although these observations are of potential concern and warrant further investigation, it is currently unknown whether these effects on the microbiome will prove bad, good, or indifferent to persons eating a low-FODMAP diet. Further, it is important to consider that different types of FODMAPs could exert different effects on specific bacterial communities. As recent studies with fMRI have taught us, different FODMAPs can exert different effects in different regions of the gastrointestinal tract (8). The same may well be true as it relates to the effects of different FODMAPs on the gut microbiome. Further, it is important to emphasize that the low-FODMAP diet is not meant to serve as a long-term strategy for patients with IBS. Rather, the full FODMAP exclusion should be viewed as a diagnostic test to identify patients with IBS who are FODMAP sensitive. Those who respond should work with a registered dietician or other reputable resources to systematically reintroduce foods containing individual FODMAPs. This allows diversification of the diet in most patients, encouraging adherence and minimizing any potentially hazardous effects on the microbiome (73).
Gastrointestinal Symptoms
Studies in healthy individuals
As previously mentioned, malabsorption of polyols may induce laxative effects, as well as flatulence, bloating, and abdominal discomfort in sensitive individuals (10). However, a great deal of intra- and intersubject variability exists in the threshold of polyol ingestion required to induce gastrointestinal symptoms, and many healthy adults, even with moderate doses, will experience no symptoms at all (74). Similar to the relation seen with malabsorption, symptom provocation with polyols also appears to be dose dependent and increases when polyols are consumed in conjunction with other carbohydrates (24, 75). For example, most healthy individuals tolerate ∼10 g sorbitol/d with only mild gastrointestinal discomfort, such as flatulence or bloating (22, 23). However, doses of 20 g sorbitol/d can evoke more distressing symptoms of abdominal pain and diarrhea (23). This dose-dependent relation has also been seen with isomalt, lactitol, and maltitol (76).
Although malabsorption accounts for a substantial proportion of symptoms, some studies have shown that gastrointestinal symptoms do not necessarily correlate with the degree of hydrogen production during breath testing, suggesting that factors other than malabsorption are involved in the development of gastrointestinal symptoms (22, 28, 77). For instance, disaccharide polyols (lactitol) are generally better tolerated than monosaccharide polyols (sorbitol) secondary to the latter exerting a greater osmotic load in the gastrointestinal tract, leading to an increased concentration of water in the colon and a consequent greater laxative effect (78, 79). Accelerated small bowel transit time seen with polyol ingestion also results in increased osmosis and water retention within the small bowel lumen and correlates with increased abdominal discomfort in healthy individuals (46). Of note, just as erythritol is more completely absorbed than the other polyols, it also produces fewer intestinal symptoms. This may be because only a small portion of erythritol enters the colon, therefore exerting little osmotic effect and limited gas production (80).
Gastrointestinal symptoms in patients with IBS
In patients with IBS, symptom provocation has also been shown to be dose dependent, although the dose-response curve appears to be shifted to the left, presumably as a consequence of underlying abnormalities in motility, transit, and visceral sensation. A study by Symons et al. (77) examined the symptoms provoked by the ingestion of fructose and sorbitol in patients with and without IBS using breath hydrogen testing after administration of high- and low-dose fructose and sorbitol mixtures. The total symptom score in patients with IBS but not in controls was greater after the higher dose of the fructose-sorbitol mixture. Of note, no correlation was observed between the increase in symptoms and the increase in peak breath hydrogen concentration (77). In the studies previously mentioned by Yao et al., both sorbitol and mannitol induced abdominal symptoms in patients with IBS, despite evidence suggesting superior absorption, compared with in healthy controls (29, 30).
These findings are consistent with the conventional wisdom that states that polyols and other poorly absorbed substrates trigger symptoms in patients with IBS via direct osmotic effects and secondary effects induced by bacterial fermentation, which produces SCFAs and gases. It is, however, likely that this prevailing wisdom is an oversimplification. For example, it is conceivable, although currently unproven, that the byproducts of fermentation, through effects on the intraluminal microenvironment, could alter a variety of other factors of relevance to patients with IBS including the colonic microbiome or metabolome, mucosal permeability, mucosal immune activation, and primary compared with secondary bile acid concentrations.
Conclusion
Polyols are sugar alcohols, which are slowly and incompletely absorbed in the small intestine via the process of passive diffusion. Studies of malabsorption have been conducted with the use of hydrogen breath testing, testing from ileal effluents, jejunal perfusion techniques, and ileostomy models. Overall, available work has shown that polyol malabsorption varies from substrate to substrate but in general occurs in a dose-dependent fashion, and malabsorption increases when polyols are ingested in combination with other carbohydrates. This is pertinent to the FODMAP hypothesis, which suggests that cumulative doses rather than individual carbohydrates are the main drivers of symptomatic IBS. An exception to this is seen with erythritol, which is relatively well absorbed compared with the other polyols. Regarding IBS, studies have shown conflicting results pertaining to polyol malabsorption. Early studies showed that fructose-sorbitol malabsorption was frequently observed in patients with IBS, but this was not significantly different from in healthy controls. Other studies showed that patients with IBS had greater evidence of malabsorption after polyol ingestion, and still other studies found that patients with IBS absorb polyols more completely than healthy controls do.
Studies showing accelerated intestinal transit time provide indirect evidence of an effect of polyols on motility. This is presumed secondary to the effects of osmosis and water retention within the small bowel lumen, which by increasing biomass could increases peristalsis. In IBS, intestinal dysmotility has been seen after polyol ingestion. Although the data addressing the effects of polyols on visceral sensation are quite limited, one study found intestinal hypersensitivity in patients with IBS after polyol ingestion. However, neither the abnormalities in motility nor visceral sensation was found to correlate with the extent of malabsorption or gastrointestinal symptoms, making the clinical significance of these findings unclear. In recent years, there has been interest in the human microbiome and its influence on gastrointestinal health and disease. In healthy individuals, moderate doses of isomalt and lactitol have been shown to shift the microbiome toward an increase in bifidobacteria and may therefore be beneficial as prebiotics. Data, however, are lacking regarding the specific relation between polyols and the microbiome in IBS.
Polyols can induce dose-dependent gastrointestinal symptoms of flatulence, bloating, abdominal discomfort, and laxative effects when consumed in healthy volunteers and patients with IBS. However, there is a substantial degree of intra and intersubject variability in symptoms, and patients with IBS tend to report symptoms after consumption of lower doses of polyols than healthy volunteers do. Although malabsorption likely accounts for a portion of symptoms, other factors, including the type of polyol ingested, the consumption pattern, and the colon’s ability to reabsorb water, are likely also involved in inducing gastrointestinal symptoms.
A relevant limitation to the review of this topic is the lack of studies evaluating polyols in isolation rather than in combination with other carbohydrates. Therefore, the individual effect of polyols on factors such as absorption, gastrointestinal motility, and visceral sensation is difficult to determine with confidence. There are also little to no data differentiating the effects of polyols on the different subtypes of IBS [IBS-D, constipation predominant (IBS-C), or mixed]. When evaluating the absorption patterns of polyols, Yao et al. (29) identified subjects with IBS as having IBS-D, IBS-C, or mixed IBS. However, baseline characteristics were similar between the 3 subtypes, and all patients with IBS were ultimately analyzed collectively and compared with controls (29). No subgroup analysis was performed. Without evidence from clinical trials, we cannot make conclusions regarding potential differences in polyol-associated effects depending on IBS subtype. However, we would anticipate that the effects in IBS-D would be more predictable than they would in IBS-C. In IBS-D, generation of SCFA from fermentation would be expected to create an osmotic load and increase biomass, both of which one would expect to worsen issues with diarrhea. On the other hand, polyol fermentation could lead to gas production (hydrogen, carbon dioxide, and methane) regardless of IBS subtype. Further research is needed to understand the effects of specific polyols on gastrointestinal function, sensation, microbiome, and metabolome in health and disorders such as IBS.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide, and polyol; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome, constipation predominant; IBS-D, irritable bowel syndrome, diarrhea predominant; ppm, parts per million.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21. [DOI] [PubMed] [Google Scholar]
- 2.Ohman L, Simren M.. Pathogenesis of IBS: role of inflammation, immunity and neuroendocrine interactions. Nat Rev Gastroenterol Hepatol 2010;7:163–73. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, Presson AP, Yuan PQ, Cortina G, Gong H, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol 2012;107:262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol 2014;11:497–505. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc 2006;106:1631–9. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008;6:765–71. [DOI] [PubMed] [Google Scholar]
- 7.Oku T, Nakamura S. Digestion, absorption, fermentation, and metabolism of functional sugar substitutes and their available energy. Pure Appl Chem 2002;74:1253–61. [Google Scholar]
- 8.Spiller R. Irritable bowel syndrome: new insights into symptom mechanisms and advances in treatment. F1000Res 2016:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grembecka M. Natural sweeteners in a human diet. Rocz Panstw Zakl Hig 2015;66:195–202. [PubMed] [Google Scholar]
- 10.Grembecka M. Sugar alcohols—their role in the modern world of sweeteners: a review. Eur Food Res Technol 2015;241:1–14. [Google Scholar]
- 11.Barrett JS, Irving PM, Shepherd SJ, Muir JG, Gibson PR. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther 2009;30:165–74. [DOI] [PubMed] [Google Scholar]
- 12.Evans PR, Piesse C, Bak YT, Kellow JE. Fructose-sorbitol malabsorption and symptom provocation in irritable bowel syndrome: relationship to enteric hypersensitivity and dysmotility. Scand J Gastroenterol 1998;33:1158–63. [DOI] [PubMed] [Google Scholar]
- 13.Langkilde AM, Andersson H, Schweizer TF, Wursch P. Digestion and absorption of sorbitol, maltitol and isomalt from the small bowel. A study of ileostomy subjects. Eur J Clin Nutr 1994;48:768–75. [PubMed] [Google Scholar]
- 14.Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Role of FODMAPs in patients with irritable bowel syndrome. Nutr Clin Pract 2015;30:665–82. [DOI] [PubMed] [Google Scholar]
- 15.Beaugerie L, Flourié B, Marteau P, Pellier P, Franchisseur C, Rambaud JC. Digestion and absorption in the human intestine of three sugar alcohols. Gastroenterology 1990;99:717–23. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnason I, Peters TJ, Levi AJ. Intestinal permeability: clinical correlates. Dig Dis 1986;4:83–92. [DOI] [PubMed] [Google Scholar]
- 17.Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Lactuose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986;71:71–80. [DOI] [PubMed] [Google Scholar]
- 18.Livesey G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev 2003;16:163–91. [DOI] [PubMed] [Google Scholar]
- 19.Zumbé A, Lee A, Storey D. Polyols in confectionery: the route to sugar-free, reduced sugar and reduced calorie confectionery. Br J Nutr 2001;85 Suppl 1:S31–45. [DOI] [PubMed] [Google Scholar]
- 20.Patil DH, Grimble GK, Silk DB. Lactitol, a new hydrogenated lactose derivative: intestinal absorption and laxative threshold in normal human subjects. Br J Nutr 1987;57:195–9. [DOI] [PubMed] [Google Scholar]
- 21.Grimble GK, Patil DH, Silk DB. Assimilation of lactitol, an ‘unabsorbed’ disaccharide in the normal human colon. Gut 1988;29:1666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corazza GR, Strocchi A, Rossi R, Sirola D, Gasbarrini G. Sorbitol malabsorption in normal volunteers and in patients with coeliac disease. Gut 1988;29:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyams JS. Sorbitol intolerance: an unappreciated cause of functional gastrointestinal complaints. Gastroenterology 1983;84:30–3. [PubMed] [Google Scholar]
- 24.Rumessen JJ, Gudmand-Hoyer E. Malabsorption of fructose-sorbitol mixtures. Interactions causing abdominal distress. Scand J Gastroenterol 1987;22:431–6. [DOI] [PubMed] [Google Scholar]
- 25.Rumessen JJ, Gudmand-Hoyer E. Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology 1988;95:694–700. [DOI] [PubMed] [Google Scholar]
- 26.Hiele M, Ghoos Y, Rutgeerts P, Vantrappen G. Metabolism of erythritol in humans: comparison with glucose and lactitol. Br J Nutr 1993;69:169–76. [DOI] [PubMed] [Google Scholar]
- 27.Nelis GF, Vermeeren MA, Jansen W. Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology 1990;99:1016–20. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Bañares F, Esteve-Pardo M, de Leon R, Humbert P, Cabré E, Llovet JM, Gassull MA. Sugar malabsorption in functional bowel disease: clinical implications. Am J Gastroenterol 1993;88:2044–50. [PubMed] [Google Scholar]
- 29.Yao CK, Tan HL, van Langenberg DR, Barrett JS, Rose R, Liels K, Gibson PR, Muir JG. Dietary sorbitol and mannitol: food content and distinct absorption patterns between healthy individuals and patients with irritable bowel syndrome. J Hum Nutr Diet 2014;27:263–75. [DOI] [PubMed] [Google Scholar]
- 30.Yao CK, Tan HL, Van Langenber DR, Barrett JB, Gibson PR, Muir JG. Abnormal intestinal handling of sorbitol and mannitol in patients with IBS: mechanistic insights and potential clinical implications. J Gastroenterol Hepatol 2011;26:70. [Google Scholar]
- 31.Menzies IS. Absorption of intact oligosaccharide in health and disease. Biochem Soc Trans 1974;2:1042–7. [Google Scholar]
- 32.Menzies IS. Transmucosal passage of inert molecules in health and disease. In: Skadhauge E, editors. Intestinal absorption and secretion. Lancaster (United Kingdom): MTP Press; 1984. p. 527. [Google Scholar]
- 33.Cobden I, Dickinson RJ, Rothwell J, Axon AT. Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. BMJ 1978;2:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juby LD, Rothwell J, Axon AT. Cellobiose/mannitol sugar test—a sensitive tubeless test for coeliac disease: results on 1010 unselected patients. Gut 1989;30:476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjarnason I, O’Morain C, Levi AJ, Peters TJ. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology 1983;85:318–22. [PubMed] [Google Scholar]
- 37.Bjarnason I, Peters TJ, Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet 1983;1:323–5. [DOI] [PubMed] [Google Scholar]
- 38.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol 2001;96:1499–506. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz L, Farrar JT. Intraluminal small intestinal pressures in normal patients and in patients with functional gastrointestinal disorders. Gastroenterology 1962;42:455–64. [PubMed] [Google Scholar]
- 40.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology 1987;92:1885–93. [DOI] [PubMed] [Google Scholar]
- 41.Thor P, Laskiewicz J, Konturek JW, Konturek SJ, Creutzfeldt W. Role of GIP and insulin in glucose-induced changes in intestinal motility patterns. Am J Physiol 1987;252:G8–12. [DOI] [PubMed] [Google Scholar]
- 42.Lin HC, Elashoff JD, Kwok GM, Gu YG, Meyer JH. Stimulation of duodenal motility by hyperosmolar mannitol depends on local osmoreceptor control. Am J Physiol 1994;266:G940–3. [DOI] [PubMed] [Google Scholar]
- 43.Asao T, Kuwano H, Nakamura J, Morinaga N, Hirayama I, Ide M. Gum chewing enhances early recovery from postoperative ileus after laparoscopic colectomy. J Am Coll Surg 2002;195:30–2. [DOI] [PubMed] [Google Scholar]
- 44.Hirayama I, Suzuki M, Ide M, Asao T, Kuwano H. Gum-chewing stimulates bowel motility after surgery for colorectal cancer. Hepatogastroenterology 2006;53:206–8. [PubMed] [Google Scholar]
- 45.Gong Y, Zhang Q, Qiao L, Lv D, Ruan J, Chen H, Gong J, Shi G. Xylitol gum chewing to achieve early postoperative restoration of bowel motility after laparoscopic surgery. Surg Laparosc Endosc Percutan Tech 2015;25:303–6. [DOI] [PubMed] [Google Scholar]
- 46.Madsen JL, Linnet J, Rumessen JJ. Effect of nonabsorbed amounts of a fructose-sorbitol mixture on small intestinal transit in healthy volunteers. Dig Dis Sci 2006;51:147–53. [DOI] [PubMed] [Google Scholar]
- 47.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002;122:1140–56. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 1990;98:1187–92. [DOI] [PubMed] [Google Scholar]
- 49.Posserud I, Ersryd A, Simrén M. Functional findings in irritable bowel syndrome. World J Gastroenterol 2006;12:2830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995;109:40–52. [DOI] [PubMed] [Google Scholar]
- 51.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mearin F, Pérez-Oliveras M, Perelló A, Vinyet J, Ibañez A, Coderch J, Perona M. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology 2005;129:98–104. [DOI] [PubMed] [Google Scholar]
- 53.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994;107:271–93. [DOI] [PubMed] [Google Scholar]
- 54.Sequeira IR, Lentle RG, Kruger MC, Hurst RD. Assessment of the effect of intestinal permeability probes (lactulose and mannitol) and other liquids on digesta residence times in various segments of the gut determined by wireless motility capsule: a randomised controlled trial. PLoS One 2015;10:e0143690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trimble KC, Farouk R, Pryde A, Douglas S, Heading RC. Heightened visceral sensation in functional gastrointestinal disease is not site-specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci 1995;40:1607–13. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009;146:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis LM, Martinez I, Walter J, Hutkins R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol 2010;144:285–92. [DOI] [PubMed] [Google Scholar]
- 58.Bouhnik Y, Flourié B, Riottot M, Bisetti N, Gailing MF, Guibert A, Bornet F, Rambaud JC. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutr Cancer 1996;26:21–9. [DOI] [PubMed] [Google Scholar]
- 59.Tamura M, Hoshi C, Hori S. Xylitol affects the intestinal microbiota and metabolism of daidzein in adult male mice. Int J Mol Sci 2013;14:23993–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salminen S, Salminen E, Koivistoinen P, Bridges J, Marks V. Gut microflora interactions with xylitol in the mouse, rat and man. Food Chem Toxicol 1985;23:985–90. [DOI] [PubMed] [Google Scholar]
- 61.Gostner A, Blaut M, Schäffer V, Kozianowski G, Theis S, Klingeberg M, Dombrowski Y, Martin D, Ehrhardt S, Taras D, et al. Effect of isomalt consumption on faecal microflora and colonic metabolism in healthy volunteers. Br J Nutr 2006;95:40–50. [DOI] [PubMed] [Google Scholar]
- 62.Ballongue J, Schumann C, Quignon P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol Suppl 1997;222:41–4. [DOI] [PubMed] [Google Scholar]
- 63.Finney M, Smullen J, Foster HA, Brokx S, Storey DM. Effects of low doses of lactitol on faecal microflora, pH, short chain fatty acids and gastrointestinal symptomology. Eur J Nutr 2007;46:307–14. [DOI] [PubMed] [Google Scholar]
- 64.Salonen A, de Vos WM, Palva A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology 2010;156:3205–15. [DOI] [PubMed] [Google Scholar]
- 65.Jeffery IB, Quigley EM, Öhman L, Simrén M, O’Toole PW. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes 2012;3:572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durbán A, Abellán JJ, Jiménez-Hernández N, Artacho A, Garrigues V, Ortiz V, Ponce J, Latorre A, Moya A. Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol Ecol 2013;86:581–9. [DOI] [PubMed] [Google Scholar]
- 67.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome–a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol 2005;43:213–22. [DOI] [PubMed] [Google Scholar]
- 68.Maukonen J, Satokari R, Mättö J, Söderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol 2006;55:625–33. [DOI] [PubMed] [Google Scholar]
- 69.Pistoli S, Smejkal C, McCartney A, Gibson GR. Differences in the faecal flora of healthy individuals and patients with irritable bowel syndrome, and in vitro effects of a synbiotic upon gut flora composition. Clin Nutr 2003;22:60. [Google Scholar]
- 70.McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 2016. Mar 14 (Epub ahead of print; DOI: 10.1136/gutjnl-2015-311339). [DOI] [PubMed] [Google Scholar]
- 71.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012;142:1510–8. [DOI] [PubMed] [Google Scholar]
- 72.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015;64:93–100. [DOI] [PubMed] [Google Scholar]
- 73.Chey WD. Food: the main course to wellness and illness in patients with irritable bowel syndrome. Am J Gastroenterol 2016;111:366–71. [DOI] [PubMed] [Google Scholar]
- 74.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75. e5. [DOI] [PubMed] [Google Scholar]
- 75.Kim Y, Park SC, Wolf BW, Hertzler SR. Combination of erythritol and fructose increases gastrointestinal symptoms in healthy adults. Nutr Res 2011;31:836–41. [DOI] [PubMed] [Google Scholar]
- 76.Koutsou GA, Storey DM, Lee A, Zumbe A, Flourie B, leBot Y, Oliver Ph. Dose-related gastrointestinal response to the ingestion of either isomalt, lactitol or maltitol in milk chocolate. Eur J Clin Nutr 1996;50:17–21. [PubMed] [Google Scholar]
- 77.Symons P, Jones MP, Kellow JE. Symptom provocation in irritable bowel syndrome. Effects of differing doses of fructose-sorbitol. Scand J Gastroenterol 1992;27:940–4. [DOI] [PubMed] [Google Scholar]
- 78.Zumbé A, Brinkworth RA. Comparative studies of gastrointestinal tolerance and acceptability of milk chocolate containing either sucrose, isomalt or sorbitol in healthy consumers and type II diabetics. Z Ernahrungswiss 1992;31:40–8. [DOI] [PubMed] [Google Scholar]
- 79.Lee A, Zumbe A, Storey D. Breath hydrogen after ingestion of the bulk sweeteners sorbitol, isomalt and sucrose in chocolate. Br J Nutr 1994;71:731–7. [DOI] [PubMed] [Google Scholar]
- 80.Oku T, Mitsuko O. Laxative threshold of sugar alcohol erythritol in human subjects. Nutr Res 1996;16:577–89. [Google Scholar]