Abstract
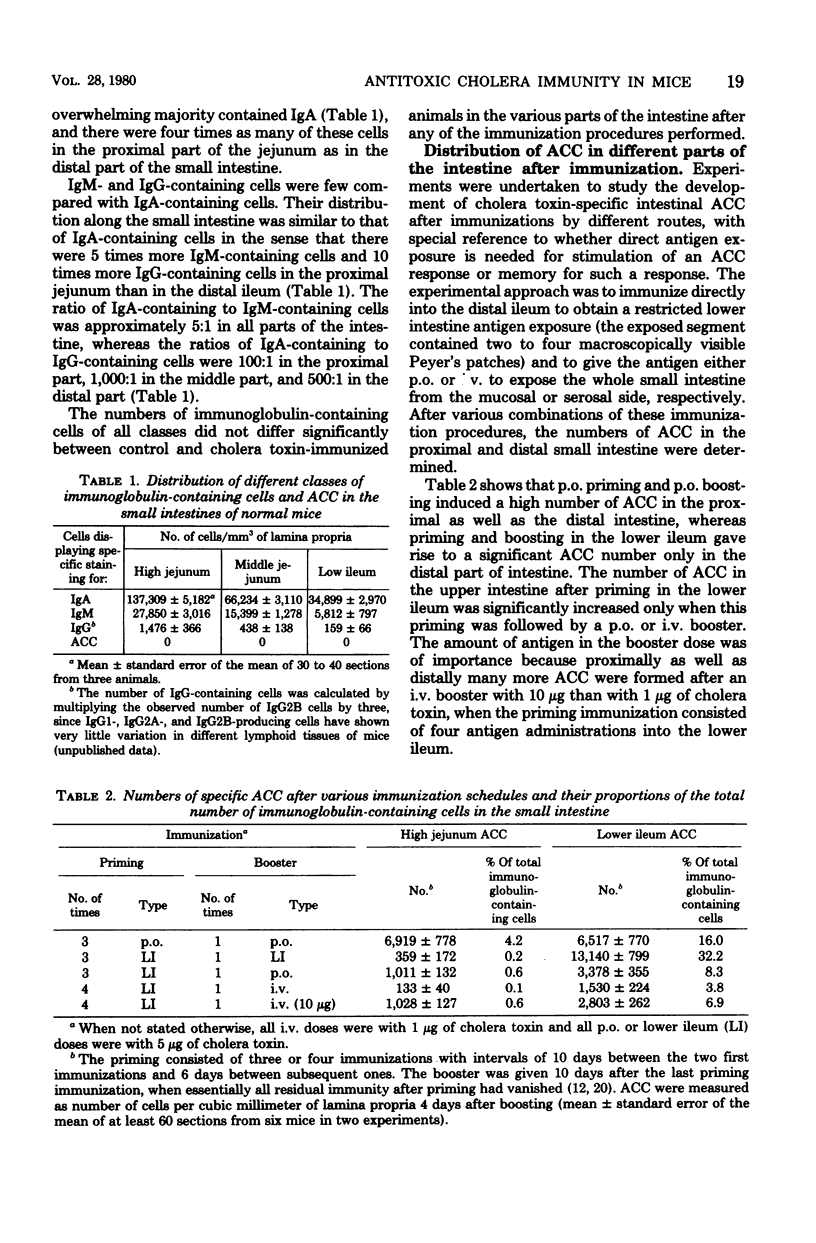
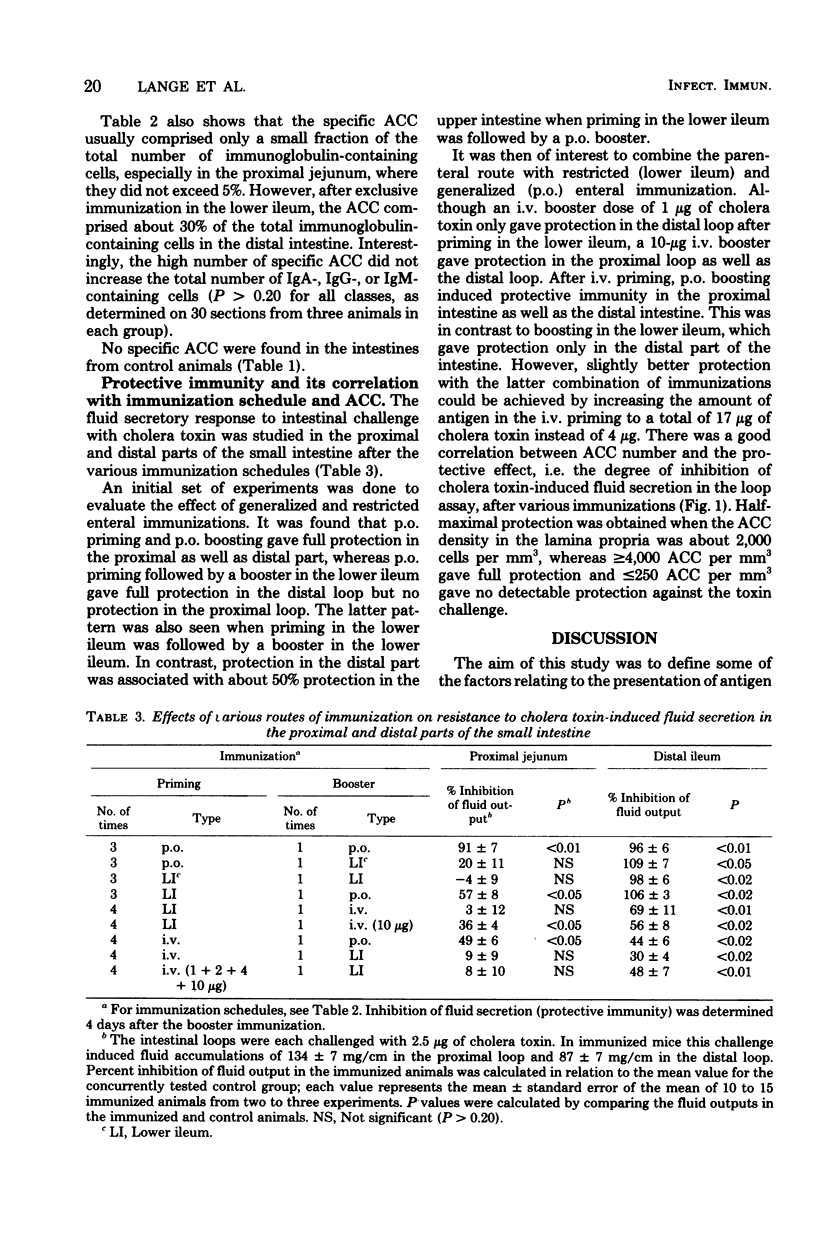
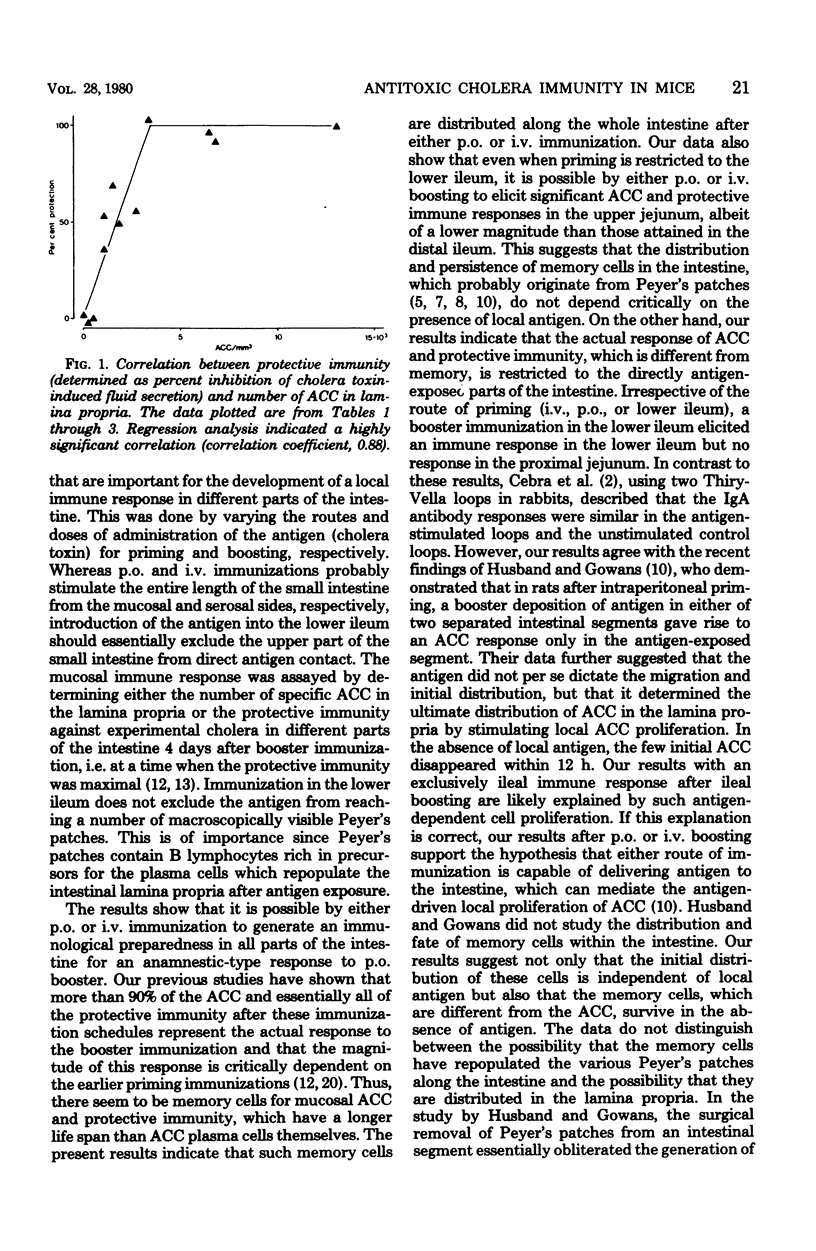
The importance of the mode of antigen presentation (intravenous, oral, or enteral restricted to the lower ileum) in the development of a local immune response and immunological memory for such a response in different parts of the intestine was studied in mice. Cholera toxin was used as antigen and the immune response was assayed by determining both the number of specific antitoxin-containing cells in the lamina propria and protection against experimental cholera. The results showed that all of these routes of antigen presentation could induce significant memory along the entire small intestine. In contrast, the actual production of antitoxin-containing cells or protective immune response elicited by booster immunization was restricted to those parts of the intestine that were directly exposed to antigen; i.e., lower ileum boosting resulted in immunity in the distal ileum but not in the proximal jejunum, whereas oral or intravenous boosting gave a response in both jejunum and ileum. Protection correlated closely with the number of antitoxin-containing cells in the lamina propria (correlation coefficient, 0.88); ≥4,000 antitoxin-containing cells per mm3 conferred solid immunity to cholera toxin-induced diarrhea. The total number of immunoglobulin-containing cells in intestines was not significantly influenced by the specific immunizations. There were four times as many of these cells in the upper jejunum (167,000 cells per mm3) as in the lower ileum, but the proportions of immunoglobulin A-containing cells (80 to 85%), immunoglobulin M-containing cells (14 to 20%), and immunoglobulin G-containing cells (0.4 to 0.9%) were similar in various parts of the intestine. The results indicate a differential dependence on local tissue antigen for the intestinal antibody-secreting cells and their memory cell precursors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienenstock J., McDermott M., Befus D., O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv Exp Med Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Kamat R., Gearhart P., Robertson S. M., Tseng J. The secretory IgA system of the gut. Ciba Found Symp. 1977 Apr 26;(46):5–28. doi: 10.1002/9780470720288.ch2. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Heremans J. F. The distribution of immunoglobulin-containing cells along the human gastrointestinal tract. Gastroenterology. 1966 Sep;51(3):305–316. [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Orlans E. Studies on the lymphocytes of sheep. III. Destination of lymph-borne immunoblasts in relation to their tissue of origin. Eur J Immunol. 1977 Jan;7(1):30–37. doi: 10.1002/eji.1830070108. [DOI] [PubMed] [Google Scholar]
- Halstead T. E., Hall J. G. The homing of lymph-borne immunoblasts to the small gut of neonatal rats. Transplantation. 1972 Sep;14(3):339–346. doi: 10.1097/00007890-197209000-00009. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Beh K. J., Lascelles A. K. IgA-containing cells in the ruminant intestine following intraperitoneal and local immunization. Immunology. 1979 Jul;37(3):597–601. [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Hansson H. A., Molin S. O., Nygren H. Local cholera immunity in mice: intestinal antitoxin-containing cells and their correlation with protective immunity. Infect Immun. 1979 Mar;23(3):743–750. doi: 10.1128/iai.23.3.743-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978 Aug;86C(4):145–152. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Mattioli C. A., Tomasi T. B., Jr The life span of IgA plasma cells from the mouse intestine. J Exp Med. 1973 Aug 1;138(2):452–460. doi: 10.1084/jem.138.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Fisher R. IgA-containing plasma cells in the lamina propria of the gut: failure of a thoracic duct fistula to deplete the numbers in rat small intestine. Eur J Immunol. 1979 Jan;9(1):85–91. doi: 10.1002/eji.1830090118. [DOI] [PubMed] [Google Scholar]
- Moore A. R., Hall J. G. Evidence for a primary association between immunoblasts and small gut. Nature. 1972 Sep 15;239(5368):161–162. doi: 10.1038/239161a0. [DOI] [PubMed] [Google Scholar]
- Nygren H., Hansson H. A., Lange S. Studies on the conjugation of horseradish peroxidase to immunoglobulin G via glutaraldehyde. Med Biol. 1979 Jun;57(3):187–191. [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. L., Lascelles A. K. The influence of systemic immunisation during mammary involution on subsequent antibody production in the mammary gland. Res Vet Sci. 1975 Mar;18(2):182–185. [PubMed] [Google Scholar]


