Abstract
Importance
In 2008, Medicare implemented the Hospital-Acquired Conditions (HACs) Initiative, a policy denying incremental payment for 8 complications of hospital care, also known as never events. The regulation's effect on these events has not been well studied.
Objective
To measure the association between Medicare's nonpayment policy and 4 outcomes addressed by the HACs Initiative: central line–associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), hospital-acquired pressure ulcers (HAPUs), and injurious inpatient falls.
Design, Setting, And Participants
Quasi-experimental study of adult nursing units from 1381 US hospitals participating in the National Database of Nursing Quality Indicators (NDNQI), a program of the American Nurses Association. The NDNQI data were combined with American Hospital Association, Medicare Cost Report, and local market data to examine adjusted outcomes. Multilevel models were used to evaluate the effect of Medicare's nonpayment policy on never events.
Exposures
United States hospitals providing treatment for Medicare patients were subject to the new payment policy beginning in October 2008.
Main Outcomes And Measures
Changes in unit-level rates of HAPUs, injurious falls, CLABSIs, and CAUTIs after initiation of the policy.
Results
Medicare's nonpayment policy was associated with an 11% reduction in the rate of change in CLABSIs (incidence rate ratio [IRR], 0.89; 95% CI, 0.83-0.95) and a 10% reduction in the rate of change in CAUTIs (IRR, 0.90; 95% CI, 0.85-0.95), but was not associated with a significant change in injurious falls (IRR, 0.99; 95% CI, 0.99-1.00) or HAPUs (odds ratio, 0.98; 95% CI, 0.96-1.01). Consideration of unit-, hospital-, and market-level factors did not significantly alter our findings.
Conclusions And Relevance
The HACs Initiative was associated with improvements in CLABSI and CAUTI trends, conditions for which there is strong evidence that better hospital processes yield better outcomes. However, the HACs Initiative was not associated with improvements in HAPU or injurious fall trends, conditions for which there is less evidence that changing hospital processes leads to significantly better outcomes.
The Hospital-Acquired Conditions (HACs) Initiative, mandated by Congress in the Deficit Reduction Act of 2005 and implemented in 2008, was one in a series of Centers for Medicare & Medicaid Services (CMS) payment reforms intended to increase emphasis on value-based purchasing.1 Eight complications, known as never events, were identified by the Department of Health and Human Services as high-cost or high-volume events that could reasonably be prevented through the application of evidence-based guidelines.2 Injury from falls, hospital-acquired pressure ulcers (HAPUs), catheter-associated urinary tract infections (CAUTIs), and central line–associated bloodstream infections (CLABSIs) were among the 8 never events covered by the HACs Initiative. Under the HACs Initiative, hospitals could no longer justify a higher-level Medicare severity diagnosis related group (MS-DRG) to recover costs incurred in caring for patients who developed 1 of the 8 never events.
Initial assessments of the policy focused on financial impact, estimated to be quite small.3-6 A recent analysis7 of CLABSIs and CAUTIs found no evidence that the policy had any measureable effect. However, this analysis was limited to 398 of the 1166 hospitals participating in the National Healthcare Safety Network, and the findings were limited by the small sample size and low response rate. In addition, no analysis has looked at the policy's effect on a wider range of HACs. Although recent quality improvement efforts8-10 have yielded dramatic improvements in CLABSIs and CAUTIs, hospitals still struggle to identify evidence-based practices that significantly improve HAPUs and injurious falls. This heterogeneous experience suggested that the effect of the HACs Initiative might vary by type of outcome. Using outcome data reported to the National Database of Nursing Quality Indicators (NDNQI) for 4 HACs, we conducted a comprehensive impact assessment of the CMS nonpayment policy.
Methods
Data Sources
This study was approved by the institutional review boards of the University of Tennessee Health Science Center, University of Florida, and Virginia Commonwealth University. Because data were reported at the unit level, informed consent was not required.
Established by the American Nurses Association in 1998, the NDNQI is a data collection project administered by the University of Kansas School of Nursing. Hospitals join the NDNQI to benchmark their performance on nursing-sensitive indicators and, in some cases, to facilitate achieving and maintaining magnet designation. Participation in the NDNQI is voluntary, and hospitals pay an annual membership fee based on the number of beds. Member hospitals choose their data coordinator, who serves as a liaison with the NDNQI and ensures accurate data collection and reporting. The NDNQI provides training and support to data coordinators and their local designees (eg, infection control personnel), centralized data management, and quarterly dashboards for benchmarking outcomes with national peer comparison data. Participating hospitals agree to provide reliable data according to the NDNQI measure guidelines. Secure, web-based data entry with preprogrammed validations and postentry audits for errors and outliers are also used to ensure data integrity.
Currently, more than 1900 US hospitals contribute data to the NDNQI. We obtained data on 4 common HACs: HAPUs, injurious inpatient falls, CLABSIs, and CAUTIs. Unit-level HACs, volume (patients and patient-days), and unit characteristics (type and nurse staffing) data for periods before and after implementation of the CMS nonpayment policy change were obtained for adult medical, surgical, step-down, and intensive care units (ICUs) participating in the NDNQI.
American Hospital Association (AHA) annual survey data were merged with the NDNQI data to gather information on hospital ownership type, teaching status, system membership, services offered, staffed number of beds, adjusted patient-days, and payer market shares. We supplemented this information with Medicare case mix and financial performance data (total profit margin) available from CMS cost reports and inpatient, prospective payment system, final-rule impact files. Additional county-level data were abstracted from the Area Resource File, the Census Bureau, HealthLeaders-InterStudy, the Bureau of Labor Statistics, and the Bureau of Economic Analysis.
Outcome Measures
Pressure Ulcers
Trained nurses assessed the prevalence of pressure ulcers on a preselected day in the quarter (stages I-IV; hospital- and community-acquired). The NDNQI pressure ulcer indicator has been demonstrated to be reliable and is endorsed by the National Quality Forum.11,12 To maintain consistency with the CMS HACs, we selected only data on stage III/IV HAPUs, which are those that have resulted in full-thickness tissue loss. We constructed quarterly HAPU rates (patients with stage III/IV HAPUs per total patients present during the prevalence check) for all participating adult nursing units (ICU and non-ICU) for July 1, 2006, to December 31, 2010.
Injurious Falls
Using their incident reporting system combined with appropriate follow-up, hospitals report all inpatient falls to the NDNQI; to maintain some consistency with the CMSHACs, we selected patient falls with injury levels in the categories of minor and greater during each calendar month for all adult surgical, medical, and medical-surgical units, excluding ICUs and step-down units, where falls are most common.13,14 Monthly injurious fall rates (injurious falls per 1000 patient-days) were constructed for these nursing units for July 1, 2006, to December 31, 2010.
Central Line–Associated Bloodstream Infections
Hospitals reporting to the NDNQI identified all infections meeting Centers for Disease Control and Prevention (CDC) case definitions for laboratory-confirmed bloodstream infections in ICU patients with 1 or more central lines.15 The NDNQI infection definitions (CLABSI, CAUTI) have always matched those used by the National Hospital Safety Network. Through December 31, 2010, the NDNQI captured infections only for ICUs in the participating hospitals. Monthly CLABSI rates (infections per 1000 central line–days) were constructed for all participating adult ICUs for January 1, 2008, to December 31, 2010. Because infection rates were not added to the NDNQI until the end of 2007, we could not capture earlier time periods. Hospitals reporting CLABSIs and CAUTIs on January 1, 2008, had already been reporting other measures to the NDNQI for a mean of 9.24 years.
Catheter-Associated Urinary Tract Infections
Participating NDNQI hospitals reported all CAUTIs in ICUs meeting CDC definitions.15 Monthly CAUTI rates (infections per 1000 indwelling urinary catheter–days) were constructed for all participating adult ICUs from January 1, 2008, to December 31, 2010.
Statistical Analysis
We included all nonfederal US hospitals participating in the NDNQI in our analyses. Our examination of the outcome data (rates and proportions) indicated that Poisson and binomial regression models would not be appropriate due to overdispersion; for this reason, we fitted negative- and β-binomial models to predict monthly (or quarterly) outcomes. The impact of the CMS policy change was captured by including a dummy variable for time periods affected by the policy change (0, before; 1, after) and an interaction term between this dummy variable and a time trend. This approach allowed for the detection of changes in the rate level (intercept) as well as changes in the time trend before and after the CMS policy change (slope). An incidence rate ratio (IRR) or odds ratio (OR) of less than 1 associated with the slope would suggest that the CMS policy change significantly reduced the outcome trajectory.
For each outcome, we fit a negative binomial (β-binomial) model using the interaction term between this dummy variable and a time trend (base model). We then estimated 4 other versions of the model that added explanatory variables in blocks based on their level of observation (unit-level variables, then hospital variables, followed by market variables, and then all variables). We used this approach because we were primarily interested in whether inclusion of these additional variables had any effect on the direction, magnitude, or significance of the estimated policy effect.
We considered several model adjustments to account for correlation within units and over time and anticipatory or lagged responses. Models with lagged outcomes (time correlation) and alternative change points (up to ±3 months) did not yield qualitatively different results, so we restricted inferences to our final models. Unit-level random intercepts were included to account for correlation of outcomes within the same unit. The few missing outcomes data were assumed to be missing at random.
Results
Study Population
The 1381 hospitals contributing data to our study were located in all 50 states and the District of Columbia. Characteristics of the hospitals reporting each outcome measure are listed in Table 1. Compared with the average community hospital reporting to the AHA Annual Survey of Hospitals, our reporting hospitals tended to be larger (14.6%-l8.3% had <100 beds vs 50.7% of AHA hospitals; P < .001), less likely to be located in rural areas (1.7%-3.3% vs 25.3%; P < .001), more likely to be teaching (Council of Teaching Hospital member or have residency training programs; 33.8%-38.5% vs 17.4%; P < .001), and more likely to be nonprofit (82.9%-85.8% vs 59.9%; P < .001). Although participating hospitals were more likely to be located in the Northeast and less likely to be located in the West, our sample contains substantial representation from all 4 census regions.
Table 1. Characteristics of Study Hospitals vs Short-term General Hospitals Reporting to the AHA.
| Characteristic | No. (%) of Hospitals Reportinga | Nonfederal AHA Hospitals (n = 4561) | |||
|---|---|---|---|---|---|
| Falls (n = 1381) | Pressure Ulcers (n = 1341) | CLABSIs (n = 699) | CAUTIs (n = 601) | ||
| No. of reporting units, mean (SD)b | 8.0 (6.1) | 7.9 (6.0) | 1.8 (1.4) | 1.7 (1.6) | NA |
| Regionc | |||||
| Midwest | 405 (29.3) | 395 (29.5) | 211 (30.2) | 180 (30.0) | 1367 (30.0) |
| Northeast | 329 (23.8) | 321 (23.9) | 158 (22.6) | 130 (21.6) | 568 (12.5) |
| South | 464 (33.6) | 440 (32.8) | 264 (37.8) | 229 (38.1) | 1708 (37.5) |
| West | 183 (13.3) | 185 (13.8) | 66 (9.4) | 62 (10.3) | 918 (20.1) |
| Locationc | |||||
| Metropolitan | 1177 (85.2) | 1153 (86.0) | 598 (85.6) | 507 (84.4) | 2579 (56.5) |
| Micropolitan | 159 (11.5) | 147 (11.0) | 89 (12.7) | 83 (13.8) | 830 (18.2) |
| Rural | 45 (3.3) | 41 (3.0) | 12 (1.7) | 11 (1.8) | 1152 (25.3) |
| No. of bedsc | |||||
| <100 | 253 (18.3) | 234 (17.5) | 104 (14.9) | 88 (14.6) | 2314 (50.7) |
| 100-399 | 817 (59.2) | 801 (59.7) | 446 (63.8) | 391 (65.1) | 1807 (39.6) |
| ≥400 | 311 (22.5) | 306 (22.8) | 149 (21.3) | 122 (20.3) | 440 (9.7) |
| Ownershipc | |||||
| Public | 96 (7.0) | 90 (6.7) | 57 (8.2) | 48 (8.0) | 1057 (23.2) |
| For-profit | 109 (7.9) | 100 (7.5) | 60 (8.6) | 55 (9.2) | 774 (17.0) |
| Not-for-profit | 1176 (85.2) | 1151 (85.8) | 582 (83.3) | 498 (82.9) | 2730 (59.9) |
| Teaching statusc | |||||
| COTH | 207 (15.0) | 203 (15.1) | 95 (13.6) | 74 (12.3) | 273 (6.0) |
| Residency training (not COTH) | 320 (23.2) | 314 (23.4) | 148 (21.2) | 129 (21.5) | 518 (11.4) |
| Nonteaching | 854 (61.8) | 824 (61.5) | 456 (65.2) | 398 (66.2) | 3770 (82.7) |
| Medicare % of total admissions, median (IQR), %c | 48.2 (15.5) | 48.1 (15.6) | 49.0 (15.8) | 49.6 (16.5) | 52.2 (18.8) |
| Nurse hours covered by RNs, median (IQR), % | 69.9 (19.8) | 69.9 (19.9) | 90.3 (12.3) | 91.1 (12.5) | NA |
Abbreviations: AHA, American Hospital Association; CAUTIs, catheter-associated urinary tract infections; CLABSIs, central line–associated bloodstream infections; COTH, Council of Teaching Hospitals; IQR, interquartile range; NA, not available; RN, registered nurse.
Hospitals reporting at least once.
Number of units reporting at least once during the study period.
Each group of hospitals is significantly different from nonfederal AHA hospitals at P < .05, χ2 test.
Effect of the 2008 CMS Policy Change
Table 2 provides the results from the β-binomial model for HAPUs and negative binomial models for the other 3 outcomes. Our results suggest that Medicare's nonpayment policy had no effect on the trajectories of stage III/IV HAPUs and injurious falls. The financial penalties were associated with more substantial changes in the infection outcomes, with an 11% reduction in the rate of change in CLABSIs (IRR, 0.89; 95% CI, 0.83-0.95) and a 10% reduction in the rate of change in CAUTIs (IRR, 0.90; 95% CI, 0.85-0.95). These results were stable after adjustment for unit-, hospital- and market-level factors.
Table 2. Changes in Monthly and Quarterly Rates Over Time for HACs.
| Estimated Change in Proportion of HACs | Basea | Base + Unitb | Base + Hospitalc | Base + Marketd | Base + Alle |
|---|---|---|---|---|---|
| Stage III/IV HAPUs, OR (95% CI)f | |||||
| Slope before October 2008 | 0.98 (0.96-0.99) | 0.97 (0.96-0.99) | 0.97 (0.96-0.99) | 0.97 (0.95-0.98) | 0.97 (0.96-0.99) |
| Slope after October 2008 | 0.96 (0.95-0.98) | 0.97 (0.95-0.98) | 0.97 (0.96-0.99) | 0.96 (0.94-0.97) | 0.98 (0.96-1.00) |
| Change in slope (after vs before Oct 2008) | 0.98 (0.96-1.01) | 1.00 (0.98-1.02) | 1.00 (0.98-1.02) | 1.00 (0.97-1.01) | 1.00 (0.98-1.03) |
| Injurious falls, IRR (95% CI)g | |||||
| Slope before October 2008 | 0.99 (0.98-0.99) | 0.99 (0.98-0.99) | 0.99 (0.99-0.99) | 0.99 (0.98-0.99) | 0.99 (0.98-0.99) |
| Slope after October 2008 | 0.98 (0.98-0.99) | 0.99 (0.98-0.99) | 0.99 (0.98-0.99) | 0.98 (0.98-0.99) | 0.98 (0.98-0.99) |
| Change in slope (after vs before Oct 2008) | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) |
| CLABSIs, IRR (95% CI)h | |||||
| Slope before October 2008 | 1.06 (0.99-1.13) | 1.07 (1.00-1.14) | 1.06 (0.99-1.13) | 1.06 (0.99-1.13) | 1.07 (1.00-1.15) |
| Slope after October 2008 | 0.94 (0.93-0.95) | 0.94 (0.93-0.95) | 0.94 (0.93-0.95) | 0.94 (0.93-0.95) | 0.94 (0.93-0.95) |
| Change in slope (after vs before Oct 2008) | 0.89 (0.83-0.95) | 0.88 (0.82-0.94) | 0.89 (0.83-0.96) | 0.89 (0.83-0.95) | 0.88 (0.82-0.94) |
| CAUTIs, IRR (95% CI)i | |||||
| Slope before October 2008 | 1.04 (0.99-1.09) | 1.03 (0.98-1.09) | 1.05 (0.99-1.10) | 1.04 (0.99-1.10) | 1.04 (0.99-1.10) |
| Slope after October 2008 | 0.93 (0.93-0.94) | 0.93 (0.93-0.94) | 0.94 (0.93-0.95) | 0.93 (0.92-0.94) | 0.94 (0.93-0.95) |
| Change in slope (after vs before October 2008) | 0.90 (0.85-0.95) | 0.91 (0.86-0.96) | 0.90 (0.85-0.95) | 0.89 (0.85-0.94) | 0.90 (0.85-0.95) |
Abbreviations: CAUTIs, catheter-associated urinary tract infections; CLABSIs, central line–associated bloodstream infections; HACs, hospital-acquired conditions; HAPUs, hospital-acquired pressure ulcers; IRR, incidence rate ratio; OR, odds ratio.
Adjusted only for time trend before and after the rule change.
Unit-level covariates: unit type, percentage of nursing (registered nurse) hours.
Hospital-level covariates: specialty (yes/no), ownership (for profit, not-for-profit, church, other nonfederal), bed size, case mix, disproportionate share hospital status (yes/no), Centers for Medicare & Medicaid Services area wage index, magnet status (yes/no), ratio of outpatient to inpatient visits, multihospital system membership (yes/no).
County-level covariates: per capita income, physicians per 1000 patients, log population, unemployment rate, and percentage of populations (≤high school education, aged >65 years, black, Hispanic, income below federal poverty line, covered by commercial health maintenance organization [HMO], covered by commercial preferred provider organization, covered by Medicaid HMO, covered by Medicare HMO, and uninsured).
Includes all covariates.
Per patients present at quarterly census.
Per 1000 patient-days.
Per 1000 central line–days.
Per 1000 catheter-days.
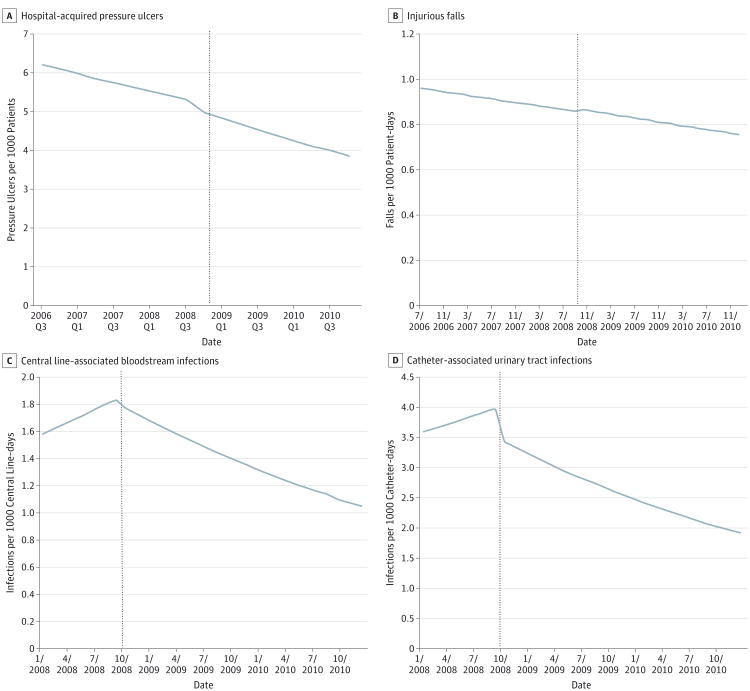
The Figure illustrates the study results by presenting the fitted trajectories of HACs over time (from the base model). Stage III/IV HAPUs and inpatient injurious falls declined somewhat steadily during the study period (July 1, 2006, to December 31, 2010), with the policy introduction having little, if any, effect on their downward trend. Slight upward, but statistically insignificant, trends occurred in CLABSIs and CAUTIs during the first 9 months of our study period (January 1 to September 30, 2008), followed by significant downward trends in the subsequent 27 months (October 1, 2008, to December 31, 2010).
Figure. Timing of Centers for Medicare & Medicaid Services Nonpayment Rule and Trends in Hospital-Acquired Conditions.
Dashed vertical line indicates the introduction of the Medicare Nonpayment policy in the fourth quarter [Q] of 2008 (A) and October 2008 (B, C, and D).
Discussion
Our results related to CLABSIs and CAUTIs differ from those reported by Lee et al,7 who found no evidence that the 2008 CMS nonpayment rule change had a statistically significant effect on the rates of CLABSIs or CAUTIs. There are several possible reasons for this discrepancy. First, the hospitals included in our samples were different; the Lee et al sample included 398 hospitals or health systems located primarily in the Northeast (40.7%) and our sample included 1381 hospitals, with strong representation in the South (32.8%-38.1%) and Midwest (29.3%-30.2%). Our sample also included more non-teaching hospitals (61.5%-66.2% vs 49.7%). Our data collection study time frame also differs from that of Lee et al7: they included CLABSIs and CAUTIs from January 1, 2006, to March 31, 2011, and our data for these measures covered January 1, 2008, to December 31, 2010. Although some might argue that longer time frames are desirable, our sample size was large enough to establish trends before and after the rule change in 2008.
Our findings suggest that the HACs Initiative was associated with at least a 10% reduction in the rate of change in infections (CLABSIs and CAUTIs) but had no effect on the rates of injurious falls or HAPUs. There are several factors that may account for this pattern of results.
Although prevention guidelines existed for all measures included in the 2008 rule,16-20 not all were supported by the same level of scientific evidence. Evidence-based procedures for the prevention of CLABSIs and CAUTIs were relatively well developed prior to 2008.20-22 It is arguable that the evidence base supporting the prevention of injurious falls and HAPUs was less robust. In fact, one comprehensive review23 of inpatient falls prevention found that, at best, multifaceted strategies may be able to reduce falls by 20%. Evidence-based preventive strategies for pressure ulcers were widely available in 2008,24 but clinicians expressed significant concerns over identifying stage I pressure ulcers on admission before they became serious stage III/IV ulcers and viewing all pressure ulcers as preventable.
Medicare's nonpayment policy may have been particularly successful in driving change for infection outcomes because the science supporting infection prevention practices was already well developed in 2008.21,22 Also of critical significance was the 2006 publication8 of the Michigan Keystone project results, which demonstrated dramatic declines of up to 66% in CLABSIs for more than 100 ICUs participating in the statewide collaborative. These results generated a sweeping culture shift in critical care medicine, leading providers to no longer view hospital-acquired infections as simply inevitable.25 In 2007, on the heels of their successful CLABSI results, the Michigan Hospital Association Keystone Center launched a statewide hospital initiative to reduce all hospital-acquired infections.9
In contrast, evidence-based falls prevention was not well developed, and there were significant questions about the preventability of some HAPUs.23 With respect to fall injuries, authors of the CMS final rule noted, “we have not identified specific prevention guidelines for the conditions…. We believe these types of injuries and trauma should not occur in the hospital, and we look forward to working with CDC and the public in identifying research…that will assist hospitals…to prevent these conditions from occurring.”1(p47215) With respect to pressure ulcers, CMS noted, “we believe the selection of this condition will result in closer examination of the patient's skin on admission and better quality of care…. We acknowledge the…concern that…some pressure ulcers are ‘unavoidable.’ However, we believe improved screening to identify pressure ulcers upon admission…will improve the quality of care.”1(p47205) Thus, for both fall injury and HAPUs, CMS appeared to be relying on penalties to drive more science rather than relying on the existing science to support improvement.
Prevention of CLABSIs and CAUTIs may also be more amenable to standardization, facilitating effective dissemination and implementation of process improvements. Best practice guidelines for the prevention of CLABSIs and CAUTIs call for attention to a limited set of critical events (eg, sterile insertion procedures10,20 and earlier withdrawal of catheters26,27) and specific changes to purchasing patterns (antimicrobial catheters for CLABSIs,22 chlorhexidine insertion-site patches21). In addition, these infections are more likely to occur in ICUs or specialized units where a limited set of patients are under the vigilant eye of a focused medical team and infection control departments. The CLABSI and CAUTI “care bundles” that involve an ordered series of clearly defined, evidence-based practices have gained widespread popularity.9,10 Adoption of bundles and checklists has been associated with significant reductions in CLABSIs and CAUTIs.28,29 In contrast, constant vigilance and teamwork across hospital units are critical to identifying patients at risk for HAPUs or falls; the need for ongoing and comprehensive prevention in these areas makes standardization far more challenging.23,24
Our study has several limitations. Hospitals reporting to the NDNQI during our study period were somewhat larger, more often located in urban areas, and more likely to be non-profit compared with the average nonfederal hospital reporting data to the AHA. Reporting to the NDNQI is voluntary, and hospitals may choose not to report in a particular month or quarter. Rates of reporting, however, are high, with 90% of eligible units reporting data and low rates of missing data among outcomes (2%-4%). Also, the rates of these events are consistent with those reported in other data sources,30-33 and nurse staffing trends at hospitals reporting to NDNQI are similar to those reported in AHA data.34 There is evidence that NDNQI participation is associated with improvements in nursing-sensitive quality indicators,35-37 but our study was designed to test the effect of the HACs Initiative beyond secular trends that might be introduced by participating in NDNQI. Hospitals participating in NDNQI tend to be larger and more urban and are more likely to be academically affiliated than are all US hospitals, but we found that hospital- and market-level co-variates had little effect on our findings.
Another limitation of the NDNQI data was that our measure of pressure ulcers represented prevalence rather than incidence, but evaluators indicate whether the pressure ulcer was present on admission vs acquired in the hospital. Although the falls data in NDNQI provide incidence rates, they originate from hospital incident reports, which may not capture all falls.38 The NDNQI CLABSI and CAUTI data were limited to ICU events, but the CMS policy was not. Although ICUs are a very important site for these infections—2009/2010 National Healthcare Safety Network reports indicate that 63% of CAUTIs and 74% of CLABSIs occurred in ICUs31,32—infections outside the ICU are a growing concern that our data cannot address.
Finally, because we were evaluating the effect of a nationwide policy change, our study design was, by necessity, a pre-post comparison of outcomes. It is not possible to attribute the changes we observed to the policy alone, without consideration of other programs or events that encouraged hospitals and providers to enhance prevention efforts related to the targeted HACs. However, our analytical approach used appropriate statistical methods to detect changes in both the (rate) levels and time trends. In addition, we adjusted for an array of unit, hospital, and market characteristics and found no effect on our conclusions.
Despite these limitations, we believe that the NDNQI sample provided credible national information on trends in HACs covered by the CMS policy change. One particular advantage of this data set was a focus on nursing quality of care that was unrelated to coding, which can reflect bias because of changes in Medicare reimbursement. It is not likely that changes in CMS payment policy affected reporting to the NDNQI. Another advantage of these data is that they provide unit-level information on adverse events and staffing. To the extent that these outcomes were affected by unit-level factors, this disaggregation may provide insights not offered by hospital-level data.
Conclusions
Despite the relatively modest financial effect of Medicare's HACs Initiative, the policy appears to have been effective in the reduction of specific never events. In particular, penalties may have been most effective where evidence for prevention was clearest or prevention was more conducive to standardized dissemination and implementation. Our results provide important insights relevant to other CMS initiatives related to HACs, including public reporting of health care–associated infections (Hospital Compare) and assessment of hospital penalties under the Hospital-Acquired Condition Reduction Program to be implemented in fiscal year 2015 (mandated by the Affordable Care Act). Although the former initiative uses transparency and the latter uses penalties, both initiatives provide incentives for hospitals to improve their performance. Our results suggest that initiatives focusing on areas with a well-developed evidence base for prevention and areas amenable to standardization are more likely to be successful in driving improvement. Conversely, when preventability and standardization are absent, our results suggest that the intended objectives may not be achieved. When selecting new areas for quality improvement focus, policymakers may wish to invest directly in the science, rather than rely on incentives to drive scientific development, when a strong evidence base and standardization are lacking.
Acknowledgments
Funding/Support: Drs Waters, Daniels, Bazzoli, Perencevich, Dunton, Staggs, Fareed, and Shorr and Ms Potter were supported by grant 1R01HS020627-01 from the Agency for Healthcare Research and Quality during the conduct of this study. Drs Waters, Daniels, Dunton, Staggs, and Shorr and Ms Potter were also supported by grant R01 AG033005 from the National Institute on Aging.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Waters had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Waters, Daniels, Bazzoli, Shorr.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Waters, Daniels, Bazzoli, Fareed, Shorr.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Waters, Daniels, Bazzoli, Liu.
Obtained funding: Waters, Daniels, Bazzoli, Dunton, Shorr.
Administrative, technical, or material support: Waters, Dunton, Potter.
Study supervision: Waters, Daniels, Bazzoli, Perencevich, Dunton.
Conflict of Interest Disclosures: None reported.
References
- 1.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program: changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist. 2007;72(162):47129–48175. [PubMed] [Google Scholar]
- 2.Rosenthal MB. Nonpayment for performance? Medicare's new reimbursement rule. N Engl J Med. 2007;357(16):1573–1575. doi: 10.1056/NEJMp078184. [DOI] [PubMed] [Google Scholar]
- 3.Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305–312. doi: 10.7326/0003-4819-157-5-201209040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNair PD, Luft HS, Bindman AB. Medicare's policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood) 2009;28(5):1485–1493. doi: 10.1377/hlthaff.28.5.1485. [DOI] [PubMed] [Google Scholar]
- 5.McHugh M, Martin TC, Orwat J, Dyke KV. Medicare's policy to limit payment for hospital-acquired conditions: the impact on safety net providers. J Health Care Poor Underserved. 2011;22(2):638–647. doi: 10.1353/hpu.2011.0058. [DOI] [PubMed] [Google Scholar]
- 6.McNutt R, Johnson TJ, Odwazny R, et al. Change in MS-DRG assignment and hospital reimbursement as a result of Centers for Medicare & Medicaid changes in payment for hospital-acquired conditions: is it coding or quality? Qual Manag Health Care. 2010;19(1):17–24. doi: 10.1097/QMH.0b013e3181ccbd07. [DOI] [PubMed] [Google Scholar]
- 7.Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428–1437. doi: 10.1056/NEJMsa1202419. [DOI] [PubMed] [Google Scholar]
- 8.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 9.Saint S, Olmsted RN, Fakih MG, et al. Translating health care-associated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf. 2009;35(9):449–455. doi: 10.1016/s1553-7250(09)35062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One. 2011;6(1):e15452. doi: 10.1371/journal.pone.0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black JM, Edsberg LE, Baharestani MM, et al. National Pressure Ulcer Advisory Panel. Pressure ulcers: avoidable or unavoidable? results of the National Pressure Ulcer Advisory Panel consensus conference. Ostomy Wound Manage. 2011;57(2):24–37. [PubMed] [Google Scholar]
- 12.National Quality Forum. National voluntary consensus standards for developing a framework for measuring quality for prevention and management of pressure ulcers. [Accessed October 12, 2014]; http://www.qualityforum.org/Projects/n-r/Pressure_Ulcer/Pressure_Ulcers.aspx.
- 13.He J, Dunton N, Staggs V. Unit-level time trends in inpatient fall rates of US hospitals. Med Care. 2012;50(9):801–807. doi: 10.1097/MLR.0b013e31825a8b88. [DOI] [PubMed] [Google Scholar]
- 14.Lake ET, Shang J, Klaus S, Dunton NE. Patient falls: association with hospital magnet status and nursing unit staffing. Res Nurs Health. 2010;33(5):413–425. doi: 10.1002/nur.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Joint Commission on Accreditation of Healthcare Organizations. The Implementation Guide for the NQF Endorsed Nursing-Sensitive Performance Measures. Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations; 2006. [Google Scholar]
- 17.Barker A, Kamar J, Morton A, Berlowitz D. Bridging the gap between research and practice: review of a targeted hospital inpatient fall prevention programme. Qual Saf Health Care. 2009;18(6):467–472. doi: 10.1136/qshc.2007.025676. [DOI] [PubMed] [Google Scholar]
- 18.Schwendimann R, De Geest S, Milisen K. Evaluation of the Morse Fall Scale in hospitalised patients. Age Ageing. 2006;35(3):311–313. doi: 10.1093/ageing/afj066. [DOI] [PubMed] [Google Scholar]
- 19.Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line–associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S22–S30. doi: 10.1086/591059. [DOI] [PubMed] [Google Scholar]
- 20.Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S41–S50. doi: 10.1086/591066. [DOI] [PubMed] [Google Scholar]
- 21.Timsit JF, Schwebel C, Bouadma L, et al. Dressing Study Group. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 22.Casey AL, Mermel LA, Nightingale P, Elliott TS. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(12):763–776. doi: 10.1016/S1473-3099(08)70280-9. [DOI] [PubMed] [Google Scholar]
- 23.Oliver D, Connelly JB, Victor CR, et al. Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: systematic review and meta-analyses. BMJ. 2007;334(7584):82. doi: 10.1136/bmj.39049.706493.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974–984. doi: 10.1001/jama.296.8.974. [DOI] [PubMed] [Google Scholar]
- 25.Dixon-Woods M, Perencevich EN. When counting central line infections counts. Infect Control Hosp Epidemiol. 2013;34(6):555–557. doi: 10.1086/670630. [DOI] [PubMed] [Google Scholar]
- 26.Davis MBH. Pediatric central venous catheter management: a review of current practice. J Assoc Vasc Access. 2013;18(2):93–98. [Google Scholar]
- 27.Trautner BW. Management of catheter-associated urinary tract infection. Curr Opin Infect Dis. 2010;23(1):76–82. doi: 10.1097/QCO.0b013e328334dda8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulman J, Stricof R, Stevens TP, et al. New York State Regional Perinatal Care Centers. Statewide NICU central-line–associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 29.Clarke K, Tong D, Pan Y, et al. Reduction in catheter-associated urinary tract infections by bundling interventions. Int J Qual Health Care. 2013;25(1):43–49. doi: 10.1093/intqhc/mzs077. [DOI] [PubMed] [Google Scholar]
- 30.VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008-2009 International Pressure Ulcer Prevalence Survey and a 3-year, acute care, unit-specific analysis. Ostomy Wound Manage. 2009;55(11):39–45. [PubMed] [Google Scholar]
- 31.Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network (NHSN) report, data summary for 2010, device-associated module. Am J Infect Control. 2011;39(10):798–816. doi: 10.1016/j.ajic.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network (NHSN) report, data summary for 2009, device-associated module. Am J Infect Control. 2011;39(5):349–367. doi: 10.1016/j.ajic.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Bouldin EL, Andresen EM, Dunton NE, et al. Falls among adult patients hospitalized in the United States: prevalence and trends. J Patient Saf. 2013;9(1):13–17. doi: 10.1097/PTS.0b013e3182699b64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staggs VS, He J. Recent trends in hospital nurse staffing in the United States. J Nurs Adm. 2013;43(7-8):388–393. doi: 10.1097/NNA.0b013e31829d620c. [DOI] [PubMed] [Google Scholar]
- 35.Duncan J, Montalvo I, Dunton N. NDNQI Case Studies in Nursing Quality Improvement. Silver Spring, MD: American Nurses Association; 2011. [Google Scholar]
- 36.Dunton N, Montalvo I. Sustained Improvement in Nursing Quality: Hospital Performance on NDNQI Indicators, 2007-2008. Silver Spring, MD: American Nurses Association; 2009. [Google Scholar]
- 37.Montalvo I, Dunton N. Transforming Nursing Data Into Quality Care: Profiles of Quality Improvement in US Healthcare Facilities: Nursesbooks.org. Silver Spring, MD: American Nurses Association; 2007. [Google Scholar]
- 38.Shorr RI, Mion LC, Chandler AM, Rosenblatt LC, Lynch D, Kessler LA. Improving the capture of fall events in hospitals: combining a service for evaluating inpatient falls with an incident report system. J Am Geriatr Soc. 2008;56(4):701–704. doi: 10.1111/j.1532-5415.2007.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]