Abstract
Tissue trauma is a frequent complication of cochlear implantation (CI) surgery, but the relationship between intracochlear trauma, electrode insertion forces, and surgeons’ ability to perceive these forces is poorly understood. In this study, we simulated CI surgery using a benchtop apparatus to repeatably apply small forces to subject’s hands while reducing variability in their hand movements. We used a psychophysical testing procedure to estimate the force perception thresholds of ten otologic surgeons, and found a median threshold of 20.4 mN. The results suggest that surgeons have the capability to sense at least some insertion forces and are likely to perceive severe trauma such as occurs when the electrode crosses from one scala to the other.
Keywords: cochlear implantation surgery, trauma, insertion forces, psychophysics, tactile perception
1. Introduction
Cochlear implants have been described as the most successful neural prostheses [Wilson and Dorman, 2008], yet estimates of complication rates in the literature range from 4–40 percent [Brito et al., 2012], and suboptimal hearing outcomes are one potential effect of these complications. Surgeons do not usually report significant physical resistance when advancing an electrode into the cochlea, but intracochlear trauma is known to occur frequently. For example, electrodes translocate from the scala tympani to the scala vestibuli in approximately one third of cochlear implantation (CI) surgeries [Finley et al., 2008; Skinner et al., 2007; Aschendorff et al., 2007]. In previous work, we measured forces while advancing a probe representing an electrode from the scala tympani to the scala vestibuli of cadavers [Schuster et al., 2015], passing through the basilar membrane and other structures separating the vestibuli. We were not able to characterize the perceptibility of the forces recorded in these experiments because to the best of our knowledge, no previous study has investigated surgeons’ abilities to perceive electrode insertion forces during CI surgery.
Perceptual abilities are challenging to measure because they relate to subjective states of awareness which cannot be directly observed. Rigorous methods that address this measurement problem are found in the field of psychophysics [Jones and Tan, 2013; Kingdom and Prins, 2010], a discipline concerned with the relation between physical stimuli and human perception. Systematic measurement of force perception began with the work of Fechner and Weber in the 19th century [Gescheider, 2013], and psychophysical methods are now used widely in applications requiring quantification of sensory abilities. Testing procedures used routinely in audiometry and optometry, for example, are informed by the extensive psychophysics literature.
In modern psychophysics, perception is viewed as a random process in which the perceptibility of a fixed stimulus intensity varies with repeated application of the stimulus. Perception is understood to occur in the presence of noise, including both external noise and the intrinsic noise of the sensory system. Thus, multiple measurements are needed to estimate the minimum stimulus intensity that individual can perceive (i.e., the absolute threshold; hereafter abbreviated as the ‘threshold’). Staircase procedures are one widely used method for estimating thresholds from multiple trials [Kingdom and Prins, 2010; Leek, 2001]. Typically, staircase trials begin with presentation of a large, easily perceivable stimulus intensity, which is reduced by fixed increments until a subject can no longer perceive the stimulus. The stimulus intensity is then increased by fixed steps until it is again perceptible, at which time the direction of the steps is again reversed. A threshold (quantified as a targeted performance level on a psychometric function [Leek, 2001]) is estimated by averaging all the reversal points at which the stimulus changes direction. Often, an “n down, 1-up” rule is used in which n successive correct responses are required before the stimulus can be decreased. The convergence of a given staircase sequence to a threshold depends on the stimulus intensities, step sizes, number of trials and other factors [Garcia-Perez, 1998].
Psychophysical experiments are prone to errors caused by subjects’ desire to perform well. For example, subjects may affirm the presence of a stimulus even when it is absent or imperceptibly small. Such bias is of particular concern in the present study because the subjects, who are surgeons, may interpret success at the experimental task as an indicator of occupational skill. A standard method to eliminate such bias in psychophysics experiments is the two-alternative forced choice (2AFC) method. Rather than replying “yes” or “no” to the presence of a stimuli, subjects are forced to choose between randomly assigned noise and stimulus alternatives. The effects of subjective preference are mitigated, because the correct alternative is presented in a random sequence.
Much of the psychophysics literature relates to the hand and fingertips [Jones, 1986; Jones and Lederman, 2006], but few studies have examined absolute force perception thresholds under conditions comparable to CI surgery. Force thresholds have been measured in the context of diabetic neuropathy testing [Bell-Krotoski and Tomancik, 1987; Voerman et al., 1999], but the point forces applied in neuropathy testing do not resemble the distribution of pressure across a surgeon’s gloved palm and fingertips when grasping a forceps to implant an electrode. Loads distributed across one or more fingertips have been tested by developers of haptic devices. Dosher and Hannaford used a forced-choice, adaptive testing protocol to measure forces applied to the fingertip with a haptic device [Dosher and Hannaford, 2005], and reported average detection thresholds ranging from 30.1 mN to 50.4 mN. Using a similar pyschophysical testing protocol, King, Donlin, and Hannaford [King et al., 2010] applied forces to multiple combinations of fingertips, and reported a threshold range of 27.8 mN to 34.0 mN. Baud-Bovy and Gatti transmitted force via a robot end effector to a spherical handle grasped by a subject, and reported a minimum threshold of 49 mN when subjects were allowed to move the handle to seek the direction of the force [Baud-Bovy and Gatti, 2010]. These studies advance our understanding of the hand’s sensitivity, but the configurations of these devices did not resemble the grasp and motion used in CI surgery.
In this study, we use a 2AFC staircase procedure to estimate the absolute force perception thresholds of a group of otologic surgeons, using an apparatus specifically designed to replicate the arm posture and forceps grasp used in CI surgery.
2. Methods
We recruited twelve subjects for this study by email, phone, and personal communications, and stipulated that all were otolaryngologists who actively performed CI surgery in their clinical practice, or otolaryngology residents at a residency training program approved by the Accreditation Council for Graduate Medical Education (ACGME). Postgraduate year 1 (PGY-1) residents were excluded, as they have little or no training or experience in otologic surgery in the first year of residency. Seven subjects were residents or fellows, and five were attending surgeons. Two subjects were excluded from the study for reasons discussed in Section 3. All subjects were male. This study was approved by the Vanderbilt University Institutional Review Board, and all subjects signed informed consent after reviewing the risks and benefits of participation.
Threshold testing requires application of stimuli that have known intensities. We used Semmes-Weinstein Monofilaments (SWM) to apply forces, which are handles embedded with a nylon monofilament (a single strand of filament). Each filament is calibrated to buckle when a force applied along the filament axis exceeds a particular intensity. SWMs are a standard clinical tool for cutaneous sensory function, and are used regularly to evaluate diabetic peripheral neuropathy. They are recommended by the American Diabetes Association [Mayfield et al., 2004], and their mechanical reliability has been thoroughly demonstrated [Haloua et al., 2011].
In clinical usage, a physician grasps a SWM handle and presses the filament tip into a patient’s skin until the filament buckles. In our experiment, subjects grasped SWM handles as they would hold a forceps to implant a cochlear implant electrode, with the filament in the approximate position of an electrode. By buckling the filament, a calibrated force was applied to subjects’ hands in a way that resembled the transmission of force through CI forceps (we shall describe how the filaments were buckled shortly).
SWM’s are provided in a standard set of buckling forces, which follow a logarithmic scale [Mueller, 1996]. A logarithmic scale is used because the perceived intensity of sensory stimuli is approximately proportional to the logarithm of the intensities of those stimuli. We selected a range of eight SWM force intensities, listed in Table 2, to span the anticipated threshold range of all subjects.
Table 2.
Semmes-Weinstein monofilaments (SWMs) were selected from the standard clinical set to span the expected range for the force threshold during CI surgery. The SWMs are presented in both grams, which are customary for SWMs, and millinewton force units
| Force (g) | Force (mN) |
|---|---|
| 0.4 | 3.9 |
| 0.6 | 5.9 |
| 1 | 9.8 |
| 1.4 | 13.7 |
| 2 | 19.6 |
| 4 | 39.2 |
| 6 | 58.8 |
| 8 | 78.5 |
| 10 | 98.1 |
| 15 | 147.1 |
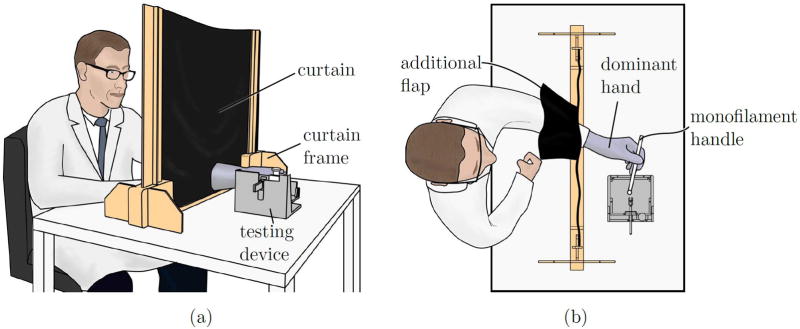
Prior to testing, subjects were seated behind an opaque curtain, shown in Figure 1, which prevented them from seeing both their tested hand and the experimenter. Subjects donned a suitably sized surgical glove on the dominant hand and passed this hand through a slit in the curtain, which was covered by an additional flap of fabric to prevent observation of the tested hand through the slit.
Figure 1.
a) An opaque curtain is placed between subjects and the testing device to prevent visual observation of the experimental apparatus and experimenter during trials. The curtain ensures that a subject experiences only tactile feedback and cannot see his or her tested hand, which may contain an experimental SWM handle (with filament) or a control handle (with filament removed). (b) A top view of a seated subject. The subject’s dominant hand is inserted through a slit in the curtain, which is covered with an additional flap of curtain fabric to prevent observation of the testing apparatus through gaps around the wrist. The experimenter (not shown) sits opposite to the subject. The curtain prevents the subject from visually observing the experimenter.
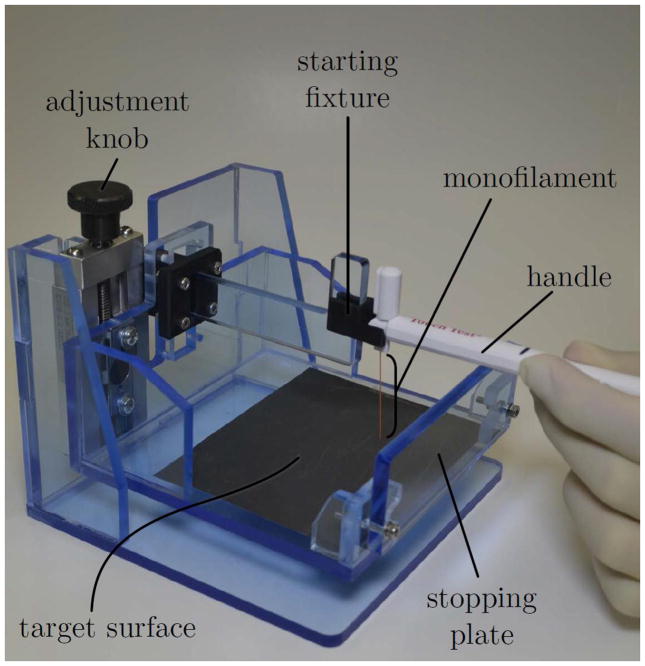
A fixture, shown in Figure 2 was located behind the curtain. We designed this fixture to cause reliable buckling of SWM filaments and to reduce variability in the motion of the SWM handles among subjects. Because subjects could not see their tested hands, the fixture was needed to guide the motion of the handle between two fixed positions. Before commencing each trial, the experimenter guided the filament-bearing end of the SWM handle into a starting fixture, shown in Figure 3(a), which cradled the handle above a target surface. The distance between the starting fixture and the target surface was fixed, but the mutual height of these components above the tabletop was adjustable. The experimenter adjusted this height, as shown in Figure 3(b), such that the SWM handle was approximately parallel to the tabletop when the tip of the handle was in the starting fixture. Without a means to adjust the height of the starting fixture, the SWM handle would be initially inclined with respect to the target surface at an angle determined by the width of a subject’s palm. Such initial angulation of the handle could have caused the filament to contact the target surface at a steep angle and possibly skid or bend rather than buckle.
Figure 2.
A custom-built testing device used to model the advancement of forceps and transmission of electrode-basilar membrane contact forces during cochlear implantation surgery.
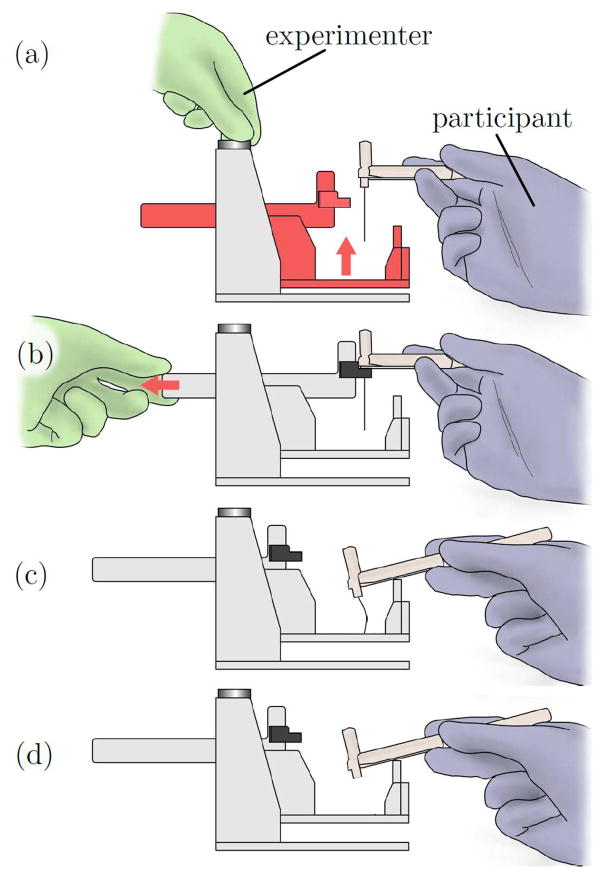
Figure 3.
(a) In each trial, a subject lowers a Semmes-Weinstein Monofilament (SWM) handle from the starting fixture until it contacts the stopping plate. If a monofilament is attached to the SWM handle (control trials omit the monofilament), the monofilament will buckle against the target surface when the handle contacts the stopping plate, causing a calibrated force to be applied to the subject’s hand. (b) Before testing, the device is adjusted to the height of each subject’s hand, such that the initial orientation of the SWM handle is approximately parallel to the tabletop (c). To begin each trial, the experimenter places the SWM handle in the starting fixture, which is at a fixed height above the stopping plate and target surface. The experimenter then retracts the starting fixture and instructs the subject to lower the SWM handle. (d) The handle rests on the stopping plate at the end of each trial. (e) For control trials, a SWM handle with no filament is used.
To commence a trial, the experimenter pulled a handle to release the end of the SWM handle from the starting fixture, as shown in Figure 3(c). The subject then followed instructions to lower the handle until it contacted a stopping plate, in which position the filament was buckled against the target surface, as shown in Figure 3(d). The target surface was covered in sandpaper to prevent sliding of the filament tip. The height of the stopping plate was chosen to be slightly shorter than the shortest filament length in order to stop advancement just after buckling, preventing the filament from bending severely or skidding on the target surface.
Our testing procedure with the apparatus followed a 2AFC, three-down, one-up (3D1U) staircase procedure. Previously, Tracey, Greene, and Doty evaluated the test-retest of several 2AFC procedures for SWM testing of tactile thresholds [Tracey et al., 2012]. The authors found that a test-retest reliability coefficient greater than 0.80 could be achieved with as few as two reversal pairs using a 3D1U method. Encouraged by these results, we adapted the 2AFC-3D1U procedure estimate surgeons’ force perception thresholds.
Before commencing trials, the experimenter read instructions to each subject from a script. To implement the 2AFC method, a control SWM was prepared by removing the filament from a standard SWM handle, as illustrated in Figure 3(d). In each trial, the control SWM device and one SWM device from the set listed in Table 2 were presented in random order. The experimenter placed a SWM handle (either a normal SWM or the control) in a subject’s hand, and then placed the handle in the starting fixture. After retracting the starting fixture, the experimenter read scripted instructions to the subject to begin lowering the SWM handle at the speed he would use to insert a CI electrode, until the SWM handle contacted the stopping plate. This sequence was then repeated with the second device of the 2AFC pair. Following each 2AFC pair, subjects were instructed to report which device (the first or the second) was associated with force sensation.
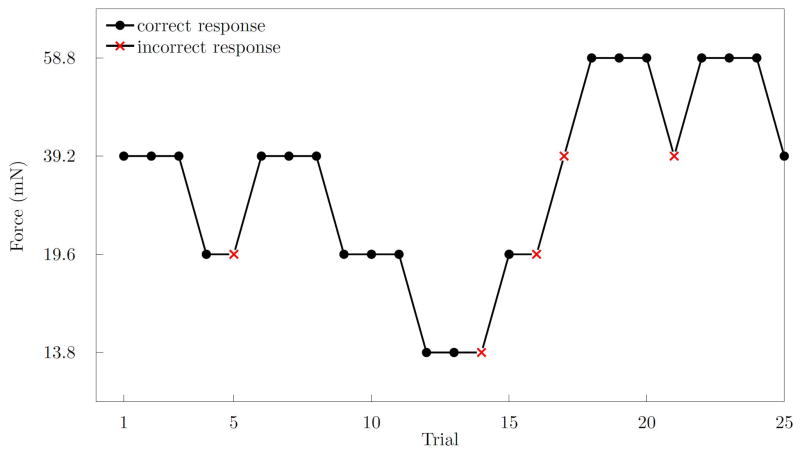
Our staircase procedure began with a filament buckling strength of 39.2 mN which we predicted would be perceivable by all subjects at all times. After three consecutive correct 2AFC trials, the filament strength was decreased by one step in the series listed in Table 2. Conversely, an incorrect response, implying an inability to perceive the presented force, was followed by an increase in filament strength in the subsequent trial. All subjects were tested with 25 pairs of filaments. A force perception threshold was calculated for each surgeon using the arithmetic mean of the log-transformed stimulus intensity values (thresholds are calculated in units in which the steps are constant [Garcia-Perez, 1998]). One representative staircase plot is shown in Figure 4.
Figure 4.
A plot generated from the responses of a single subject demonstrating correct (black) and incorrect (red) responses, and the resulting staircase. Three correct responses were required before decreasing the stimulus intensity. This plot shows three reversal pairs consisting of six reversal points. A reversal point is defined as a change in direction of the plot.
3. Results
Using the 2AFC-3D1U staircase procedure, we found a median threshold of 20.4 mN, with a range of 10.8 mN to 36.5 mN, as listed in Table 3. All twelve subjects completed testing, but two (1 resident, 1 attending) were excluded from the analysis because their performance exceeded the range of SWM filaments given in Table 2 during staircase testing. One subject exceeded the maximum strength filament in our set, and the other exceeded the weakest filament. Thus, we were not able to compute thresholds for these two individuals.
Table 3.
Force perception threshold statistics computed from ten otolaryngological surgeon subjects
| Resident/Fellow (n=6) | Attending (n=4) | All (n=10) | |
|---|---|---|---|
| Median (mN) | 20.4 | 26.6 | 22.3 |
| Min (mN) | 10.8 | 17.4 | 10.8 |
| Max (mN) | 33.6 | 36.5 | 36.5 |
4. Discussion
Electrode insertion forces during CI surgery range from approximately 0 mN to 250 mN. We reported a median force perception threshold of 22.3 mN, which suggests that surgeons have the capability to perceive at least some forces in CI surgery. To date, no study has measured force perception thresholds as they pertain to CI surgery. Force thresholds for other surgical tasks have been examined [Zhou et al., 2008], but to our knowledge, this study is the first to measure surgeon’s force perception thresholds using psychophysical methods. The results may aid the design of less traumatic CI surgical techniques and devices.
Electrode insertion forces may depend on the speed of insertion, the type of electrode, the insertion tool, intracochlear anatomy, and other factors. Furthermore, forces vary continuously during insertion, and force profiles vary from surgery to surgery. To control for the many possible sources of variation in the clinical environment, we standardized subjects’ hand motions and applied forces of constant magnitude. By applying forces abruptly and in the absence of pre-existing forces, we sacrificed some fidelity to clinical conditions. Rapidly changing forces that are applied in the absence of other forces may be easier to perceive than forces that rise gradually [Jones and Lederman, 2006]. Thus, the ability to identify traumatic forces may be diminished when experienced in combination with other forces of benign origin. Before an electrode rounds the basal turn, frictional forces on the electrode are often very small, thus our results are directly applicable to the perceptibility of forces during this phase of the surgery.
Previously, we reported a median force of 88 mN to puncture the inner membrane of the scala tympani with a probe [Schuster et al., 2015]. Puncture of this membrane occurs when electrodes translocate from the scala tympani to the scala vestibuli, a traumatic event that occurs in approximately one-third of CI surgeries. Our present results suggest that this frequent form of trauma is perceptible to surgeons, though the puncture forces we measured directly at the membrane may be higher than the forces transmitted to the hand during puncture.
5. Conclusion
Surgeons’ force perception thresholds can be estimated using a simple, benchtop model of CI surgery. We estimated force thresholds using psychophysical testing methods, which are beneficial for handling variability and bias.
To improve measurement of force perception thresholds, future work should aim for greater fidelity to clinical conditions. Time varying forces could be applied by an actuator, but haptic rendering of small forces is technically challenging (see [Gurari and Baud-Bovy, 2014], for example). Force sensors have been used to continuously measure insertion forces in both cochlear phantoms and cadaveric specimens [Majdani et al., 2010], and instrumentation could be developed to acquire in vivo force measurements. However, when recording forces over time, threshold estimation will be complicated by the reaction time delay between the onset of force perception and a surgeon’s signal that a force has been perceived. Reaction times range from approximately 100–200 ms, which would make it difficult to distinguish a perceived force from nearby values on a rapidly fluctuating force recording. Furthermore, methods will be needed to control for false positives in cadaveric or in vivo experiments. Our experimental model is easily replicable, controls for false positives, and could be used to enlarge the available data while more elaborate methods for estimating force perception thresholds in CI surgery are developed.
Table 1.
Demographics of subjects included in analysis of force perception thresholds. All subjects were male and right-handed
| Resident/Fellow (n=6) | Attending (n=4) | |
|---|---|---|
| Average Age (years)† | 30.5 (27, 36) | 53.5 (37, 79) |
| Fellowship Training Completed | ||
| Otology/Neurotology | n/a | 3 (75 %) |
| Pediatrics | n/a | 1 (25 %) |
| Number of CI Surgeries per Month† | n/a | 4 (3, 9) |
Median (min, max)
Acknowledgments
Funding
The project described was supported by Award Number R01DC008408 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Footnotes
Conflicts of Interest
Author Labadie consulted for Advanced Bionics Corporation during the two years prior to submission of this article. The remaining authors declare that there are no conflicts of interest.
References
- Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear and Hearing. 2007;28(2):75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- Baud-Bovy G, Gatti E. Hand-held object force direction identification thresholds at rest and during movement. Haptics: Generating and Perceiving Tangible Sensations. 2010:231–236. [Google Scholar]
- Bell-Krotoski J, Tomancik E. The repeatability of testing with Semmes-Weinstein monofilaments. The Journal of Hand Surgery. 1987;12(1):155–161. doi: 10.1016/s0363-5023(87)80189-2. [DOI] [PubMed] [Google Scholar]
- Brito R, Monteiro TA, Leal AF, Tsuji RK, Pinna MH, Bento RF. Surgical complications in 550 consecutive cochlear implantation. Brazilian Journal of Otorhinolaryngology. 2012;78(3):80–85. doi: 10.1590/S1808-86942012000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher J, Hannaford B. Human interaction with small haptic effects. Presence: Teleoperators and Virtual Environments. 2005;14(3):329–344. [Google Scholar]
- Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, Skinner MW. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otology & Neurotology. 2008;29(7):920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez MA. Forced-choice staircases with fixed step sizes: asymptotic and small-sample properties. Vision Research. 1998;38(12):1861–1881. doi: 10.1016/s0042-6989(97)00340-4. [DOI] [PubMed] [Google Scholar]
- Gescheider GA. Psychophysics: the Fundamentals. 3. Psychology Press; 2013. [Google Scholar]
- Gurari N, Baud-Bovy G. Customization, control, and characterization of a commercial haptic device for high-fidelity rendering of weak forces. Journal of Neuroscience Methods. 2014;235:169–180. doi: 10.1016/j.jneumeth.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Haloua MH, Sierevelt I, Theuvenet WJ. Semmes-Weinstein monofilaments: influence of temperature, humidity, and age. The Journal of Hand Surgery. 2011;36(7):1191–1196. doi: 10.1016/j.jhsa.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Jones LA. Perception of force and weight: theory and research. Psychological Bulletin. 1986;100(1):29. [PubMed] [Google Scholar]
- Jones LA, Lederman SJ. Human Hand Function. 1. New York, Oxford: University Press; 2006. [Google Scholar]
- Jones LA, Tan HZ. Application of psychophysical techniques to haptic research. IEEE Transactions on Haptics. 2013;6(3):268–284. doi: 10.1109/TOH.2012.74. [DOI] [PubMed] [Google Scholar]
- King HH, Donlin R, Hannaford B. Perceptual thresholds for single vs. multi-finger haptic interaction. IEEE Haptics Symposium; 2010; pp. 95–99. [Google Scholar]
- Kingdom F, Prins N. Psychophysics: a Practical Introduction. 1. New York: Academic Press; 2010. [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Perception & Psychophysics. 2001;63(8):1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- Majdani O, Schurzig D, Hussong A, Rau T, Wittkopf J, Lenarz T, Labadie RF. Force measurement of insertion of cochlear implant electrode arrays in vitro: comparison of surgeon to automated insertion tool. Acta Oto-laryngologica. 2010;130(1):31–36. doi: 10.3109/00016480902998281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM American Diabetes Association. Preventative Foot Care in Diabetes. Diabetes Care. 2004;27(1 Suppl):S63–4. doi: 10.2337/diacare.27.2007.s63. [DOI] [PubMed] [Google Scholar]
- Mueller MJ. Identifying patients with diabetes mellitus who are at risk for lower-extremity complications: use of Semmes-Weinstein monofilaments. Physical Therapy. 1996;76(1):68–71. doi: 10.1093/ptj/76.1.68. [DOI] [PubMed] [Google Scholar]
- Schuster D, Kratchman LB, Labadie RF. Characterization of intracochlear rupture forces in fresh human cadaveric cochleae. Otology & Neurotology. 2015;36(4):657–661. doi: 10.1097/MAO.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JG, Saxon EA, Hullar TE, Finely CC. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. The Annals of Otology, Rhinology & Laryngology Supplement. 2007;116(4):2–24. [PubMed] [Google Scholar]
- Tracey EH, Greene AJ, Doty RL. Optimizing reliability and sensitivity of Semmes-Weinstein monofilaments for establishing point tactile thresholds. Physiology & Behavior. 2012;105(4):982–986. doi: 10.1016/j.physbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Voerman VF, van Egmond J, Crul BJ. Normal values for sensory thresholds in the cervical dermatomes: A critical note on the use of Semmes-Weinstein monofilaments. American Journal of Physical Medicine & Rehabilitation. 1999;78(1):24–29. doi: 10.1097/00002060-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: current designs and future possibilities. Journal of Rehabilitation Research & Development. 2008;45(5):695–730. doi: 10.1682/jrrd.2007.10.0173. [DOI] [PubMed] [Google Scholar]
- Zhou M, Perreault J, Schwaitzberg S, Cao C. Effects of experience on force perception threshold in minimally invasive surgery. Surgical Endoscopy. 2008;22(2):510–515. doi: 10.1007/s00464-007-9499-y. [DOI] [PubMed] [Google Scholar]