Abstract
Objectives:
HIV and herpes simplex virus type 2 (HSV-2) infections are sexually transmitted and propagate in sexual networks. Using mathematical modeling, we aimed to quantify effects of key network statistics on infection transmission, and extent to which HSV-2 prevalence can be a proxy of HIV prevalence.
Design/methods:
An individual-based simulation model was constructed to describe sex partnering and infection transmission, and was parameterized with representative natural history, transmission, and sexual behavior data. Correlations were assessed on model outcomes (HIV/HSV-2 prevalences) and multiple linear regressions were conducted to estimate adjusted associations and effect sizes.
Results:
HIV prevalence was one-third or less of HSV-2 prevalence. HIV and HSV-2 prevalences were associated with a Spearman's rank correlation coefficient of 0.64 (95% confidence interval: 0.58–0.69). Collinearities among network statistics were detected, most notably between concurrency versus mean and variance of number of partners. Controlling for confounding, unmarried mean/variance of number of partners (or alternatively concurrency) were the strongest predictors of HIV prevalence. Meanwhile, unmarried/married mean/variance of number of partners (or alternatively concurrency), and clustering coefficient were the strongest predictors of HSV-2 prevalence. HSV-2 prevalence was a strong predictor of HIV prevalence by proxying effects of network statistics.
Conclusion:
Network statistics produced similar and differential effects on HIV/HSV-2 transmission, and explained most of the variation in HIV and HSV-2 prevalences. HIV prevalence reflected primarily mean and variance of number of partners, but HSV-2 prevalence was affected by a range of network statistics. HSV-2 prevalence (as a proxy) can forecast a population's HIV epidemic potential, thereby informing interventions.
Keywords: epidemiology, herpes simplex virus type 2, HIV, mathematical model, sexual behavior, sexual network
Introduction
Acquisition of a sexually transmitted infection (STI) depends on the probability of engaging in a sexual partnership with an STI-infected individual, being susceptible to the infection, and the efficiency of transmission in a sex act [1,2]. Even though STIs share the same mode of transmission, the nature of sexual behavior may favor transmission of specific STIs over others [1–6]. Moreover, risk of STI acquisition does not depend only on an individual's behavior, but on the ‘ecology’ of the individual within the sexual network, that is the partner and partner's partners behavior [7–11].
The combination of an individual's partners and their partners’ partners forms a small part of a bigger sexual network that connects individuals (directly or indirectly) through their present or past partnerships [1,8]. Networks play an essential role in STI patterns; individuals who engage in serial monogamous partnerships are probably less likely to spread STIs than those who engage in concurrent partnerships [11,12]. STIs’ natural history and infectiousness profile also contribute to the observed variability in STI epidemiology [5].
STI epidemiology is, in principle best studied by collecting and analyzing data from biobehavioral surveys and case–control and longitudinal studies. Although these remain a standard practice, their implementation and cost is challenging. They involve investigation of sensitive behaviors, are logistically difficult, and require costly biological assays and sophisticated technologies to ascertain current infection or previous exposure [1]. In addition to selection bias in study participation, social desirability bias can result in distorted and uninterpretable findings [13]. As individuals are not likely to know the partners of their partners, there are also inherent informational limitations in individual egocentric data [14,15].
With these challenges to empirical data, we used in-silico simulations of HIV and herpes simplex virus type 2 (HSV-2) transmission in a population to explore ‘microscopically’ the link between sexual networks and HIV/HSV-2 patterns. We explored infection transmission in diverse network structures informed by observed behavior patterns and tuned by ranges of different network statistics. This approach enabled us to explore associations and risks of exposure beyond what can be implemented in an actual observational study.
We aimed specifically to determine the mean effects of key network statistics on infection transmission, and to what extent they are independent of each other. We further aimed to assess whether HSV-2 prevalence, despite differences in natural history and infectiousness profile, is associated with HIV prevalence and can (as a proxy) forecast HIV epidemic potential in a population yet to experience a full-blown epidemic. An overarching goal of our study is to inform the investigation of the network factors that have led to such large HIV epidemics in Sub-Saharan Africa (SSA), but nowhere else.
Methods
Model structure
We constructed an individual-based Monte Carlo simulation model describing sex partnering, birth and death, and HIV/HSV-2 transmission in a given population. Each individual is born, dies, forms/dissolves marital/nonmarital partnerships, or acquires HIV/HSV-2 at event-specific probabilities at each time step in each simulation run. Transmission was assumed to occur only through sex. An initial population of 2000 individuals was assumed (for computational reasons), but this population can vary with time by random birth/death events. The model was structured as a one-sex model to avoid complexities in the definitions and analytics of network statistics. As our present interest is on the natural dynamics as the HIV epidemics emerged in SSA, we did not incorporate more recent intervention scale-up such as HIV antiretroviral therapy. Final analyses were conducted at endemic equilibrium for both infections.
Infection natural history and transmission
HIV/HSV-2 natural history and transmission parameters, and infection stage structures, were based on earlier work and recent data updates (Table 1) [16,17]. HIV progression was divided into the stages of acute, chronic, and advanced with durations of 49 days, 9 years, and 2 years, respectively – based on compilation of data and analyses of cohort studies [18–22]. AIDS mortality was included as a hazard rate. HIV transmission probability per coital act per stage was based on analyses of the Rakai study [18–20,23,24].
Table 1.
Key model parameters.
| Parameter | Value | Source |
| HIV transmission probability per coital act per HIV stage | ||
| Acute | 0.0360 | Published literature [18,19,23] |
| Chronic | 0.0008 | Published literature [18,20] |
| Advanced | 0.0042 | Published literature [18,20] |
| Duration of each HIV stage | ||
| Acute | 49 days | Published literature [18–20] |
| Chronic | 9.0 years | Published literature [20–22] |
| Advanced | 2.0 years | Published literature [18,20] |
| Frequency of coital acts per HIV stage | ||
| Acute | 10.6 per month | Published literature [18] |
| Latent | 11.0 per month | Published literature [18] |
| Late | 10.6 per month | Published literature [18] |
| HSV-2 transmission probability per coital act per HSV-2 stage | ||
| Primary infection | 0.0189 | Published literature [16] |
| Latent infection | 0.0 | Published literature [16] |
| Reactivation | 0.0189 | Published literature [16] |
| HSV-2 shedding frequency | 14% | Published literature [16,25] |
| Duration of each HSV-2 stage | ||
| Primary infection | 20.0 days | Published literature [16] |
| Latent infection | 78.5 days | Published literature [16] |
| Reactivation | 12.8 days | Published literature [16] |
| Frequency of coital acts per HSV-2 stage | ||
| All stages | 11.0 per month | Published literature [18] |
| Marriage rate | 0.12 | Estimated from Kenya's 2008–2009 DHS data using modeling to fit the age-specific prevalence of current marriage [33] |
| Mean duration of marital partnership | 20 years | Representative value informed by Kenya's 2008–2009 DHS data of the distribution of marital partnership duration as well as these data for other Sub-Saharan African countries [33] |
| Mean duration of nonmarital (casual) sexual partnership | 6 months | Representative value and informed by previous work [20,35] |
| Number of premarital sex partners among unmarried individuals over the last year | Estimated from Kenya's 2008–2009 DHS distribution of premarital sex partners [33]. Range is estimated based on DHS distribution of premarital sex partners for 20 Sub-Saharan African countries [34] | |
| Mean | 0.32 and a range of 0.0–1.0 | |
| Variance | 0.0087 and a range of 0.0–2.0 | |
| Number of extramarital sex partners among married individuals over the last year | Estimated from Kenya's 2008–2009 DHS distribution of extramarital sex partners [33]. Range is estimated based on DHS distribution of extramarital sex partners for 20 Sub-Saharan African countries [34] | |
| Mean | 0.0088 and a range of 0.0–0.5 | |
| Variance | 0.0 with a range of 0.0–1.0 | |
DHS, Demographic and Health Survey; HSV-2, herpes simplex virus type 2.
HSV-2 natural history was described by the stages of primary infection, latent infection, and reactivation [16]. Viral shedding was assumed to occur only during primary infection and reactivations at a frequency of 14% of the time [16,25]. HSV-2 transmission probability per coital act during shedding was determined through several analyses [16]. Based on evidence of the pattern, sites, and frequency of subclinical reactivations [26], all seropositive persons were assumed equally infectious regardless of clinical symptoms.
With the conflicting evidence on the biological interaction of HIV and HSV-2, and on the potential effects of any interaction on HIV acquisition and transmission [16,27–32], no HIV/HSV-2 biological interaction was assumed.
Sexual behavior and networks
Marital partnerships were assumed to form/dissolve at a specific rate with no polygamy. Marital rate (probability per unit time for a person to marry) was estimated using a mathematical model for partnership formation/dissolution and maximum likelihood applied to the current marriage age-specific prevalence in accordance with Demographic and Health Survey (DHS) data from Kenya [33]. Average marriage duration was set at 20 years, also according to DHS data from Kenya and other countries in SSA [33].
Nonmarital partnerships were assumed to form/dissolve among both unmarried (premarital sex) and married (extramarital sex) individuals, but at different rates. Rates of premarital/extramarital partnership formation followed a gamma distribution with parameters estimated using DHS data from 20 SSA countries [33] per Omori et al.[34] methodology. An average duration of 6 months was assumed for nonmarital partnerships, as a representative value informed by previous work [20,35].
In a weighted random network, such as our baseline network, degree correlation, and clustering coefficient (both defined below and in Text S1) are determined by the degree (number of partners) distribution [34]. A weighted random network is a network where each individual randomly selects partners from among all individuals in the population, but each individual may have different propensity to form a partnership – a situation known as proportionate mixing [36].
We aimed to investigate STI dynamics in diverse networks where degree correlation and clustering coefficient can vary independently. To control these factors while maintaining the degree distribution, ‘rewiring’ of newly formed partnerships is required [37]. We used a rewiring prescription introduced by Yuasa and Shirayama [38] utilizing a tuning factor with two parameters, kcorr and kclu, controlling degree correlation and clustering coefficient, respectively. If
, the network is a random weighted network, otherwise, degree correlation and clustering coefficient can be varied.
The age-specific population distribution was estimated using Kenya's 2008–2009 DHS data [33]. The age-specific natural death rate was set using Kenya's demographic indicators [39].
Network statistics
Based on relevance to STI epidemiology, we investigated five types of network statistics: population mean and variance of the number of partners over the last year for married/unmarried individuals, degree correlation for nonmarital/all partnerships, clustering coefficient for nonmarital/all partnerships, and concurrency prevalence for nonmarital/all partnerships. The 1-year timeframe for the number of partners was chosen based on DHS data availability [33,34]. Degree correlation measures the correlation between the number of partners of each partner in a partnership (Text S1) [40]. Clustering coefficient measures the degree to which individuals in the network tend to cluster together (Text S1) [40]. Concurrency prevalence measures the proportion of individuals who have at least two partners at a specific time point (Text S1) [41].
Plan of analysis
We generated 500 sexual networks by varying the mean and variance of number of partners for married/unmarried individuals, and κcorr and κclu. The choice of 500 is a balance between computing time versus the need to have diverse networks and to estimate measures with sufficiently narrow confidence intervals to make valid inferences. Parameters were sampled from plausible ranges. For each dynamical network, HIV/HSV-2 transmission was also simulated. Each simulation was seeded with 10 infected individuals for each of HIV/HSV-2, and was run with a ‘burn-in’ time of 200 years to reach equilibrium. Accordingly, we generated 500 HIV/HSV-2 epidemics in 500 different networks.
Network statistics and HIV/HSV-2 prevalences were calculated in each simulation. Spearman's rank correlation coefficient (SRCC) was calculated to estimate the associations between HIV/HSV-2 prevalences and each of the network statistics. Associations were considered strong if SRCC was at least 0.6, of intermediate strength if SRCC was between 0.2 and 0.6, and weak if SRCC was less than 0.2. Subsequently, to examine potential collinearities between network statistics and to control for confounding, multiple linear regression analyses were conducted and adjusted associations assessed. These analyses were conducted on the population-based summary measures of the simulated networks/epidemics. The dependent variable was HIV (or HSV-2) prevalence, as a continuous variable, and the independent variables were the network statistics.
The regression statistical model was selected using the variance inflation factor (VIF) [42] and Akaike's information criterion [43] as criteria. To account for the collinearities while building the models, the VIF was calculated and model selection was based on VIF less than 10. Among models satisfying the VIF criterion, the final model was selected by minimizing Akaike's information criterion. The final model was then used to calculate associations and estimate effect sizes, that is, the standardized partial regression coefficients (estimated parameters of the independent variables in the regression equation) [42]. Effect sizes were considered strong if effect size was at least 0.6, of intermediate strength if effect size was between 0.2 and 0.6 and weak if effect size was less than 0.2. A strong effect size would imply that the independent variable (a network statistic) has a strong influence on the dependent variable (infection prevalence). Statistical analyses were implemented in R version 3.2.3. (R Foundation, Vienna, Austria).
One aim of the study was to assess whether HSV-2 prevalence, given its ecological association with HIV prevalence with the shared mode of transmission, can inform us about HIV epidemic potential, that is the endemic level of HIV prevalence, by acting as a proxy ‘summary collective measure’ of sexual risk behavior. To address this question, we conducted multiple regression analyses for HIV prevalence where HSV-2 prevalence was included as an independent variable, whether as the only variable or with other network statistics.
Results
Figure 1a–f shows the association between HIV/HSV-2 prevalences across the 500 simulations at different scales of network statistics. HSV-2 prevalence was consistently higher than HIV prevalence, and apart from settings with HSV-2 prevalence greater than 60%, HIV prevalence was one-third or less of HSV-2 prevalence. HIV/HSV-2 prevalences were associated with an SRCC of 0.64 (95% confidence interval: 0.58–0.69).
Fig. 1.
Association between HIV prevalence and herpes simplex virus type 2 (HSV-2) prevalence.
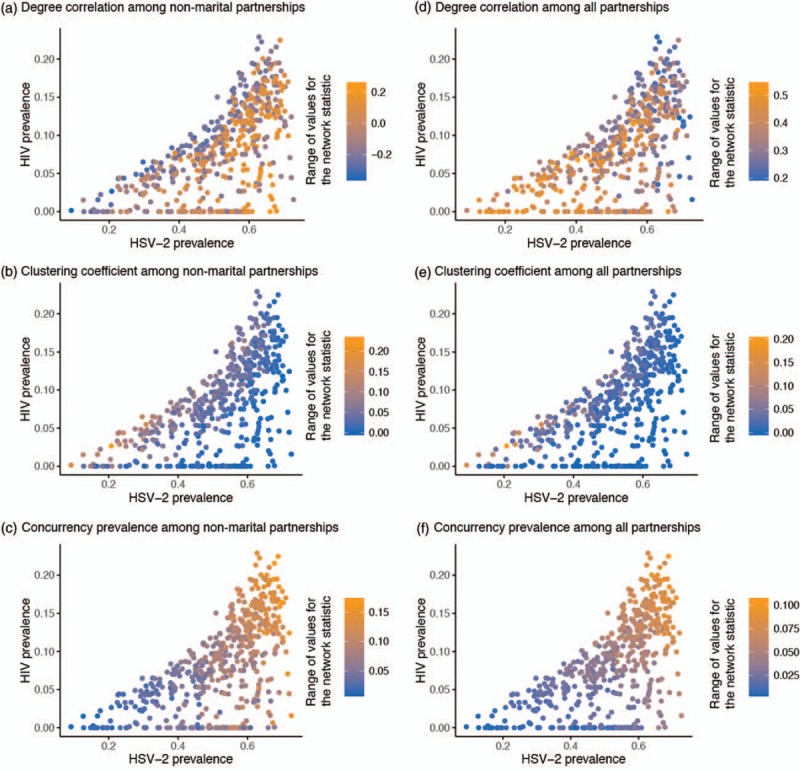
Association between HIV prevalence and HSV-2 prevalence across 500 epidemic and sexual network simulations at variable levels of population mean and variance of number of sexual partners over the last year for both unmarried and married individuals, degree correlation for nonmarital and all partnerships, clustering coefficient for nonmarital and all partnerships, and concurrency prevalence for nonmarital and all partnerships. The shading scale in each panel describes the range of simulated values for the sexual network statistic of that specific panel. Each of the panels describes the association between HIV prevalence and HSV-2 prevalence in the context of the variation in a specific sexual network statistic. HSV-2, herpes simplex virus type 2.
In examining the univariable (unadjusted) associations between each network statistic and HIV/HSV-2 prevalences, it was evident that network statistics sometimes affected HIV/HSV-2 prevalences differentially. Higher concurrency, for example, drove higher prevalence for both infections (Fig. 1c and f). In contrast, degree correlation among nonmarital partnerships affected each prevalence differently (Fig. 1a).
Table 2 shows the associations between network statistics and HIV prevalence as measured by SRCC. HIV prevalence was strongly and positively correlated with unmarried mean/variance of number of partners and concurrency prevalence among nonmarital/all partnerships. Meanwhile, it had intermediate-strength positive associations with married mean/variance of number of partners and clustering coefficient among all partnerships, but intermediate-strength negative association with degree correlation among all partnerships. Lastly, HIV prevalence had weak associations with degree correlation and clustering coefficient, both among nonmarital partnerships.
Table 2.
Associations between each of HIV and herpes simplex virus type 2 (HSV-2) prevalences and sexual network statistics, and sexual network predictors of HIV and HSV-2 prevalence.
| HIV infection | ||||||||||||
| Number of partners over the last year | Degree correlation | Clustering coefficient | Concurrency | Goodness of fit | ||||||||
| Unmarried individuals | Married individuals | Nonmarital partnerships (95% CI) | All partnerships (95% CI) | Nonmarital partnerships (95% CI) | All partnerships (95% CI) | Nonmarital partnerships (95% CI) | All partnerships (95% CI) | Adjusted R2 | AIC | |||
| Mean (95% CI) | Variance (95% CI) | Mean (95% CI) | Variance (95% CI) | |||||||||
| Spearman's rank correlation coefficient with HIV prevalence | 0.86 (0.83, 0.88) | 0.86 (0.83, 0.88) | 0.34 (0.26, 0.42) | 0.37 (0.29, 0.45) | −0.03 (−0.11, 0.06) | −0.44 (−0.51, −0.37) | 0.18 (0.08, 0.27) | 0.33 (0.24, 0.41) | 0.92 (0.90, 0.93) | 0.73 (0.68, 0.78) | ||
| Standardized partial regression coefficients for the Concurrency Class model | −0.14 (−0.17, −0.11) | 0.16 (0.13, 0.19) | 0.96 (0.93, 0.99) | 0.88 | −3916 | |||||||
| Standardized partial regression coefficients for the Number of Partners Class model | 0.62 (0.55,0.68) | 0.28 (0.21, 0.35) | 0.19 (0.11, 0.26) | 0.16 (0.09, 0.23) | −0.08 (−0.11, −0.05) | 0.10 (0.06, 0.15) | 0.90 | −3967 | ||||
| HSV-2 infection | ||||||||||||
| Number of partners over the last year | Degree correlation | Clustering coefficient | Concurrency | Goodness of fit | ||||||||
| Unmarried individuals | Married individuals | Nonmarital partnerships (95% CI) | All partnerships (95% CI) | Nonmarital partnerships (95% CI) | All partnerships (95% CI) | Nonmarital partnerships (95% CI) | All partnerships (95% CI) | Adjusted R2 | AIC | |||
| Mean (95% CI) | Variance (95% CI) | Mean (95% CI) | Variance (95% CI) | |||||||||
| Spearman's rank correlation coefficient with HSV-2 prevalence | 0.77 (0.73, 0.80) | 0.44 (0.37, 0.50) | 0.52 (0.45, 0.58) | 0.42 (0.34, 0.49) | 0.35 (0.27, 0.42) | −0.45 (−0.51, −0.38) | −0.48 (−0.55, −0.40) | −0.31 (−0.40, −0.23) | 0.82 (0.79, 0.85) | 0.86 (0.82, 0.89) | ||
| Standardized partial regression coefficients for the Concurrency Class model | 0.09 (0.04, 0.13) | −0.41 (−0.45, −0.37) | 0.70 (0.66, 0.74) | 0.79 | −2814 | |||||||
| Standardized partial regression coefficients for the Number of Partners Class model | 0.46 (0.39,0.53) | 0.23 (0.16, 0.30) | 0.49 (0.42, 0.57) | −0.20 (−0.27, −0.12) | 0.12 (0.08, 0.15) | −0.42 (−0.46, −0.37) | 0.88 | −3099 | ||||
The table shows the Spearman's rank correlations between each sexual network statistic and the prevalence of HIV and HSV-2. The table also shows the predictors of HIV and HSV-2 prevalences as determined through multiple regression analyses on model simulations. The sexual network predictors were investigated through two model types, Concurrency Class model and Number of Partners Class model.
AIC, Akaike's information criterion; CI, confidence interval.
Table 2 also shows the associations between network statistics and HSV-2 prevalence as measured by SRCC. HSV-2 prevalence was strongly and positively correlated with unmarried mean number of partners and concurrency prevalence of nonmarital/all partnerships. Meanwhile, it had intermediate-strength positive associations with unmarried variance of number of partners, married mean/variance of number of partners, and degree correlation among nonmarital partnerships, but intermediate-strength negative associations with degree correlation among all partnerships and clustering coefficient among nonmarital/all partnerships. HSV-2 prevalence was not weakly associated with any of the network statistics.
Several collinearities were detected in the regression analyses (Fig. 2a). These included: concurrency prevalence among nonmarital/all partnerships versus unmarried/married mean/variance of number of partners, concurrency prevalence among nonmarital versus all partnerships, degree correlation among nonmarital versus all partnerships, and clustering coefficient among nonmarital versus all partnerships. We used, therefore, two classes (i.e., two types) of models in the final regression analyses to control for collinearities: Concurrency Class, which included one of the two concurrency measures, but no measure of number of partners; and Number of Partners Class including unmarried/married mean/variance of number of partners, but no concurrency measure.
Fig. 2.
Collinearities among key sexual network statistics and predictors of higher HIV or herpes simplex virus type 2 (HSV-2) prevalence.
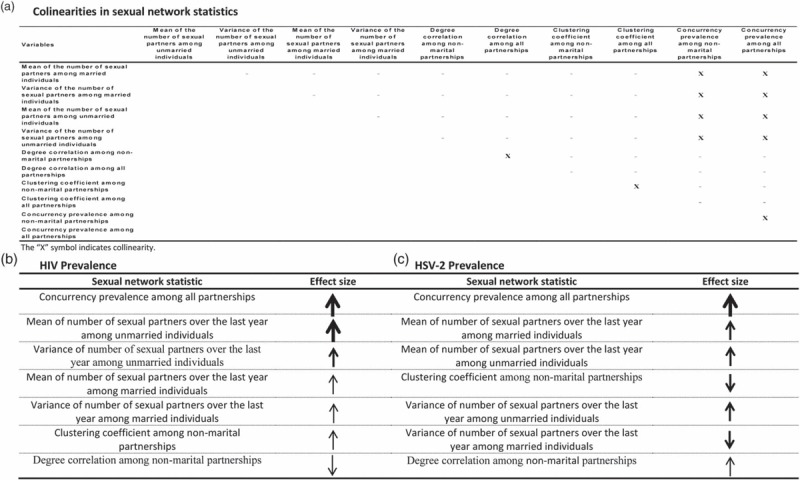
(a) Identified collinearities among key sexual network statistics across 500 sexual network simulations. Predictors of higher HIV (b) or HSV-2 (c) prevalence and their effect sizes as identified across 500 epidemic and sexual network simulations. In b and c, network statistics are ordered according to descending strength of effect size. Size of arrow denotes strength of effect size: large arrow for strong effect size (≥0.6), medium arrow for intermediate-strength effect size (0.2≤ effect size <0.6), and small arrow for weak effect size (<0.2). HSV-2, herpes simplex virus type 2.
Table 2 shows the best-fit models in the Concurrency Class. For each infection, the final model included degree correlation and clustering coefficient, both for nonmarital partnerships, and concurrency prevalence for all partnerships. The HIV model explained 88% of the variation. In this model, concurrency prevalence was a strong predictor of HIV prevalence, with weak effects for degree correlation and clustering coefficient. The HSV-2 model explained 79% of the variation. In this model, concurrency prevalence was also a strong predictor of HSV-2 prevalence, with intermediate strength but negative effect for clustering coefficient, and weak effect for degree correlation.
Table 2 also shows the best-fit models in the Number of Partners Class. For each infection, the final model included unmarried/married mean/variance of number of partners, and degree correlation and clustering coefficient, both for nonmarital partnerships. The HIV model explained 90% of the variation. In this model, unmarried mean number of partners was a strong predictor of HIV prevalence, with intermediate-strength effect for unmarried variance of number of partners and weak effects for the other variables. The HSV-2 model explained 88% of the variation. In this model, unmarried/married mean/variance of number of partners and clustering coefficient had intermediate-strength effects (that was negative for clustering coefficient), and degree correlation had a weak effect. Figure 2b and c summarize these effects and their scales.
Table 3 shows the results of HIV models where HSV-2 prevalence was included as an independent variable. HSV-2 prevalence was a strong predictor of HIV prevalence whether alone, or with the addition of degree correlation and clustering coefficient. However, with the addition of concurrency prevalence, HSV-2 prevalence was a weak predictor as concurrency prevalence alone explained most of HIV prevalence variation. With inclusion of concurrency prevalence, clustering coefficient, and degree correlation in the model, HSV-2 prevalence was not predictive of HIV prevalence and had a vanishing effect size – inclusion of network statistics explicitly as independent variables in the model prevented HSV-2 prevalence from ‘acting’ as a collective measure of sexual risk behavior.
Table 3.
HSV-2 prevalence as a proxy ‘biomarker’ of HIV prevalence.
| Goodness of fit | ||||||
| HSV-2 prevalence | Degree correlation among nonmarital partnerships | Clustering coefficient among nonmarital partnerships | Concurrency prevalence among all partnerships | Adjusted R2 | AIC | |
| Spearman's rank correlation coefficient between HIV and HSV-2 prevalences | 0.64 (0.58, 0.69) | |||||
| Spearman's rank correlation coefficients between network statistics and HSV-2 prevalence | 0.35 (0.27, 0.42) | −0.48 (−0.55, −0.40) | 0.82 (0.79, 0.85) | |||
| Standardized partial regression coefficient for the concurrency only model | 0.91 (0.87, 0.95) | 0.83 | −3725 | |||
| Standardized partial regression coefficient for the HSV-2 only model | 0.62 (0.55, 0.69) | 0.38 | −3085 | |||
| Standardized partial regression coefficients for the HSV-2 and degree correlation model | 0.70 (0.63, 0.77) | −0.24 (−0.31, −0.17) | 0.43 | −3126 | ||
| Standardized partial regression coefficients for the HSV-2 and clustering coefficient model | 0.92 (0.85, 0.98) | 0.54 (0.47, 0.61) | 0.59 | −3286 | ||
| Standardized partial regression coefficients for the HSV-2 and concurrency prevalence model | −0.23 (−0.28, −0.17) | 1.09 (1.03, 1.14) | 0.85 | −3786 | ||
| Standardized partial regression coefficients for the HSV-2, degree correlation, and clustering coefficient model | 0.96 (0.89, 1.03) | −0.18 (−0.24, −0.13) | 0.52 (0.45, 0.58) | 0.62 | −3322 | |
| Standardized partial regression coefficients for the HSV-2, degree correlation, clustering coefficient, and concurrency prevalence model | 0.02 (-0.05, 0.08) | −0.14 (−0.17, −0.11) | 0.17 (0.13, 0.21) | 0.95 (0.89, 1.00) | 0.88 | −3914 |
This table investigates whether herpes simplex virus type 2 (HSV-2) prevalence can act as a ‘summary collective measure’ of sexual risk behavior. The table shows the results of multiple regression analyses for HIV prevalence where HSV-2 prevalence was included as an independent variable, whether as the only variable or with other sexual network statistics.
CI, confidence interval.
AIC, Akaike's information criterion.
Discussion
We explored the sexual network determinants of HIV/HSV-2 prevalences and the epidemiological overlap between these two infections. Not surprisingly, we found that each infection prevalence is predicted by key network summary statistics. However, the network effects on each infection showed similarity and variation in nature and scale. Unmarried mean/variance of number of partners and concurrency prevalence were overall strong predictors for both infections. The other network statistics had smaller effects that varied by infection. The network statistics considered here explained most of the variation in HIV/HSV-2 prevalences across these simulations.
Importantly, we found that network statistics effects on prevalence were not independent of each other. Concurrency prevalence captured the same effect as that of mean/variance of number of partners. Our results demonstrated also that HSV-2 prevalence is a proxy of HIV prevalence, and therefore, of HIV epidemic potential among populations where HIV has not yet been introduced, or has not yet reached its endemic equilibrium (stable endemic level). With both infections being transmitted sexually and propagating in the same sexual network, HSV-2 prevalence acts as a ‘summary collective measure’ of the network statistics that drive HIV transmission.
HIV prevalence was also found to be one-third or less of HSV-2 prevalence. If HSV-2 prevalence was less than 20% in a given population, such as among many female sex worker populations in the Middle East and North Africa [3,44], our results indicate that HIV is unlikely to reach a concentrated epidemic level among these sex workers (>5% prevalence). Meanwhile, if HSV-2 prevalence was at 50%, there is sufficient sexual risk behavior in the network to sustain a large HIV epidemic, as found in the general population HIV epidemics in SSA [45]. It would be of interest to assess whether these modeling predictions are consistent with the HIV/HSV-2 association in empirical data. The latter could be assessed through a global systematic review and meta-analyses of existing studies with primary data for both infections.
HSV-2 prevalence was found to be a proxy of HIV prevalence, acting as a ‘biomarker’ or a ‘temperature scale’ of the sexual risk behavior that drives HIV transmission in a population. This is akin to hepatitis C virus, which was shown to be a ‘temperature scale’ of the injecting risk behavior that drives HIV transmission among people who inject drugs [46–48]. HSV-2 prevalence, together with other factors such as costs and health benefits, can be used as a criterion in the prioritization, optimization, and resource allocation of HIV prevention interventions, as has been suggested previously [15]. HSV-2 prevalence can help in identifying which populations and/or sexual networks to target while designing cost-effective HIV/STI-specific prevention interventions.
Though HIV and HSV-2 propagate in the same network, there are distinctions in how network statistics affect each infection transmission. Clustering had an opposite effect on each infection, driving higher HIV transmission but lower HSV-2 transmission. HIV is possibly more difficult to propagate in networks, with its lower infectiousness and shorter infection duration, and much of its transmission could be occurring in clusters, reminiscent of core group STI dynamics [5]. Meanwhile, HSV-2 spreads more easily in networks, with its higher infectiousness and longer infection duration, and therefore, can reach a larger population segment. High clustering in a network may limit HSV-2's ability to reach remote areas of the network, just as observed in an analysis of generic epidemic transmission [37].
Higher degree correlation among nonmarital partnerships was associated with higher HSV-2, but lower HIV transmission. Degree correlation may facilitate the formation of larger connected sections within a network (larger components), thereby probably allowing HSV-2 to reach a larger population segment and remote areas of the network. Meanwhile, higher degree correlation among all partnerships, that is, with inclusion of marital partnerships, was negatively associated with HIV/HSV-2 transmission, as it indicated tendency for monogamy.
Our findings indicate hidden complexity, if not danger, in concluding that specific network aspects are core drivers of STI epidemics without cautious analysis that control for other network factors and their interdependency. Our results, for example, showed that concurrency was strongly predictive of HIV prevalence, apparently supporting the literature implicating concurrency as the key factor to explain SSA fulminant HIV transmission [49]. However, such a conclusion seems unwarranted as our results indicate that concurrency's effect is not independent of the number of partners effect. Concurrency appeared largely as a proxy for the high mean/variance of number of partners – not a surprising outcome considering the analytical association between concurrency and mean/variance of number of partners [41,50]. HIV prevalence in our analysis reflected primarily mean/variance of number of partners, with smaller effects for clustering and degree correlation. These factors alone explained 90% of HIV prevalence variation (Table 2).
Our study has limitations. We focused on specific network statistics based on presumptive relevance to HIV/HSV-2. Other network measures or behavioral attributes may shed further light on STI dynamics. For example, we did not explicitly explore role of commercial sex, but this may have a sizable independent effect. Our analyses were conducted on population-based summary data, and technically could be subject to ecological fallacy. For analytical tractability, we used a one-sex model, but this limitation is not perceived to be critical as the core transmission dynamics is already captured allowing the model to effectively simulate same sex or heterosexual transmission in a population. We assumed no HIV/HSV-2 biological interaction, despite earlier observational evidence [16,27,28], as subsequent clinical trials have questioned such biological interaction [30–32].
We included broad ranges for the network statistics in context of absence of empirical measures for some network statistics, such as degree correlation and clustering. We focused on studying the ‘mean’ overall association between each network statistic and HIV/HSV-2 prevalences over broad ranges for these variables. Each of these associations, nevertheless, is nonlinear and complex, and may change in strength or nature at different scales of network statistics or prevalence levels. Moreover, different interventions, whether they affect both infections directly (such as condom use), or whether they affect one of them differentially (such as antiretroviral therapy) can further influence these nonlinear associations and their interactions. These intricacies warrant detailed examination in further work.
In conclusion, HIV transmission and HSV-2 transmission in sexual networks share similarities that allow us to use HSV-2 prevalence as a proxy of HIV epidemic potential, and accordingly inform HIV prevention interventions. There are also distinctions in the network factors that drive each infection transmission. HIV prevalence reflected primarily mean/variance of number of partners (or alternatively concurrency), whereas HSV-2 prevalence was affected by a range of network statistics. Key infection effects of network statistics were found not to be independent – concurrency captured similar effects as those of mean/variance of number of partners. This genre of epidemic analyses, particularly when extended to more infections, can elucidate the behavioral factors that drive STI transmission, how STIs can inform us about the structure of sexual networks, and how to best design interventions to control transmission.
Acknowledgements
The authors gratefully acknowledge the fine support of Ms Adona Canlas in the preparation of this manuscript, and the manuscript language review of Dr Ross MacDonald, both from Weill Cornel Medicine. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine-Qatar.
Funding: The publication was made possible by NPRP grant number 5–752–3–177 from the Qatar National Research Fund (a member of Qatar Foundation). Ryosuke Omori acknowledges also the support of Japan Science and Technology Agency (JST), PRESTO, and Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Young Scientists (B) 15K19217. The statements made herein are solely the responsibility of the authors and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
R.O. designed the study, developed the mathematical model, and performed the analyses. L.J.A-R. led the conception and conduct of the study.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Neslon KE, Williams CM. Infectious disease epidemiology: theory and practice. Second ed.Massachussets: Jones and Bartlett Publishers; 2007. [Google Scholar]
- 2.Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, Hawkes S. Global control of sexually transmitted infections. Lancet 2006; 368:2001–2016. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Raddad LJ, Akala FA, Semini I, Riedner G, Wilson D, Tawil O. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa: Time for Strategic Action, Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. Washington DC: The World Bank Press; 2010. [Google Scholar]
- 4.Boily MC, Masse B. Mathematical models of disease transmission: a precious tool for the study of sexually transmitted diseases. Can J Public Health 1997; 88:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunham RC, Plummer FA. A general model of sexually transmitted disease epidemiology and its implications for control. Med Clin North Am 1990; 74:1339–1352. [DOI] [PubMed] [Google Scholar]
- 6.Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis 1978; 5:51–56. [DOI] [PubMed] [Google Scholar]
- 7.Day S, Ward H, Ison C, Bell G, Weber J. Sexual networks: the integration of social and genetic data. Soc Sci Med 1998; 47:1981–1992. [DOI] [PubMed] [Google Scholar]
- 8.Ghani AC, Swinton J, Garnett GP. The role of sexual partnership networks in the epidemiology of gonorrhea. Sex Transm Dis 1997; 24:45–56. [DOI] [PubMed] [Google Scholar]
- 9.Potterat JJ, Rothenberg RB, Muth SQ. Network structural dynamics and infectious disease propagation. Int J STD AIDS 1999; 10:182–185. [DOI] [PubMed] [Google Scholar]
- 10.Morris M. Network epidemiology: a handbook for survey design and data collection. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 11.Morris M. Sexual networks and HIV. AIDS 1997; 11:S209–S216. [PubMed] [Google Scholar]
- 12.Manhart LE, Aral SO, Holmes KK, Foxman B. Sex partner concurrency: measurement, prevalence, and correlates among urban 18-39-year-olds. Sex Transm Dis 2002; 29:133–143. [DOI] [PubMed] [Google Scholar]
- 13.Morris M. Telling tails explain the discrepancy in sexual partner reports. Nature 1993; 365:437–440. [DOI] [PubMed] [Google Scholar]
- 14.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Statist Rev 1994; 62:229–243. [Google Scholar]
- 15.Abu-Raddad LJ, Schiffer JT, Ashley R, Mumtaz G, Alsallaq RA, Akala FA, et al. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics 2010; 2:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, Corey L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008; 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awad SF, Sgaier SK, Tambatamba BC, Mohamoud YA, Lau FK, Reed JB, et al. Investigating voluntary medical male circumcision program efficiency gains through subpopulation prioritization: insights from application to Zambia. PLoS One 2015; 10:e0145729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005; 191:1403–1409. [DOI] [PubMed] [Google Scholar]
- 19.Pinkerton SD. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav 2008; 12:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS 2008; 22:1055–1061. [DOI] [PubMed] [Google Scholar]
- 21.Morgan D, Whitworth J. The natural history of HIV-1 infection in Africa. Nat Med 2001; 7:143–145. [DOI] [PubMed] [Google Scholar]
- 22.UNAIDS. UNAIDS reference group on estimates, modelling and projections. Geneva, Switzerland: UNAIDS; 2007. [Google Scholar]
- 23.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis 2008; 198:687–693. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 2006; 314:1603–1606. [DOI] [PubMed] [Google Scholar]
- 25.Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, Corey L. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008; 198:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 2000; 342:844–850. [DOI] [PubMed] [Google Scholar]
- 27.Tanton C, Abu-Raddad LJ, Weiss HA. Time to refocus on HSV interventions for HIV prevention?. J Infect Dis 2011; 204:1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 29.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Partners in Prevention HSV/HIV Transmission Study Team. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Partners in Prevention HSV/HIV Transmission Study Team. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, et al. HPTN 039 Protocol Team. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, et al. HSV trial team; Steering and Data Monitoring Committees. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med 2008; 358:1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. [[Accessed 15 June 2016]]. http://www.measuredhs.com/ [Google Scholar]
- 34.Omori R, Chemaitelly H, Abu-Raddad LJ. Dynamics of noncohabiting sex partnering in sub-Saharan Africa: a modelling study with implications for HIV transmission. Sex Transm Infect 2015; 91:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awad SF, Abu-Raddad LJ. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s?. Epidemics 2014; 8:9–17. [DOI] [PubMed] [Google Scholar]
- 36.Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual activity classes. Philos Trans R Soc Lond B Biol Sci 1993; 342:137–159. [DOI] [PubMed] [Google Scholar]
- 37.Ball F, Britton T, Sirl D. A network with tunable clustering, degree correlation and degree distribution, and an epidemic thereon. J Math Biol 2013; 66:979–1019. [DOI] [PubMed] [Google Scholar]
- 38.Yuasa T, Shirayama S. Extraction method of principal factor in epidemic propagation on network. IPSJ SIG Technical Report (ICS). In; 2010. pp. 1–6. [Google Scholar]
- 39.United Nations Department of Economic and Social Affairs, Population Division, Population Estimates and Projections Section. World population prospects, the 2012 revision. 2012. Available at: http://esa.un.org/wpp/Excel-Data/population.htm [Accessed 5 March 2016] [Google Scholar]
- 40.Newman M. Networks: an introduction. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 41.Kretzschmar M, Morris M. Measures of concurrency in networks and the spread of infectious disease. Math Biosci 1996; 133:165–195. [DOI] [PubMed] [Google Scholar]
- 42.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. Chicago, IL: Irwin; 1996. [Google Scholar]
- 43.Akaike H. Akaike's Information Criterion. International encyclopedia of statistical science. 2011; Berlin: Springer, pp. 25–25. [Google Scholar]
- 44.Abu-Raddad LJ, Hilmi N, Mumtaz G, Benkirane M, Akala FA, Riedner G, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS 2010; 24 suppl 2:S5–S23. [DOI] [PubMed] [Google Scholar]
- 45.Auvert B, Buve A, Ferry B, Carael M, Morison L, Lagarde E, et al. Study Group on the Heterogeneity of HIV Epidemics in African Cities. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS 2001; 15 suppl 4:S15–S30. [DOI] [PubMed] [Google Scholar]
- 46.Vickerman P, Hickman M, May M, Kretzschmar M, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction 2010; 105:311–318. [DOI] [PubMed] [Google Scholar]
- 47.Mumtaz GR, Weiss HA, Vickerman P, Larke N, Abu-Raddad LJ. Using hepatitis C prevalence to estimate HIV epidemic potential among people who inject drugs in the Middle East and North Africa. AIDS 2015; 29:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akbarzadeh V, Mumtaz GR, Awad SF, Weiss HA, Abu-Raddad LJ. HCV prevalence can predict HIV epidemic potential among people who inject drugs: mathematical modeling analysis. BMC Public Health 2016; 16:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS 1997; 11:641–648. [DOI] [PubMed] [Google Scholar]
- 50.Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci 1992; 108:89–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


