Supplemental Digital Content is available in the text.
Keywords: J591, molecular imaging, PC3LN3, prostate carcinoma, prostate-specific membrane antigen, single-chain variable fragment, technetium-99m
Abstract
Introduction
Prostate-specific membrane antigen (PSMA) is an extensively studied antigen for imaging prostate cancer. We prepared a single-chain variable fragment (scFv) of J591, a monoclonal antibody that recognises an external epitope of PSMA, incorporating a His-tag for labelling with 99mTc tricarbonyl, and evaluated its binding using human PCa cell lines.
Methods
J591(scFv) was expressed in HEK-293T cells and purified by metal ion affinity chromatography, followed by size exclusion chromatography. Stability and monomer/dimer ratios of purified scFv under different storage conditions were analysed by SDS-PAGE and analytical size exclusion chromatography. J591(scFv) was labelled with 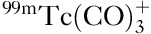 at 37°C for 60 min. The stability of 99mTc-scFv in human serum was analysed by SDS-PAGE with autoradiography. Cell-binding studies were carried out using PC3LN3 (PSMA negative) and PC3LN3-PSMA (a variant engineered to express PSMA) cell lines.
at 37°C for 60 min. The stability of 99mTc-scFv in human serum was analysed by SDS-PAGE with autoradiography. Cell-binding studies were carried out using PC3LN3 (PSMA negative) and PC3LN3-PSMA (a variant engineered to express PSMA) cell lines.
Results
J591(scFv) was most stable to dimerisation on storage at −80°C compared with −20 and 4°C. Radiochemical yields of 85–90% were obtained with the final radiochemical purity of more than 99% after purification by gel filtration. In these small-scale studies, the maximum specific activity achieved was 7 MBq/μg. Liquid chromatography–mass spectrometry showed the formation of 99mTc-J591(scFv), which was radiochemically stable in serum, with no dissociation of 99mTc over 24 h. Cell-binding assays showed specific binding to PSMA-positive cells.
Conclusion
J591(scFv) can be radiolabelled with 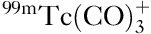 conveniently and efficiently. The labelled product was stable in serum. It showed selective binding to PSMA-positive cells compared with PSMA-negative cells. This potential radiotracer warrants evaluation in PCa xenograft models.
conveniently and efficiently. The labelled product was stable in serum. It showed selective binding to PSMA-positive cells compared with PSMA-negative cells. This potential radiotracer warrants evaluation in PCa xenograft models.
Introduction
The most extensively studied antigen in prostate cancer (PCa) for antibody-based imaging is prostate-specific membrane antigen (PSMA) 1. Radiolabelled monoclonal antibodies (mAb) against PSMA have been used in the clinic for the evaluation of PCa, primarily the 111In-labelled monoclonal antibody, capromab pendetide (Prostascint), which has been commercially available since 1997 for SPECT imaging 2. Although the hybrid technology of SPECT/CT has improved the accuracy of 111In-capromab imaging, capromab has the limitation that it binds to an internal epitope of PSMA 3. J591 is an alternative antibody that recognises an extracellular domain of PSMA and has been investigated for localisation and staging of PCa 4,5. Indeed, there have been recent reports of clinical trials with 111In-DOTA-J591 for SPECT imaging, 89Zr-DFO-J591 for PET imaging and 177Lu-DOTA-J591 for therapy 6–8.
However, the biokinetics of mAb such as J591 are not compatible with the use of short-lived radiolabels. For example, optimal images with 111In-J591 were obtained 5–7 days after injection 6. To improve upon the slow kinetics of mAb, small molecule ligands for PSMA have been investigated in recent years. Molecular Insight Pharmaceuticals (now part of Progenics) have developed a range of 99mTc and 123I-labelled PSMA ligands for imaging and 131I-labelled analogues as potential targeted therapeutic agents 9–11. The first human results with 123I MIP-1072 and MIP-1095 show rapid accumulation and prolonged retention in PSMA-expressing lesions, with tumour/background ratios of ∼10 achieved in SPECT images obtained 4 h after injection 9. In parallel work, 68Ga-labelled urea-based PSMA inhibitors have shown great promise for PSMA-specific tumour imaging with PET 11,12 and analogues labelled with 177Lu are being evaluated for therapy 13. Importantly, given the 68-min half-life of 68Ga, diagnostic images can be obtained within 60 min after injection 12.
The engineering of single-chain variable region fragments (scFv, MWt: ∼27 kDa) of mAb is another route to improve their kinetics for imaging with radionuclides with short half-lives 14. Parker et al. 15 described the construction of a J591(scFv) based on the reported complementarity determining region of J591. The nucleotide sequence was optimised for expression in Pichia pastoris and a hexa His-tag was added to facilitate affinity purification. J591(scFv) has also been used as a targeting agent for the delivery of toxins to PCa 16,17. Antibody fragments such as scFv can be conveniently labelled with 99mTc tricarbonyl through a His-tag. The basis of 99mTc tricarbonyl kit formulation is disodium boron carbonate, which serves as a CO source and a reducing agent 18. The group at the Paul Scherrer Institute developed a direct labelling protocol for scFv and minibodies carrying an N-terminal or a C-terminal His-tag with 99mTc tricarbonyl 19 and further optimisation has been performed by Badar et al. 20.
In an editorial on agents for imaging PCa, Eder et al. 11 stated that PSMA is the most promising target because of its accessibility on the cell surface, internalisation following binding for retention of the radiolabel and correlation of expression with stage and grade of PCa. With this objective, we have developed a 99mTc-labelled scFv of the mAb J591 and evaluated its binding to PSMA-expressing human PCa cells.
Materials and methods
Protein production and purification
The J591(scFv) sequence in the VH-VL orientation was PCR amplified from the SFG P28z vector as described previously 21. Mammalian HEK-293T cells were used to produce J591(scFv) in a growth medium of RPMI 1640 (PAA) with 10% FBS and antibiotics. The cells were cultured in an atmosphere of 5% CO2 at 37°C. The supernatant was collected over 4–6 weeks and stored at 4°C. When ∼1500 ml of supernatant had been collected, it was purified by Ni-NTA chromatography (Qiagen, Manchester, UK), followed by size exclusion FPLC (AKTA, Superdex 75 10/300 GL; GE Life Sciences, Little Chalfont, UK). The elution fractions were concentrated using centrifugal concentrators (Vivaspin 6 PES, MWCO 5000; Fisher-Sartorius, Loughborough, UK) before exchanging the buffer to PBS (pH 7) by size exclusion chromatography (as above). The protein was analysed by SDS-PAGE (Novex, Fisher, Loughborough, UK) using 12% gel, western blotting with 3,3-diaminobenzidine peroxidase (Sigma-Aldrich, Gillingham, UK) to detect the HRP-conjugated secondary antibody and HPLC (SEC-2000; Phenomenex, Macclesfield, UK). The protein was stored at −80°C in small aliquots on the basis of the protein concentration, which was determined using a UV–visible Spectrophotometer Nanodrop 2000c (Thermo Fisher Scientific, Paisley, UK). For stability studies, aliquots were also stored at 4°C and −20°C for 3 weeks. The effect of addition of 5% glycerol to reduce the extent of dimerisation was also evaluated.
Radiochemistry
99mTc tricarbonyl was prepared by adding 1000 MBq 99mTc-pertechnetate (Drytec generator; GE Healthcare, Little Chalfont, UK) in 500 µl to an Isolink kit (donated by Covidien Healthcare, Fareham, UK)) and heating it for 25 min at 100°C. The formation of the 99mTc tricarbonyl was monitored by TLC on glass-backed silica gel 60 plates developed with 1% HCl in methanol 20. The Rf values in this system are as follows: 99mTc tricarbonyl, 0.2–0.8; 99mTc colloids, 0.0 and 99mTc-pertechnetate, 0.9. An aliquot of the 99mTc tricarbonyl (∼200 MBq in 100 µl) was then incubated with 100 µl J591(scFv) (1 µg/µl) for 60 min at 37°C. After radiolabelling, the reaction mixture was applied to a size exclusion column (Sephadex G-25 PD Mini-trap; GE Life Sciences, Little Chalfont, UK), eluted with PBS and 30 fractions of 100 µl each were collected by gravity as described previously 22. The activity in the fractions was measured in an ionisation chamber (CRC-15R; Capintec, Southern Scientific, Henfield, UK). The fractions with the highest activity (generally 8, 9 and 10) were analysed on iTLC-SA strips (Agilent Technologies, Cheadle, UK) developed with 0.1 mol/l citrate buffer pH 5.5 and analysed by a radiochromatogram scanner (BioScan, Washington DC, USA). The Rf values in this system are as follows: 99mTc-J591(scFv), 0.0; 99mTc tricarbonyl, 0.9 and 99mTc-pertechnetate, 0.9. The fractions with the highest activity were then pooled.
A series of experiments were conducted that involved radiolabelling of J591(scFv) (10 µg) with different amounts of 99mTc tricarbonyl (10, 30, 50 and 70 MBq) to determine the maximum amount of radioactivity that could be loaded onto the J591(scFv). In another series, different concentrations of J591(scFv) ranging from 72 to 3.6 µmol/l were reacted with the same amount of tricarbonyl (10 µl, 30 MBq). Finally, J591(scFv) was radiolabelled with 99mTc tricarbonyl in two different NaCl concentrations (140 and 500 mmol/l) to determine the effect of salt concentration on radiolabelling efficiency 20.
J591(scFv) and 99mTc-J591(scFv) were characterised by mass spectrometry (MS) (Series 6520 QTOF LC–MS; Agilent Technologies, Cheadle, UK). The unlabelled scFv (20 µl, ∼30 µg) was analysed by direct injection and 99mTc-J591(scFv) (1 MBq, 10 µl, ∼16 µg) was analysed by liquid chromatography–mass spectrometry on a C18 column with an acetonitrile/0.1% formic acid gradient from 0 to 100% acetonitrile over 15 min at a flow rate of 0.5 ml/min.
Serum stability was studied by incubating 99mTc-J591(scFv) with an equal volume of human serum at 37°C and removing aliquots over the course of 24 h. Samples were tested for release of free pertechnetate or other small molecules containing 99mTc by iTLC-SA as described above; however, this method would not detect transchelation to serum proteins as both 99mTc-J591(scFv) and serum proteins would remain at the origin. Samples were analysed by SDS-PAGE 12% gel with detection by a phosphorimager (Cyclone Plus; Perkin Elmer, Beaconsfield, UK) and Coomassie blue staining.
Cell-binding studies
The PC3LN3 (parental, PSMA negative) and PC3LN3-PSMA (a variant engineered to express PSMA) cell lines were produced as described previously 22. FACS Calibur (Becton Dickinson, Oxford, UK) analysis before the cell-binding experiments with PCLN3 and PC3LN3-PSMA cell lines confirmed PMSA status using the anti-His-tag antibody penta-His Alexa 488 (Qiagen). For cell-binding experiments, 24-well plates were seeded with equal numbers of cells (4×105) in a volume of 500 µl/well and maintained at 37°C for 24 h. To achieve a range of protein concentrations, 10 serial dilutions of 99mTc-J591(scFv) were prepared in triplicate from 7200 nmol/l (10 µg in 50 µl) to 0.4 nmol/l (0.001 µg in 50 µl). The cells were incubated with 99mTc-J591(scFv) for 60 min at 4°C to minimise internalisation, after which the supernatant was aspirated and the cells were carefully washed three times with 500 µl Hanks’ balanced salt solution to remove any unbound radioactivity. The cells were then lysed with 0.5 mol/l NaOH (200 µl) and the lysate was transferred to tubes for gamma counting (1470 Wallac Wizard; Perkin Elmer). The percentage bound was plotted against protein concentration and the results were analysed by nonlinear regression using GraphPad Prism software (San Diego, Califonia, USA) with a one-site total binding algorithm to calculate an EC50 value for the cold mAb.
Statistical analysis
Results are expressed as mean±SD for n independent experiments. Differences between groups were assessed using Student’s t-test for unpaired values.
Results
Protein production and purification
J591(scFv) was produced in mammalian cells. A yield of 10–12 mg/l of J591(scFv) was obtained from the supernatant after the purification steps of the Ni-NTA column and SEC. In the Ni-NTA purification step, J591(scFv) eluted as a single peak when the column was washed with 250 mmol/l imidazole. In the SEC buffer adjustment step, the dimer fraction eluted first (10–15 ml), followed by the monomer fraction (15–20 ml) (Supplementary Fig. S1a, Supplemental digital content 1, http://links.lww.com/NMC/A116). An intense band observed at 27.7 kDa in both SDS-PAGE (Supplementary Fig. S1b, Supplemental digital content 1, http://links.lww.com/NMC/A116) and western blotting (Nawaz S, Kampmeier F, Mullen GED, Blower PJ and Ballinger JR, unpublished data) also confirmed the results. The purified sample was further analysed by size exclusion HPLC. The monomer fraction eluted at 9 min and the dimer at 8 min The problem of dimerisation over a period of 3 weeks was minimised by immediate storage at −80°C compared with 4°C and −20°C (Nawaz S, Kampmeier F, Mullen GED, Blower PJ and Ballinger JR, unpublished data). The addition of glycerol did not significantly decrease the dimerisation of J591(scFv).
Radiochemistry
For the preparation of 99mTc tricarbonyl, pertechnetate was added to the kit and heated for 25 min at 100°C. TLC showed an 85–90% radiochemical yield of 99mTc tricarbonyl, with the main impurity being free pertechnetate. The product was used for the subsequent labelling reaction without purification.
Site-specific labelling with 99mTc tricarbonyl via the His-tag of J591(scFv) resulted in a radiochemical yield of 85–100%. If the yield was less than quantitative, a final radiochemical purity of more than 99% could be achieved after a gel filtration purification step (Supplementary Fig. S2, Supplemental digital content 1, http://links.lww.com/NMC/A116). The radiochemical yield increased during incubation at 37°C up to 60 min, after which there was no further increase. For higher concentrations of J591(scFv), the time of incubation had no effect on the radiolabelling yield. However, with low concentrations, the duration of incubation played an important role (Fig. 1). For example, with 3.6 µmol/l J591(scFv), the radiochemical yield increased from 27±2% at 30 min to 80±5% at 60 min (n=3, t-test, P=0.005), whereas at 72 µmol/l, radiochemical yield was ∼100% at both time points (P=NS). The maximum amount of 99mTc tricarbonyl that could be loaded onto 1 µg J591(scFv) was 7 MBq. As previous work from this laboratory showed that the NaCl concentration can influence labelling efficiency with 99mTc tricarbonyl 20, experiments were conducted at two different salt concentrations. Increasing the NaCl concentration from 140 to 500 mmol/l slightly, but not significantly, increased the radiolabelling yield (Fig. 2). With 36 µmol/l J591(scFv), the radiochemical yield after 60 min of incubation was 75±4% at 140 mmol/l NaCl and 85±5% at 500 mmol/l NaCl (n=3, t-test, P=NS). 99mTc-J591(scFv) was radiochemically stable in serum, with no change in the speciation of 99mTc over 24 h as shown by TLC and SDS-PAGE, where there remained only an intense band at ∼30 kDa.
Fig. 1.
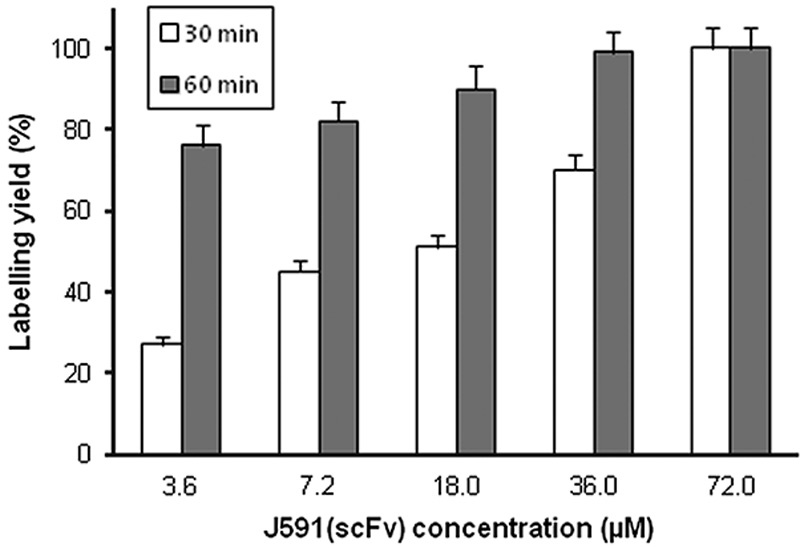
Effect of J591(scFv) concentration on labelling efficiency after 30 and 60 min of incubation. Each value is mean+SD for three measurements.
Fig. 2.

Effect of NaCl concentration (140 or 500 mmol/l) on the labelling efficiency of J591(scFv) (18 or 36 µmol/l) with 99mTc tricarbonyl. Each data point is mean±SD for three measurements.
Analysis by MS following a direct injection of J591(scFv) showed a strong peak at 27.7 kDa, which corresponds to the molecular weight of the J591(scFv). Liquid chromatography–mass spectrometry, using a C18 column, showed the formation of 99mTc-J591(scFv) detected in the radiometric chromatogram. MS of 99mTc-J591(scFv) showed a single peak at 27.7 kDa.
Cell-binding studies
Before conducting the cell-binding experiments, both the negative and the PSMA-positive cell lines were analysed by FACS for PSMA expression. The PC3LN3-PSMA cell line showed a positive shift in FL1, whereas the parental PSMA-negative PC3LN3 showed no shift in FL1. Serial dilutions of 99mTc-J591(scFv) were incubated with the both cell lines at 4°C, to minimise internalisation, for 60 min for binding equilibrium to be reached. As can be seen in Fig. 3, the binding of 99mTc-J591(scFv) showed that J591(scFv) specifically and saturably binds to the PC3LN3-PSMA cell line with an EC50 value of 3.7 nmol/l, whereas only nonspecific binding was observed in the PC3LN3 cell line.
Fig. 3.
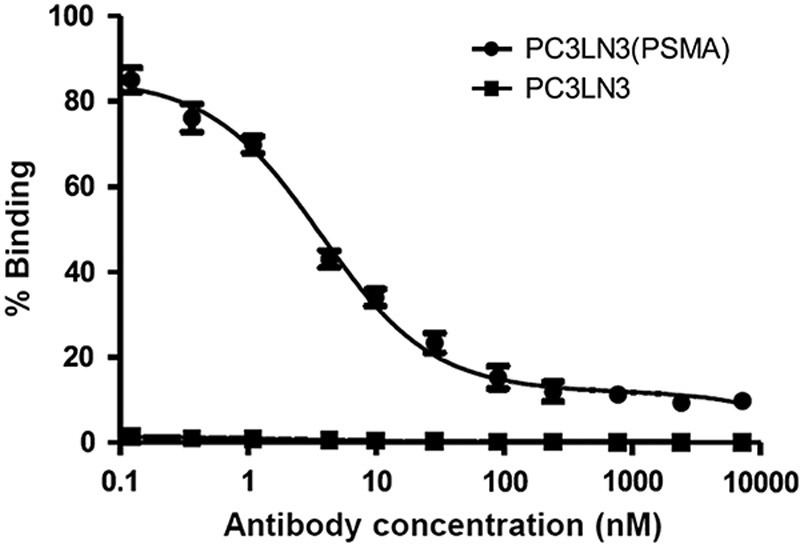
Binding of 99mTc-J591(scFv) to PC3LN3 parental and PC3LN3-PSMA overexpressing cell lines. Each data point is mean±SD for three measurements.
Discussion
The role of molecular imaging in localisation and staging of PCa is becoming increasingly important, with a variety of probes under development 11. Although recent interest has focused on small molecules, there may be a role for antibody-based targeting and the preparation of a radiolabelled scFv fragment may offer a suitable balance between binding affinity and pharmacokinetics suited to short half-lives such as those of 99mTc or 68Ga.
J591(scFv) was conveniently produced in mammalian cells at a moderate yield of 10–12 mg/l of J591(scFv). Purification by an Ni-NTA column, followed by size exclusion chromatography yielded the monomer, which was characterised by SDS-PAGE, western blotting and SEC. The product could be stored at −80°C for at least 3 weeks with minimal dimerisation of J591(scFv).
Site-specific labelling of the His-tag of J591(scFv) was performed by a two-step procedure. First, 99mTc tricarbonyl was prepared in a good yield by the addition of 99mTc-pertechnetate to a kit and heating. The 99mTc tricarbonyl was then incubated with J591(scFv), resulting in a radiochemical yield of 85–100%. At low protein concentrations, the radiochemical yield increased during incubation at 37°C up to 60 min, after which there was no further increase. For high concentrations of J591(scFv), the time of incubation had no effect on the radiolabelling yield. A high NaCl concentration (500 mmol/l) modestly increased the radiolabelling yield as we have reported previously 20.
A final radiochemical purity of more than 99% could be achieved after a gel filtration purification step in which a Sephadex G-25 column removed any free pertechnetate or tricarbonyl from the reaction mixture. This was particularly important for the serum stability experiments, although there was some loss of radiolabelled compound on the column (recovery ∼80%). J591(scFv) was radiochemically stable in serum, with no change in the speciation of 99mTc over 24 h.
Cell-binding experiments were carried out using negative and PSMA-positive PCa cell lines. In addition to specific binding to the receptor, nonspecific binding can also occur because of hydrophobic and ionic interactions with other sites on the cell surface, and it is important to identify the contribution of specific and nonspecific binding towards the total binding observed. Nonspecific binding was measured using the negative cell line. Measurement of the binding of J591(scFv) using 99mTc-J591(scFv) showed specific, saturable binding to the PC3LN3-PSMA cell line with an EC50 value of 3.7 nmol/l, whereas only nonspecific binding was observed in the PC3LN3 cell line (Fig. 3). Although this does not provide an EC50 or an affinity value for 99mTc-J591(scFv) itself, it shows that 99mTc-J591(scFv) binds specifically to PC3LN3-PSMA cells in a qualitatively similar manner to J591(scFv).
In parallel with this work, we have been evaluating the potential utility of a diabody (MWt: 54 kDa) generated from J591 22. It was labelled with 99mTc tricarbonyl in a manner similar to the present work and at a similar specific activity. Specific binding of the diabody to a PSMA overexpressing variant of the DU145 cell line was found, with an IC50 of 5 nmol/l, very similar to the value observed in the present work. The 99mTc-labelled diabody was evaluated in SCID beige mice bearing DU145-PMSA xenografts with SPECT imaging, followed by biodistribution studies. Blood pool clearance was somewhat slow and tumours were not visualised until 4 h after injection, with optimal contrast at 8 h, at which time the tumour/blood ratio was 8 and the tumour/muscle ratio was 17 22. This indicates the feasibility of the approach, but suggests that the scFv described in the present work might be more suitable as scFv fragments typically show more rapid blood clearance than a diabody.
Conclusion
J591(scFv) can be radiolabelled with 99mTc tricarbonyl conveniently and efficiently. In these small-scale studies, the maximum specific activity achieved was 7 MBq/μg. The labelled product was stable in serum. It showed selective, saturable binding to PSMA-positive cells compared with PSMA-negative cells. This potential radiotracer warrants in-vivo evaluation in PCa xenograft models.
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.nuclearmedicinecomm.com).
Acknowledgements
The authors thank Prof John maher and Dr Florian Kampmeier, King’s College London, for assistance in the preparation of the J591(scFv) sequence and PC3LN3 and PC3LN3-PSMA cell lines and protein production. Isolink kits were donated by Covidien Healthcare. 99mTc-pertechnetate was supplied by the radiopharmacy at Guy’s and St Thomas’ Hospital, London. Saima Nawaz was a recipient of a PhD studentship sponsored by Imaging Equipment Limited, Chilcompton, Somerset BA3 4HP. This research was supported by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC (WT088641/Z/09/Z), the KCL and UCL Comprehensive Cancer Imaging Centre funded by Cancer Research UK and EPSRC in association with the MRC and DoH (England), and by the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the DoH.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998; 52:637–640. [DOI] [PubMed] [Google Scholar]
- 2.Sodee DB, Nelson AD, Faulhaber PF, Maclennan GT, Resnick MI, Bakale G. Update on fused capromab pendetide imaging of prostate cancer. Clin Prostate Cancer 2005; 3:230–238. [DOI] [PubMed] [Google Scholar]
- 3.Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther 2008; 8:175–181. [DOI] [PubMed] [Google Scholar]
- 4.Nargund V, Al Hashmi D, Kumar P, Gordon S, Otitie U, Ellison D, et al. Imaging with radiolabelled monoclonal antibody (MUJ591) to prostate-specific membrane antigen in staging of clinically localized prostatic carcinoma: comparison with clinical, surgical and histological staging. BJU Int 2005; 95:1232–1236. [DOI] [PubMed] [Google Scholar]
- 5.Tsui P, Rubenstein M, Guinan P. Correlation between PSMA and VEGF expression as markers for LNCaP tumor angiogenesis. J Biomed Biotechnol 2005; 2005:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandit-Taskar N, O'Donoghue JA, Divgi CR, Wills EA, Schwartz L, Gönen M, et al. Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res 2015; 5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandit-Taskar N, O’Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res 2015; 21:5277–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallabhajosula S, Nikolopoulou A, Jhanwar YS, Kaur G, Tagawa ST, Nanus DM, et al. Radioimmunotherapy of metastatic prostate cancer with 177Lu-DOTA-huJ591 anti prostate specific membrane antigen specific monoclonal antibody. Curr Radiopharm 2016; 9:44–53. [DOI] [PubMed] [Google Scholar]
- 9.Barrett JA, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S, et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med 2013; 54:380–387. [DOI] [PubMed] [Google Scholar]
- 10.Vallabhajosula S, Nikolopoulou A, Babich JW, Osborne JR, Tagawa ST, Lipai I, et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J Nucl Med 2014; 55:1791–1798. [DOI] [PubMed] [Google Scholar]
- 11.Eder M, Eisenhut M, Babich J, Haberkorn U. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging 2013; 40:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43:42–51. [DOI] [PubMed] [Google Scholar]
- 14.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med 2010; 40:167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker SA, Diaz IL, Anderson KA, Batt CA. Design, production, and characterization of a single-chain variable fragment (ScFv) derived from the prostate specific membrane antigen (PSMA) monoclonal antibody J591. Protein Expr Purif 2013; 89:136–145. [DOI] [PubMed] [Google Scholar]
- 16.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, S adelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1999; 1:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng KW. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate 2009; 69:1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberto R, Ortner K, Wheatley N, Schibli R, Schubiger AP. Synthesis and properties of boranocarbonate: a convenient in situ CO source for aqueous preparation of [99mTc(OH2)3(CO)3]+. J Am Chem Soc 2001; 123:3135–3136. [DOI] [PubMed] [Google Scholar]
- 19.Waibel R, Alberto R, Willuda J, Finnern R, Schibli R, Stichelberger A, et al. Stable one-step technetium-99m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat Biotechnol 1999; 17:897–901. [DOI] [PubMed] [Google Scholar]
- 20.Badar A, Williams J, de Rosales RT, Tavaré R, Kampmeier F, Blower PJ, et al. Optimising the radiolabelling properties of technetium tricarbonyl and His-tagged proteins. EJNMMI Res 2014; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 2002; 20:70–75. [DOI] [PubMed] [Google Scholar]
- 22.Kampmeier F, Williams JD, Maher J, Mullen GE, Blower PJ. Design and preclinical evaluation of a 99mTc-labelled diabody of mAb J591 for SPECT imaging of prostate-specific membrane antigen (PSMA). EJNMMI Res 2014; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.nuclearmedicinecomm.com).


