Abstract
Purpose
Results from the Danish cluster-randomized trial of telehealthcare to 1,225 patients with chronic obstructive pulmonary disease (COPD), the Danish Telecare North Trial, concluded that the telehealthcare solution was unlikely to be cost-effective, by applying international willingness-to-pay threshold values. The purpose of this article was to assess potential sources of variation across subgroups, which could explain overall cost-effectiveness results or be utilized in future economic studies in telehealthcare research.
Methods
First, the cost-structures and cost-effectiveness across COPD severities were analyzed. Second, five additional subgroup analyses were conducted, focusing on differences in cost-effectiveness across a set of comorbidities, age-groups, genders, resource patterns (resource use in the social care sector prior to randomization), and delivery sites. All subgroups were investigated post hoc. In analyzing cost-effectiveness, two separate linear mixed-effects models with treatment-by-covariate interactions were applied: one for quality-adjusted life-year (QALY) gain and one for total healthcare and social sector costs. Probabilistic sensitivity analysis was used for each subgroup result in order to quantify the uncertainty around the cost-effectiveness results.
Results
The study concludes that, across the COPD severities, patients with severe COPD (GOLD 3 classification) are likely to be the most cost-effective group. This is primarily due to lower hospital-admission and primary-care costs. Telehealthcare for patients younger than 60 years is also more likely to be cost-effective than for older COPD patients. Overall, results indicate that existing resource patterns of patients and variations in delivery-site practices might have a strong influence on cost-effectiveness, possibly stronger than the included health or sociodemographic sources of heterogeneity.
Conclusion
Future research should focus more on sources of heterogeneity found in the implementation context and the way telehealthcare is adopted (eg, by integrating formative evaluation into cost-effectiveness analyses).
Trial registration
Keywords: COPD, telemonitoring, telehealth, health economics, heterogeneity, Denmark
Introduction
Trial-based evidence on the cost-effectiveness of telehealthcare for patients with chronic obstructive pulmonary disease (COPD) is accumulating.1–8 This evidence addresses whether or not the relative treatment effect of an intervention (ie, telehealthcare) compared to some alternative (ie, typically usual care) is worth any additional costs.9 Most of these studies have too few patients to make subgroup analysis meaningful, and the large-scale Whole System Demonstrator study has only reported main cost-effectiveness results from all included patients with diabetes, chronic heart failure, and COPD. Because patients are heterogeneous and have complex healthcare needs, cost-effectiveness is likely to vary with different baseline factors, such as health status, sociodemographic characteristics, or other baseline characteristics.10 Although some studies have reported a direct relationship between COPD severity and costs,11,12 there is very little knowledge of the relative cost-structures and heterogeneity of cost-effectiveness for patients with COPD that are specific for telehealthcare research.13
The recently reported Danish cluster-randomized trial of telehealthcare (Danish Telecare North Trial) among 1,225 patients with COPD reported additional costs, similar gain in quality-adjusted life-years (QALYs), and a relatively high incremental cost-effectiveness ratio (ICER) compared to international willingness-to-pay threshold (WTP) values, making the telehealthcare solution unlikely to be cost-effective.14 However, subgroups of patients with COPD within the trial could be more or less cost-effective, and these tendencies are important in order to explain the overall cost-effectiveness outcome, as input to decision-models such as the recently conducted study by Hofer et al15 or simply to create informed hypotheses for use in the design of future trial-based economic evaluations.
The objective of this article is to present cost-effectiveness results across a range of plausible subgroups in the cluster-randomized Danish Telecare North Trial. The subgroups are COPD severity in particular (classified according to the Global Initiative for Chronic Obstructive Lung Disease [GOLD]), but also three different comorbidities (coronary heart disease, diabetes, or mental health problems), gender, age, and existing resource patterns as well as delivery site.
Methods
The study protocol16 and the overall results from the economic evaluation14 have been published elsewhere, but a brief summary is provided in Table 1.
Table 1.
Overview of economic evaluation of the Danish Telecare North Trial
| Perspective of analysis | Healthcare sector and social care sector |
|---|---|
| Outcomes | Total costs per QALY gained |
| Compared alternatives | IG: a set of telehealthcare equipment and were monitored by a community-based healthcare team (based in social care sector). Patients also received disease-specific education |
| CG: usual care | |
| Duration of study | 12 months |
| Patients included | All patients with COPD that may benefit from telehealthcare. Fixed residence in North Denmark Region, Danish speaking, GSM coverage or phone line. No cognitive impairments. N=1,225 patients; 578 in IG and 647 in CG |
| Clusters | Municipality districts (social care sector), 26 clusters in total, 13 in each treatment arm. Each municipality had at least one district in both the intervention and control groups |
| Cost categories | Hospital admissions, outpatient visits including emergency contacts, primary-care contacts, prescribed medicine, personal care, practical help, at-home nursing care, and rehabilitation |
| Conclusion | Incremental QALYs was 0.0132 (rounded) |
| Incremental total costs were €728 (rounded) | |
| ICER was €55.327 per QALY gained | |
| Telehealthcare is unlikely to be cost-effective |
Abbreviations: QALY, quality-adjusted life-year; IG, intervention group; CG, control group; COPD, chronic obstructive pulmonary disease; GSM, global system for mobiles; ICER, incremental cost-effectiveness ratio.
Patients in the intervention group received a set of telehealthcare equipment and were monitored by a municipality- based healthcare team consisting primarily of nurses. Furthermore, patients received disease-specific education. The control group received usual care. In total, 1,225 patients satisfied the exclusion and inclusion criteria; 26 municipality districts across 10 different municipalities/delivery sites defined the randomization units (13 in each arm). These districts were matched, so that all municipalities/delivery sites contained municipality districts with patients who received telehealthcare or usual care16; 578 patients were randomized to telehealthcare, and 647 to usual care.
The primary outcome for the cost-effectiveness analysis was total healthcare and social sector costs per QALY gained. Costs included intervention costs, healthcare costs (patient-level hospital-, medicine-, and primary sector costs), and social sector costs (patient-level costs associated with practical help and care at home, home-based nursing care, and rehabilitation). The duration of the study was 12 months.16
No subgroup analyses were pre-defined in the trial protocol, but different baseline characteristics were collected as part of the trial. These included forced expiratory volume in 1 second (FEV1%) measured by the patients’ general practitioner. Presence of comorbidities was ascertained from questionnaires that were filled out by the patients’ general practitioner. Age and gender were identified from the patients’ social security number. Patient-level resource use from both within the trial period and 1 year prior to randomization was collected from medical registers by applying patients’ social security number. National-level patient data for all hospital contacts were collected from the Danish National Patient Register;17 all contacts between patients and the primary care sector from the National Health Insurance Service Register;18 and medication use was taken from The Danish Register of Medicinal Product Statistics.19 Patient-level community care service was taken from care systems in each of the 26 included municipality districts. Intervention costs included costs of hardware and peripherals, installation and deinstallation costs, maintenance and support costs, training costs for health care professionals, patient-specific training, monitoring costs, and project management costs. QALYs were calculated by linear interpolation of EQ5D-3L scores with Danish societal weights.20 More details on the data are described in the overall within-trial economic evaluation.14
The study was conducted in accordance with the Declaration of Helsinki. The trial has been presented to the Regional Ethical Committee for Medical Research in the North Denmark Region, where it was determined that no ethical approval was necessary. The trial has also been authorized by the Danish Data Protection Agency. All patients signed an informed consent form before taking part in the clinical trial. Trial registration: Clinicaltrials.gov, NCT01984840.
Statistical analysis
The statistical analysis employed in this article followed the analytical strategy from a cost-effectiveness article published previously.14 An intention-to-treat principle was applied. Missing data were assumed missing at random (MAR) and were imputed according to methodological guidelines.21
To allow for inclusion of particularly COPD severity-specific costs in future decision-modeling studies, the unadjusted cost structure across treatment alternatives was analyzed. These cost-structures are presented for each of the applied cost-categories mentioned earlier. Results are presented as means [standard deviation (SD)] and between-group differences are reported as raw mean difference and standardized difference (SMD = difference between randomization group averages/SD of the total sample) to allow for meta-analyses.
Estimation of incremental total costs and incremental QALYs in all subgroups was based on two separate linear mixed-effects models with treatment-by-covariate interactions. Total costs were controlled for relevant subgroup interaction on the treatment variable, baseline EQ5D score, baseline costs, age, baseline FEV1%, presence of musculo-skeletal disease, and clustering. Similarly, QALYs gained were controlled for the relevant subgroup interaction term on the treatment identifier, baseline EQ5D score, age, gender, baseline FEV1%, marital status, presence of diabetes, presence of cancer, and clustering. By applying the “mi estimate: xtmixed” command with robust standard errors in STATA12.1, a deterministic ICER estimate was calculated for each subgroup by linear combination of the relevant treatment beta-coefficients in both models. More details on the applied linear mixed-effects models are available in the Supplementary material. To quantify the uncertainty around these estimates, a series of probabilistic sensitivity analyses were conducted. The output from both models was exported to Microsoft Excel 2010 along with Cholesky’s decomposition matrix, and 5,000 new parameter estimates from the analytical models from normal distributions were drawn. Estimates of incremental QALYs and incremental total costs were created to present the probability that telehealthcare was cost-effective as a function of decision-makers; WTP for extra QALYs. The probabilities that telehealthcare is cost-effective are presented at €25,000 and €40,000, which is roughly the threshold values applied in the UK (1€=0.73 £).
Results
Complete data for both total costs (ie, all cost-categories) and EQ5D scores at baseline and follow-up were available for 751 patients (61%; 325 in the telehealthcare group; 426 in the control group). Incomplete data stemmed primarily from non-response or from incomplete registration of EQ5D questionnaire items (8% had missing EQ5D summary scores at baseline; 27% at follow-up); 12% had missing values on one cost-category – rehabilitation; 103 patients died during the trial period (8%).
The telehealthcare and usual care group were similar at baseline (Table 2). The general tendency is that patients in the intervention group have slightly worse health (higher proportion of patients with severe COPD [GOLD 3], more comorbidities, and greater resource use in municipalities prior to randomization). There were also more men in the telehealthcare group. Between-group difference across delivery sites was expected given the randomization procedure.
Table 2.
Baseline characteristics
| Subgroup category | Telehealthcare | Usual care | Difference | Fisher’s exact test |
|---|---|---|---|---|
|
| ||||
| n=578 | n=647 | Raw | P-value | |
| COPD severity (GOLD classification 1–4) | ||||
| Mild, GOLD 1 | 3.98 (n=23) | 4.64 (n=30) | −0.66 | |
| Moderate, GOLD 2 | 31.49 (n=182) | 31.07 (n=201) | 0.42 | |
| Severe, GOLD 3 | 32.18 (n=186) | 30.60 (n=198) | 1.58 | 0.937 |
| Very severe, GOLD 4 | 13.84 (n=80) | 13.91 (n=90) | −0.07 | |
| Missing | 18.51 (n=107) | 19.78 (n=128) | −1.27 | |
| Comorbidities | ||||
| Coronary heart disease | 32.70 (n=189) | 31.84 (n=206) | 0.86 | 0.927 |
| Diabetes | 10.21 (n=59) | 9.89 (n=64) | 0.32 | 0.962 |
| Mental health problem | 4.84 (n=28) | 4.79 (n=31) | 0.05 | 0.991 |
| Missing | 8.13 (n=47) | 7.88 (n=51) | 0.25 | |
| Age§ | ||||
| <60 years | 16.78 (n=97) | 14.68 (n=95) | 2.10 | |
| 60–69 years | 34.08 (n=197) | 31.68 (n=205) | 2.40 | 0.445 |
| 70–79 years | 36.33 (n=210) | 40.19 (n=260) | −3.86 | |
| ≥80 years | 12.80 (n=74) | 13.45 (n=87) | −0.65 | |
| Men§ | 48.27 (n=279) | 43.74 (n=283) | 4.53 | 0.063 |
| Existing resource pattern | ||||
| No resource use in social sector 12 months prior to randomization | 52.25 (n=302) | 57.50 (n=372) | −5.25 | |
| Resource use in social sector 12 months prior to randomization | 39.62 (n=229) | 36.32 (n=235) | 3.30 | 0.136 |
| Missing | 8.13 (n=47) | 6.18 (n=40) | 1.95 | |
| Delivery site | ||||
| Delivery site 1 | 8.13 (n=47) | 6.18 (n=40) | 1.95 | |
| Delivery site 2 | 16.09 (n=93) | 4.33 (n=28) | 11.76 | |
| Delivery site 3 | 10.73 (n=62) | 9.58 (n=62) | 1.15 | |
| Delivery site 4 | 5.54 (n=32) | 5.10 (n=33) | 0.44 | |
| Delivery site 5 | 6.40 (n=37) | 7.88 (n=51) | −1.48 | 0.000 |
| Delivery site 6 | 3.46 (n=20) | 4.17 (n=27) | −0.71 | |
| Delivery site 7 | 4.50 (n=26) | 4.33 (n=28) | 0.17 | |
| Delivery site 8 | 15.40 (n=89) | 6.49 (n=42) | 8.91 | |
| Delivery site 9 | 10.55 (n=61) | 10.05 n=65) | 0.50 | |
| Delivery site 10 | 19.30 (n=111) | 41.89 (n=271) | −22.59 | |
Note: Data are presented as percentages (number of patients).
Variable has no missing values.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Subgroups defined by COPD severity
The unadjusted cost-structure across GOLD classifications is presented in Table 3. For patients with mild COPD (GOLD 1), telehealthcare was associated with higher total costs (raw mean difference €2401) giving rise to an SMD of 23.37%. This was primarily driven by higher social sector costs – that is, help and care at home (raw mean difference €513; SMD 12.82%), home nursing care (raw mean difference €1380; SMD 26.21%), and rehabilitation (raw mean difference €89; SMD 35.85%). Patients in the telehealthcare group had fewer costs due to primary-care visits (raw mean difference −€123, standardized between-group difference; −25.01%).
Table 3.
Average costs (€) per patient across treatment groups at 12-month follow-up for all COPD severities
| Cost categories across COPD severities | Telehealthcare
|
Usual care
|
Between-group difference
|
|
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Raw mean | Standardized (%)* | |
| Mild COPD (GOLD 1) | ||||
| Hospital contacts | ||||
| Admissions | 768 (4879) | 813 (2449) | −45.16 | −1.22 |
| Outpatient/emergency department visits | 111 (291) | 97 (274) | 14.58 | 5.17 |
| Primary-care contacts | 602 (420) | 725.7 (535) | −123.32 | −25.01 |
| Municipality-care contacts | ||||
| Help and care at home | 1174 (5128) | 661 (2868) | 512.96 | 12.82 |
| Home nursing care | 1857 (7782) | 477 (1524) | 1379.50 | 26.21 |
| Rehabilitation§ | 106 (325) | 17 (157) | 88.90 | 35.85 |
| Medicine | 1025 (714) | 1085.3 (726) | −60.85 | −8.43 |
| Total service costs (excluding intervention costs) | 5645 (14486) | 3878 (6079) | 1766.58 | 17.22 |
| Total costs (including intervention costs) | 6280 (14464) | 3878 (6079) | 2401.58 | 23.37 |
| Moderate COPD (GOLD 2) | ||||
| Hospital contacts | ||||
| Admissions | 1299 (5369) | 811 (2653) | 488.67 | 11.72 |
| Outpatient/emergency department visits | 194 (450) | 123 (348) | 71.80 | 17.90 |
| Primary-care contacts | 618 (397) | 616 (475) | 1.93 | 0.44 |
| Municipality-care contacts | ||||
| Help and care at home | 946 (3495) | 1132 (4168) | −186.12 | −4.81 |
| Home nursing care | 625 (2893) | 388 (2111) | 236.36 | 9.40 |
| Rehabilitation§ | 50 (265) | 24 (191) | 25.56 | 11.14 |
| Medicine | 1312 (856) | 1240 (795) | 71.79 | 8.70 |
| Total service costs (excluding intervention costs) | 5045 (8992) | 4334 (7796) | 709.98 | 8.46 |
| Total costs (including intervention costs) | 5759 (8988) | 4334 (7796) | 1424.25 | 16.94 |
| Severe COPD (GOLD 3) | ||||
| Hospital contacts | ||||
| Admissions | 2686 (8818) | 4116 (16371) | −1429.47 | −10.73 |
| Outpatient/emergency department visits | 347 (564) | 346 (583) | 0.92 | 0.16 |
| Primary-care contacts | 602 (460) | 679 (582) | −76.39 | −14.46 |
| Municipality-care contacts | ||||
| Help and care at home | 1990 (5654) | 1968 (5934) | 22.58 | 0.39 |
| Home nursing care | 680 (1805) | 638 (2559) | 42.72 | 1.92 |
| Rehabilitation§ | 116 (462) | 106 (533) | 10.64 | 2.13 |
| Medicine | 1722 (855) | 1721 (1035) | 0.54 | 0.06 |
| Total service costs (excluding intervention costs) | 8144 (11826) | 9572 (19000) | −1428.47 | −8.94 |
| Total costs (including intervention costs) | 8855 (11807) | 9572 (19000) | −716.92 | −4.49 |
| Very severe COPD (GOLD 4) | ||||
| Hospital contacts | ||||
| Admissions | 6670 (21124) | 4619 (12368) | 2051.10 | 12.02 |
| Outpatient/emergency department visits | 728 (801) | 527 (737) | 200.35 | 25.88 |
| Primary care contacts | 571 (422) | 518 (408) | 53.01 | 12.76 |
| Municipality care contacts | ||||
| Help and care at home | 4202 (9588) | 1340 (3435) | 2862.11 | 39.93 |
| Home nursing care | 775 (2475) | 616 (1934) | 158.84 | 7.20 |
| Rehabilitation§ | 133 (422) | 57 (246) | 75.43 | 22.04 |
| Medicine | 2176 (1659) | 1863 (946) | 312.54 | 23.36 |
| Total service costs (excluding intervention costs) | 15255 (24517) | 9541 (14497) | 5713.38 | 28.52 |
| Total costs (including intervention costs) | 15941 (24506) | 9541 (14497) | 6399.81 | 31.87 |
Note:
Standardized difference, difference between randomization group averages divided by the standard deviation (SD) of the total sample.
Imputed data.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Telehealthcare was associated with higher total costs for patients with moderate COPD (GOLD 2; raw mean difference €1424; SMD 16.94%). The telehealthcare group had higher costs due to hospital admissions (raw mean difference €489; SMD 11.72%) and home nursing care (raw mean difference €236; SMD 9.40%). There were fewer costs due to help and care at home (raw mean difference −€186; SMD −4.81%).
Total costs for patients with very severe COPD (GOLD 4) was associated with higher total costs in the telehealthcare group (raw mean difference €6400; SMD 31.87%). Higher costs were accrued across all cost categories.
In contrast to other COPD severities, patients with severe COPD (GOLD 3) had fewer total costs in the telehealthcare group (raw mean difference −€717; SMD −4.49%). This was driven by cost savings in hospital admissions (raw mean difference −€1429; SMD −10.73%) and primary sector contacts (raw mean difference −€76; SMD −14.46%).
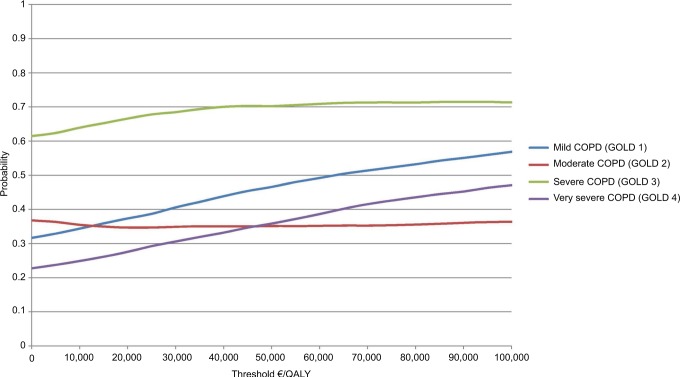
From Table 4, the ICER point estimates indicate that the telehealthcare intervention was dominant for patients with severe COPD (GOLD 3), with a probability of achieving cost-effectiveness of 68% at a WTP threshold of €25,000 and 70% at €40,000. The probability that telehealthcare is cost-effective remains consistently lower across different levels of WTP threshold values for other COPD severities (Figure 1). Usual care was dominant for moderate COPD (GOLD 2), with a probability of achieving cost-effectiveness of 35% at a WTP threshold of €25,000 and 35% at €40,000.
Table 4.
Cost-effectiveness of telehealthcare compared to usual care across COPD severities
| COPD severity (GOLD classification 1–4)# | QALY | Wald test* | Total costs (€) | Wald test* | ICER | Pr(cost-effective) | Pr(cost-effective) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Between group difference (95% CI) | P-value | Between group difference (95% CI) | P-value | (€ per QALY) | at €25,000 | at €40,000 | |
| Mild, GOLD 1 (6%) | 0.0281 (–0.0551; 0.1113) | 0.4131 | 1872 (–5337; 9082) | 0.3998 | 66,577 | 39% | 44% |
| Moderate, GOLD 2 (38%) | –0.0113 (–0.0469; 0.0243) | 1455 (–94; 3003) | UC dominant | 35% | 35% | ||
| Severe, GOLD 3 (39%) | 0.0302 (–0.0075; 0.0678) | –964 (–3750; 1822) | THC dominant | 68% | 70% | ||
| Very severe, GOLD 4 (17%) | 0.0229 (–0.0261; 0.0719) | 2959 (–2087; 8005) | 129,035 | 29% | 33% | ||
Note:
Wald test for interaction between treatment and the subgroup variables.
QALYs and total costs were not simultaneously controlled for baseline FEV1%. All results are imputed.
Abbreviations: QALY, quality-adjusted life-years; COPD, chronic obstructive pulmonary disease; UC, usual care; THC, telehealthcare; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICER, incremental cost-effectiveness ratio; Pr, probability.
Figure 1.
Probability of telehealthcare being cost-effective, depending on the severity of COPD (by GOLD classification).
Abbreviations: COPD, chronic obstructive pulmonary disease; QALY, quality-adjusted life-years; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Subgroups defined by comorbidities, gender, age, resource patterns, and delivery site
In Table 5, the ICER point estimate is dominant for telehealthcare for patients younger than 60 years, with a probability of achieving cost-effectiveness of 69% at a WTP threshold of €25,000 and 68% at €40,000. A relatively high probability of achieving cost-effectiveness is present for patients aged 80 and older (62% at a WTP threshold of €25,000 and 71% at €40,000). Telehealthcare is dominant and has a high probability of achieving cost-effectiveness, given the data for the subgroup with patients having resource use (practical help, home nursing care, and rehabilitation) in the municipalities at some point 12 months prior to randomization (89% at a WTP threshold of €25,000 and 89% at €40,000). Across municipalities/delivery sites, there are large variations in cost-effectiveness with probabilities of cost-effectiveness ranging from low probabilities of cost-effectiveness (eg, 0%) to very high (eg, 100%) at the applied WTP threshold values. This is primarily due to cost savings.
Table 5.
Cost-effectiveness of telehealthcare compared to usual care in subgroups
| Subgroup category | QALY | Wald test* | Total costs (€) | Wald test* | ICER | Pr(cost-effective) | Pr(cost-effective) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Between group difference (95% CI) | P-value | Between group difference (95% CI) | P-value | (€ per QALY) | at €25,000 | at €40,000 | |
| Comorbidities | |||||||
| Coronary heart disease | |||||||
| No (65%) | 0.0168 (–0.0059; 0.0394) | 0.636 | 445 (–1604; 2493) | 0.566 | 26,527 | 49% | 57% |
| Yes (35%) | 0.0068 (–0.0315; 0.0451) | 1290 (–650; 3229) | 189,373 | 28% | 31% | ||
| Diabetes | |||||||
| No (89%) | 0.0112 (–0.0116; 0.3402) | 0.577 | 309 (–1340; 1957) | 0.182 | 27,573 | 49% | 56% |
| Yes (11%) | 0.0294 (–0.0312; 0.090) | 4731 (–1085; 10547) | 160,728 | 8% | 12% | ||
| Mental health problem | |||||||
| No (95%) | 0.0130 (–0.0094; 0.0354) | 0.911 | 772 (–770; 2314) | 0.815 | 59,378 | 29% | 39% |
| Yes (5%) | 0.0178 (–0.0627; 0.0983) | 38 (–5837; 5913) | 2,135 | 55% | 58% | ||
| Gender | |||||||
| Female (54%) | 0.0052 (–0.0172; 0.0276) | 0.347 | 407 (–1471; 2286) | 0.573 | 77,890 | 39% | 42% |
| Male (46%) | 0.0225 (–0.0108; 0.0557) | 1122 (–888; 3132) | 49,917 | 44% | 55% | ||
| Age## | |||||||
| <60 years (16%) | 0.0046 (–0.0403; 0.0495) | 0.692 | –560 (–2972; 1851) | 0.707 | THC dominant | 69% | 68% |
| 60–69 years (33%) | 0.0009 (–0.0322; 0.0340) | 1622 (–1170; 4415) | 1,764,487 | 22% | 25% | ||
| 70–79 years (38%) | 0.01607 (–0.0211; 0.0532) | 528 (–1848; 2903) | 32,845 | 47% | 51% | ||
| ≥80 years (13%) | 0.0489 (–0.0202; 0.1180) | 484 (–2117; 3085) | 9,900 | 62% | 71% | ||
| Patients with previous resource use in social sector | |||||||
| No resource use in social sector 12 months prior to randomization (64%) | 0.0180 (–0.0054; 0.0414) | 0.894 | 1396 (290; 2503) | 0.228 | 77,622 | 7% | 18% |
| Resource use in social sector 12 months prior to randomization (36%) | 0.0143 (–0.0322; 0.0607) | –1205 (–5144; 2734) | THC dominant | 89% | 89% | ||
| Delivery site | |||||||
| Delivery site 1 (7%) | 0.0675 (0.0357; 0.0993) | 0.102 | 3952 (2952; 4952) | 0.000 | 58,520 | 0% | 6% |
| Delivery site 2 (10%) | –0.0033 (–0.0311; 0.0245) | 3375 (1870; 4881) | UC dominant | 1% | 3% | ||
| Delivery site 3 (10%) | 0.0033 (–0.0712; 0.0779) | –12 (–2344; 2319) | THC dominant | 55% | 56% | ||
| Delivery site 4 (5%) | 0.0397 (0.0061; 0.0732) | –290 (–1026; 446) | THC dominant | 90% | 93% | ||
| Delivery site 5 (7%) | –0.0007 (–0.0322; 0.0308) | –981 (–2985; 1023) | 1,389,780 | 71% | 70% | ||
| Delivery site 6 (4%) | 0.0308 (–0.0117; 0.0733) | –9138 (–12087; –6188) | THC dominant | 100% | 100% | ||
| Delivery site 7 (4%) | 0.0193 (–0.0171; 0.0557) | 262 (–248; 772) | 13,549 | 58% | 64% | ||
| Delivery site 8 (11%) | 0.0383 (–0.0027; 0.0792) | –2545 (–2848; –2242) | THC dominant | 100% | 100% | ||
| Delivery site 9 (10%) | 0.0160 (–0.0032; 0.0353) | 416 (72; 759) | 25,921 | 51% | 59% | ||
| Delivery site 10 (32%) | 0.0028 (–0.0379; 0.0435) | 1062 (94; 2029) | 376,794 | 19% | 26% | ||
Note:
Wald test for interaction between treatment and the subgroup variable.
QALYs and total costs were not simultaneously controlled for age. All results are imputed.
Abbreviations: QALY, quality adjusted life year; COPD, chronic obstructive pulmonary disease; UC, usual care; THC, telehealthcare; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICER, incremental cost-effectiveness ratio; Pr, probability.
Furthermore, from Table 5, it can be seen that no dominant courses of action can be found for comorbidities or gender. There is a tendency for coronary heart disease and diabetes to reduce the likelihood of achieving cost-effectiveness of telehealthcare (ICER point estimates increases from €26,527 per QALY with no comorbidities to €189,373 per QALY with coronary heart disease, and from €27,573 per QALY to €160,724 per QALY with diabetes). Telehealthcare for patients with mental illness, on the other hand, seems more likely to be cost-effective, since the ICER point estimate changes from €59,378 per QALY without mental illness to €2,135 per QALY with mental health problems. The results also contain tendencies for telehealthcare to men to be more cost-effective than for women (ICER €49,917 per QALY for men and €77,890 per QALY for women), because they achieve a higher QALY gain, albeit also higher costs. But uncertainties, particularly surrounding gender, are large.
Discussion
Based on the trial-based economic evaluation in the cluster-randomized Danish Telecare North Trial,14 this subgroup analysis demonstrates no statistically significant differences in incremental QALYs and incremental total costs, except for costs across municipalities/delivery sites. The tendency is that incremental QALYs are small and positive across subgroups (except for patients with moderate COPD [GOLD 2] and for patients in some delivery sites). Therefore, cost-effectiveness results in subgroups mostly reflect that cost savings have occurred here.
Telehealthcare for patients with severe COPD (GOLD 3) is more likely to be cost-effective than in other COPD severities. This was primarily driven by cost savings in hospital admissions and primary-care contacts. Telehealthcare for patients with other COPD severities is less likely to be cost-effective due to higher total costs. Results also indicate that existing resource patterns of patients and delivery site might have a strong influence on cost-effectiveness, possibly stronger than the included health or sociodemographic characteristics. Furthermore, telehealthcare for patients younger than 60 years is more likely to be cost-effective. No firm cost-effectiveness conclusions could be made of the included comorbidities and gender.
Strengths and limitations
To date, there is almost no knowledge of heterogeneity in cost-effectiveness for COPD patients in telehealthcare research. This study has sought to nuance the available evidence by presenting incremental costs, incremental QALYs, and the uncertainty around these estimates in a set of subgroups from an economic evaluation alongside a relatively large clinical trial. These subgroup analyses follow analytical good practices when presenting heterogeneity analyses in cost-effectiveness research by presenting treatment-by-covariate interaction from a single clinical trial22 that makes use of patient-level data routinely captured in Danish registers. We have also quantified the uncertainty surrounding the cost and QALY estimates by probabilistic sensitivity analysis.22
On the other hand, if the study should be used for inferential purposes, it is a limitation that analyses were conducted post hoc and that there is no statistical power to conclude that the differences found are no more than random noise in the data. However, this is a weakness that is shared with most cost-effectiveness studies conducted alongside clinical trials, which are usually only powered to test differences in some clinical measure. Another limitation of the study is that only 61% of the participants had complete registrations of all cost categories and EQ5D summary scores.
Comparison with other studies
Early studies on cost-effectiveness of patients with COPD have focused on patients with severe or very severe COPD (GOLD 3 and GOLD 4).1–6 They all demonstrate a potential for cost savings without sacrificing effect, although the methodological quality is rather low.23 This study included patients with all GOLD 1–4 severities and pinpoints that telehealthcare for GOLD 3 classified patients is more likely to be cost-effective and even potentially cost saving. Furthermore, a recently published economic evaluation for GOLD 3 patients concluded that telehealthcare was not likely to be cost-effective, except maybe for patients without comorbidities.24 Although the uncertainty around this conclusion is high, the fact that the absence of comorbidities is important for achieving cost-effectiveness – depending on the type of comorbidity – is also indicated in this study. Another recently published economic evaluation concluded that telehealthcare was unlikely to be cost-effective for the patients with COPD who were included.8 The study included 256 patients with all COPD severities, but only 34% patients with severe COPD. Moreover, 68% of subjects had one or more comorbidities. Applying the conclusions from this subgroup analysis might explain this result.
Implications for clinicians and decision-makers
A major challenge in assessing telehealth is that its adoption may give rise to various organizational impacts.25 Cost-effectiveness may depend on how it is embodied in existing healthcare delivery practices (eg, differences in healthcare practices or motivation and experiences of caregivers and patients). This study indicates that patient’s existing resource pattern is important for achieving cost-effectiveness. A plausible reason could be that, if healthcare professionals responsible for monitoring the patients are unfamiliar with a particular patient’s history or exacerbation behavior, telehealthcare might be at risk of being an add-on to usual care and not a substitute, because it is difficult for them to evaluate whether a patient is in need of hospital admission during an exacerbation or if the exacerbation could be handled in another more cost-effective way. Furthermore, when telehealthcare is implemented, patients could become more aware of their disease, or delivery sites could discover patients with COPD that had an unmet need for treatment and care that would not have been discovered otherwise. In our study, this could explain the probabilities of cost-effectiveness for patients with or without resource use in municipalities prior to randomization. This would mean that the implementation context of telehealthcare is important for achieving cost-effectiveness. Variations in practices, workflow, and management attention across healthcare delivery sites are also plausible,26,27 but not quantified in this study.
Future studies
Despite more than two decades of research, it is still not possible unequivocally to identify which types of telehealth-care technologies would be cost-effective for certain patient types.28 One possible reaction is to suggest that it is “time to pause” the widespread application of telehealthcare until well-designed longer term multicenter studies with appropriate follow-up (ie, continue summative evaluation but possibly with more ambitious or complicated analytical designs) have proven the benefits of the technology.29 Another reaction is to focus more on the context of implementation by seeking to integrate formative evaluation designs in cost-effectiveness analyses. Future research should focus more on contextual and/or implementation factors for telehealthcare adoption – for example, behavior and engagement of patients and health professionals; how organizational cultures, incentive systems, and management support the adoption of telehealthcare; or how telehealthcare could be embedded in existing workflows. This work should be focused on explaining how context and implementation factors are related to differences in included cost-categories or perceptions of health-related quality of life in order to achieve cost-effectiveness.
Some contexts and implementation factors may be difficult to identify or define a priori. In fact, one could argue that valuable information would be lost if post hoc subgroup analyses are not conducted due to clinical research practices and the fear of data-dredging. That a heterogeneity analysis has been conducted post hoc must, therefore, not routinely be an obstacle for learning more about the consequences of implementing medical technologies such as telehealthcare.
Supplementary material
Details of the applied linear mixed-effects models
This study applied linear mixed-effects models, which are also known in the literature as random effects models, multilevel models, or hierarchical linear models. Linear mixed-effects models are used to analyze data that have a hierarchical or nested structure1 (eg, patients nested in hospitals, repeated measurements taken from the same in individuals, or, as in this case, patients within municipality districts).
Linear mixed-effects models can be used in cost-effectiveness research conducted alongside cluster-randomized trials (ie, where randomization is conducted at a different level than the individual patients – in this case, municipality districts),2–4 because it is plausible that resource consumption or costs, health-related quality of life, or death to a certain degree, might be more similar within clusters than they are across clusters. This cluster effect must be taken into account in order to obtain valid parameter estimates and standard errors due to the violation of the independence assumption between observations in ordinary least squares regression.1–3
Mixed models contain both fixed effects and random effects. Fixed effects are analogous to standard OLS regression coefficients and are estimated directly. However, random effects are not directly estimated but are summarized according to the variance–covariance structure of the model.1
A basic two-level linear mixed-effects model with two covariates for both total costs and QALY gain with treatment-by-covariate interaction is presented below:
Where i is the patient identification number; j the cluster identification number; β0 and γ0 are model intercepts, and Tj the treatment indicator (Tj = 0 for clusters in control group; Tj = 1 for clusters in intervention group). γ1 and β1 are incremental total costs and incremental QALYs; Zij is the covariate variable used in the particular subgroup analysis; Xij is an additional covariate, rj and sj are random components, which represent the differences in the cluster mean costs and outcomes from the overall means in each treatment group, and, finally, εij and uij are error terms to the model that are assumed normally distributed.
In our study, this basic tow-level model was expanded with the mentioned additional covariates to allow for baseline adjustment of the treatment effects found in each subgroup analysis (total costs models were controlled for baseline EQ5D-score, baseline costs, age, baseline FEV1%, presence of musculoskeletal disease, and clustering; QALY models were controlled for baseline EQ5D score, age, gender, baseline FEV1%, marital status, presence of diabetes, presence of cancer, and clustering).
In STATA 12.1 the xtmixed procedure can be used to fit linear mixed models by maximum likelihood estimation.5
A reference category in each subgroup was used, and treatment effects for other subgroup categories were found by linear combination of treatment effects within subgroups. A deterministic incremental cost-effectiveness ratio (ICER) is then found by dividing each treatment effect found in the totalcost regression (γ1) with each treatment effect found in the QALY regression (β1).
References
- 1.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata Volume I: Continuous Responses. 3rd ed. Texas: Stata Press; 2012. [Google Scholar]
- 2.Gomes M, Grieve R, Nixon R, Ng ES, Carpenter J, Thompson SG. Methods for covariate adjustment in cost-effectiveness analysis that use cluster randomised trials. Health Econ. 2012;21(9):1101–1118. doi: 10.1002/hec.2812. [DOI] [PubMed] [Google Scholar]
- 3.Gomes M, Ng ES, Grieve R, Nixon R, Carpenter J, Thompson SG. Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med Decis Making. 2012;32(2):350–361. doi: 10.1177/0272989X11418372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann MO, Fairall L, Clark A, Mugford M. Methods for analyzing cost effectiveness data from cluster randomized trials. Cost Eff Resour Alloc. 2007;5(Icc):12. doi: 10.1186/1478-7547-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stata Press xtmixed: Multilevel mixed-effects linear regression. Stata 12 User’s Guide. 2012. [Last accessed June 17, 2017]. pp. 302–354. Available from: http://www.stata-press.com/data/r12/
Acknowledgments
This study was funded by the North Denmark Region, the 11 municipalities in North Denmark Region, The Obel Family Foundation, the Danish Agency for Digitalization Policy and Strategy, the European Social Fund, and Aalborg University.
Footnotes
Author contributions
All authors made substantial contributions to data analysis, drafting and critical revision of the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.de Toledo P, Jiménez S, del Pozo F, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Trans Inf Technol Biomed. 2006;10(3):567–573. doi: 10.1109/titb.2005.863877. [DOI] [PubMed] [Google Scholar]
- 2.Haesum LK, Soerensen N, Dinesen B, et al. Cost-utility analysis of a telerehabilitation program: a case study of COPD patients. Telemed J E Health. 2012;18(9):688–692. doi: 10.1089/tmj.2011.0250. [DOI] [PubMed] [Google Scholar]
- 3.Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33(5):1031–1038. doi: 10.1183/09031936.00063108. [DOI] [PubMed] [Google Scholar]
- 4.Paré G, Poba-Nzaou P, Sicotte C, et al. Comparing the costs of home telemonitoring and usual care of chronic obstructive pulmonary disease patients: a randomized controlled trial. Eur Res Telemed. 2013;2(2):35–47. [Google Scholar]
- 5.Paré G, Sicotte C, St-Jules D, Gauthier R. Cost-minimization analysis of a telehomecare program for patients with chronic obstructive pulmonary disease. Telemed J E Health. 2006;12(2):114–121. doi: 10.1089/tmj.2006.12.114. [DOI] [PubMed] [Google Scholar]
- 6.Vitacca M, Bianchi L, Guerra A, et al. Tele-assistance in chronic respiratory failure patients: a randomised clinical trial. Eur Respir J. 2009;33(2):411–418. doi: 10.1183/09031936.00005608. [DOI] [PubMed] [Google Scholar]
- 7.McDowell JE, McClean S, FitzGibbon F, Tate S. A randomised clinical trial of the effectiveness of home-based health care with telemonitoring in patients with COPD. J Telemed Telecare. 2015;21(2):80–87. doi: 10.1177/1357633X14566575. [DOI] [PubMed] [Google Scholar]
- 8.Stoddart A, van der Pol M, Pinnock H, et al. Telemonitoring for chronic obstructive pulmonary disease: a cost and cost-utility analysis of a randomised controlled trial. J Telemed Telecare. 2015;21(2):108–118. doi: 10.1177/1357633X14566574. [DOI] [PubMed] [Google Scholar]
- 9.Sculpher M. Reflecting heterogeneity in patient benefits: the role of subgroup analysis with comparative effectiveness. Value Health. 2010;13(Suppl 1):S18–S21. doi: 10.1111/j.1524-4733.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 10.Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ. 2004;13(5):461–475. doi: 10.1002/hec.843. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of COPD -2016. 2016. [Last accessed June 22, 2017]. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 12.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 13.McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(7):1–52. doi: 10.1002/14651858.CD007718.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udsen FW, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish “TeleCare North” cluster-randomized trial. BMJ Open. 2017;7(5):e014616. doi: 10.1136/bmjopen-2016-014616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer F, Achelrod D, Stargardt T. Cost-utility analysis of telemonitoring interventions for patients with chronic obstructive pulmonary disease (COPD) in Germany. Appl Health Econ Health Policy. 2016;14(6):691–701. doi: 10.1007/s40258-016-0267-9. [DOI] [PubMed] [Google Scholar]
- 16.Udsen FW, Lilholt PH, Hejlesen O, Ehlers LH. Effectiveness and cost-effectiveness of telehealthcare for chronic obstructive pulmonary disease: study protocol for a cluster randomized controlled trial. Trials. 2014;15:178. doi: 10.1186/1745-6215-15-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Danish Health Data Board Landspatientregisteret - Borgernes kontakter til sygehusene [The National Patient Register] [Last accessed June 17, 2017]. Available from: http://www.esundhed.dk/sundhedsregistre/LPR/Sider/LPR.aspx. Danish.
- 18.The Danish Health Data Board Dokumentation af registrene [The National Health Insurance Service Register] [Last accessed June 17, 2017]. Available from: http://www.esundhed.dk/dokumentation/Registre/Sider/Register.aspx. Danish.
- 19.The Danish Health Data Board Lægemiddelstatistik - Forbrug af lægemidler i Danmark [The Register of Medicinal Product Statistics] [Last accessed June 17, 2017]. Available from: http://esundhed.dk/sundhedsregistre/LSR/Sider/LSR.aspx. Danish.
- 20.Wittrup-Jensen KU, Lauridsen J, Gudex C, Pedersen KM. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health. 2009;37(5):459–466. doi: 10.1177/1403494809105287. [DOI] [PubMed] [Google Scholar]
- 21.Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–1170. doi: 10.1007/s40273-014-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sculpher M. Subgroups and heterogeneity in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):799–806. doi: 10.2165/00019053-200826090-00009. [DOI] [PubMed] [Google Scholar]
- 23.Udsen F, Hejlesen O, Ehlers L. A systematic review of the cost and cost-effectiveness of telehealthcare to patients suffering from chronic obstructive pulmonary disease. J Telemed Telecare. 2014;20(4):212–220. doi: 10.1177/1357633X14533896. [DOI] [PubMed] [Google Scholar]
- 24.Jódar-Sánchez F, Ortega F, Parra C, et al. Cost-utility analysis of a telehealth programme for patients with severe chronic obstructive pulmonary disease treated with long-term oxygen therapy. J Telemed Telecare. 2014;20(6):307–316. doi: 10.1177/1357633X14544421. [DOI] [PubMed] [Google Scholar]
- 25.Lamothe L, Fortin JP, Labbé F, Gagnon MP, Messikh D. Impacts of telehomecare on patients, providers, and organizations. Telemed J E Health. 2006;12(3):363–369. doi: 10.1089/tmj.2006.12.363. [DOI] [PubMed] [Google Scholar]
- 26.Skinner J. Causes and consequences of regional variations in health Care. In: Pauly MV, Mcguire TG, Barros PP, editors. Handbook of Health Economics. Vol. 2. Oxford; New York: 2012. pp. 45–93. [Google Scholar]
- 27.Krumholz HM. Variations in health care, patient preferences, and high-quality decision making. JAMA. 2013;310(2):151–152. doi: 10.1001/jama.2013.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wootton R. Twenty years of telemedicine in chronic disease management—an evidence synthesis. J Telemed Telecare. 2012;18(4):211–220. doi: 10.1258/jtt.2012.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein RS, O’Hoski S. Telemedicine in COPD: time to pause. Chest. 2014;145(5):945–949. doi: 10.1378/chest.13-1656. [DOI] [PubMed] [Google Scholar]