Abstract
Background
Ultrasound elastography is an imaging modality used to show tissue stiffness in tumor pathophysiological processes that promote the formation of stiffer tissues. Endobronchial ultrasound (EBUS) elastography is an ultrasound elastography-based technique for measuring tissue stiffness during EBUS-guided transbronchial needle aspiration (EBUS-TBNA). The diagnostic value of EBUS elastography in central lung lesions remains largely unknown.
Material/Methods
A total of 57 patients with central lung lesions underwent ultrasonic bronchoscope examination. EBUS with standard B mode evaluation and elastography with grading score measurement were performed before EBUS-guided transbronchial needle aspiration (EBUS-TBNA). Comparison of the diagnosis accuracy in malignant lung lesions between elastography and standard EBUS was made.
Results
Our data showed that the hypoechoic lesions, uneven echo, distinct boundary, and no air bronchogram were significant indicators of standard EBUS in diagnosis of malignant lung lesions (P<0.01). The differences in elastosonography grading scores between the benign and malignant lung lesions were statistically significance (P<0.01), and the elastography grading score was more sensitive and specific than the standard EBUS criteria in diagnosing malignant lung lesions. The area under the receiver operating characteristic curve (ROC) for the elastography grading score was 0.793. The best cut-off point of the elastography grading score for distinguishing malignant from benign lung lesions was 2.5. The elastography grading score had a sensitivity of 72.2%, specificity of 76.2%, positive predictive value of 83.4%, and negative predictive value of 61.5% for distinguishing malignant from benign lung lesions. The overall accuracy of elastography grading score was 73.7%.
Conclusions
BUS elastography can effectively diagnose central lung lesions. The diagnostic accuracy of elastography in malignant lung lesions is higher than that of standard EBUS criteria.
MeSH Keywords: Elasticity Imaging Techniques, Lung Neoplasms, Ultrasonography
Background
Lung cancer is one of the most common malignant tumors that damage human health [1]. Accurately discriminating between benign and malignant lung lesions is essential for the clinical treatment and prognosis of lung cancer patients. Given the number of bony structures and gas in the lung, ultrasound can be easily attenuated, so the ultrasound technique has been regarded as unsuitable for diagnosing lung disease. In recent years, with the development of ultrasound bronchoscopy, an ultrasonic probe is placed in front of the bronchoscope. It can reach the lesion position directly through the airway and decrease the influence of gas in the lung, which then greatly improves the diagnostic accuracy of ultrasound in lung disease.
Ultrasound elastography, a new technology developed in recent years, has gradually been applied to determine the elasticity of lesions and pathophysiological processes such as malignancy that make tissues stiffer or less deformable. This technique also exhibits a potential value in diagnosis of benign and malignant lesions of the breast and thyroid [2,3]. However, whether ultrasound elastography can be used for the non-invasive discrimination of benign and malignant lung lesions remains unknown. Our present study aimed to assess the value of EBUS elastography in lung lesion diagnosis.
Material and Methods
Patients
A total of 57 patients suspected of central lung cancer by chest CT were assessed by EBUS-TBNA at our hospital between 1 January 2014 and 31 October 2015. Among these patients, 40 were male and 17 were female, and their average age was 67.8±7.5 years. Patients who had bleeding tendency or coagulation dysfunction and severe cardiac insufficiency were excluded. All cases were confirmed by pathological diagnosis. The final diagnosis was made and descriptive diagnosis based on pathological specimens. Definite diagnosis was considered correct if the pathologic diagnosis confirmed malignancy. Benign diagnosis needs to be confirmed with 6-month follow-up. If the follow-up confirmed the diagnosis, the diagnosis was then considered correct. Written informed consent was obtained from all subjects. This study was approved by the Ethics Review Committee of the First People’s Hospital of Nantong.
Elastography procedure and EBUS-TBNA
Ultrasonic bronchoscopy examination was performed under general anaesthesia with etomidate and fentanyl via a laryngeal mask. The convex probe EBUS (CP-EBUS; EB-1970UK, Pentax, Japan) was inserted through an oral connecting tube. Scanning was performed at an ultrasound frequency of 7.5 MHz, and images were generated with a new dedicated ultrasound processor (HI VISION AVIUS, HITACHI, Japan). After the appearance of target lung lesions, the diameter, shape, edge definition, internal echo distribution, air bronchogram, and location of lung lesion were recorded in conventional ultrasound B mode. Ultrasound image characteristics were recorded independently and analyzed by 2 skilled endoscopists with formal training.
Elastography was performed on all lung lesions. After recording the ultrasound image characteristics in B mode, the procedure was switched to elastography mode. The scan range included the entire lung lesion and surrounding normal tissue. Elastographic images were generated based on the compressive action generated by the pulsation of vessels in the thoracic cavity and respiratory movement. If the imaging is not ideal, the surgeon can press a button on the ultrasonic bronchoscope, with the frequency of 3–5 times per second, to activate the elastic imaging. After obtaining a stable image, the “freeze” function was used. The elasticity of tissue within the scanned area was reconstructed by comparing it with the surrounding tissue. The elasticity of tissue was translated into a color signal that was overlaid on the B mode image. The colors associated with hard, intermediate, and soft tissues were blue, green, and yellow/red, respectively. Elastographic and B mode images were simultaneously displayed side-by-side on the monitor. Elastographic patterns were described according to the dominant colors and their distribution within the target lung lesions. The following grading standard was used: 1 point when over 80% of the section was green and yellow/red; 2 points when over 50% but less than 80% of the section was green and yellow/red; 3 points when over 50% but less than 80% of the section was blue; and 4 points when over 80% of the section was blue [4].
After elastography, EBUS-TBNA was performed with a Cook 22-gauge needle (c976006, Cook Ireland, Ltd., Ireland). Three passes were obtained, and histological and cytological specimens were collected and sent to the laboratory for subsequent analysis by pathologists who were blinded to the elastography values. A definitive diagnosis of malignancy from the EBUS-TBNA specimens was considered a positive result. Lack of clear evidence of malignancy by EBUS-TBNA was deemed a negative result. A follow-up for 6 months was performed for confirmation of each negative case.
Statistical analysis
All the data was analyzed using the SPSS statistical software package (ver. 17.0; SPSS Inc., USA). Comparison of the categorical variable between benign and malignant lung lesions was conducted via contingency table (χ2) analysis. All numerical variables were tested using the independent samples t test. Differences were considered statistically significant when P<0.05. Sensitivity, specificity, accuracy, positive predictive values (PPVs), and NPVs were calculated. ROC analysis was performed to show the specificity/sensitivity for different elastography grading scores and conventional EBUS criteria.
Results
Characteristics of study patients
EBUS elastography and EBUS-TBNA was performed on 57 lung lesions at different locations in 57 patients (40 males and 17 females) with an average age of 67.8±7.5 years. Histological and cytological results confirmed 36 malignant lung lesions, and among these specimens, 11 lung lesions were squamous cell carcinoma, 8 lung lesions were adenocarcinoma, 14 lung lesions were small cell lung cancer, and 3 lung lesions were low-differentiation cancer. By the end of the study, 21 patients had benign diagnoses (6 tuberculosis, 10 pneumonia, 3 sarcoidosis, and 2 lung abscess). Among 57 cases, 12 lung lesions were visible in routine bronchoscopy examination. Among the observed lung lesions, 8 were located in the upper lobe of the left lung, 12 in the lower lobe of the left lung, 9 in the upper lobe of the right lung, 18 in the right middle lobe, and 10 in the right lower lobe. The clinical characteristics of the 57 patients are summarized in Table 1.
Table 1.
Characteristics of study patients.
| Characteristics | No. or median |
|---|---|
| Gender | |
| Male | 40 |
| Female | 17 |
| Age (years) | 67 |
| Location | |
| Upper lobe of left lung | 8 |
| Lower lobe of left lung | 12 |
| Upper lobe of right lung | 9 |
| Middle lobe of right lung | 18 |
| Lower lobe of right lung | 10 |
| Pathology | |
| Malignant | 36 |
| Squamous cell carcinoma | 11 |
| Adenocarcinoma | 8 |
| Small-cell lung cancer | 14 |
| Low-differentiation cancer | 3 |
| Benign | 21 |
EBUSB-mode features of lung lesions
The B mode features of EBUS imaging of 57 lung lesions are shown in Table 2. There were significant differences in hyperechogenicity, distinct boundary, homogeneous echogenicity, and no air bronchogram between the benign and malignant lung lesions (P<0.01).
Table 2.
EBUS B-mode features of lung lesions.
| Group | No. | Boundary | Lesions echo | Echogenicity | Air-bronchogram | ||||
|---|---|---|---|---|---|---|---|---|---|
| Distinct | Indistinct | Homo-genous | Hetero-genous | Hypo-echonic | Hyper-echonic | Not have | Have | ||
| Malignant | 36 | 25* | 11* | 22* | 14* | 26* | 10* | 27* | 9* |
| Benign | 21 | 5 | 16 | 7 | 14 | 6 | 15 | 8 | 13 |
Diagnostic value of elastography grading score
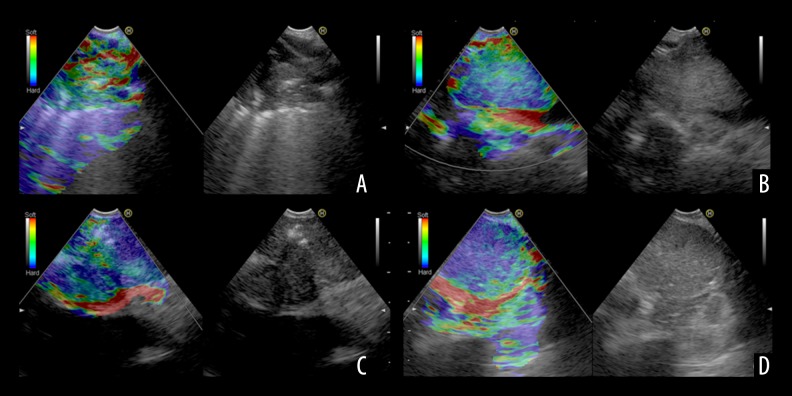
Among the 57 lung lesions included in this work, 36 malignant and 21 benign lung lesions were confirmed. Representative lung lesions on EBUS elastography are showed in Figure 1. Figure 1A: Representative images showing the indistinct boundary and low and heterogeneous echo of lung lesions. The elastography grading score in Figure 1 was 1 point. Histopathological specimen from EBUS-TBNA demonstrated the existence of inflammation. Figure 1B: Representative images showing the unclear boundary and the low internal echogenicity of lung lesions. The elastography grading score was 2 points. The pathological data confirmed that there were inflammatory cell infiltrations. Figure 1C: Representative images showing it had distinct boundary and low and uneven echogenicity of lung lesions. The elastography grading score in this Figure 1 was 3 points. The pathological data showed a diagnosis of adenocarcinoma. Figure 1D: Representative images showing the distinct boundary, low echogenicity, and homogeneous echogenicity of lung lesions. The elastography grading score was 4 points. The pathological data showed a diagnosis of small cell lung cancer. The elastography grading score was higher in the malignant lesions group than in the benign lesions group (3.027±0.909 vs. 1.952±0.864, P=0.000) (Figure 2). The area under the curve (ROC) for the elastography grading score was 0. 793. The optimal cut-off point for distinguishing malignant from benign lung lesions was at the elastography grading score of 2.5 (Figure 3). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were 72.2%, 76.2%, 83.4%, 61.5%, and 73.7%, respectively.
Figure 1.
(A–D) Representative lung lesions on EBUS elastography.
Figure 2.
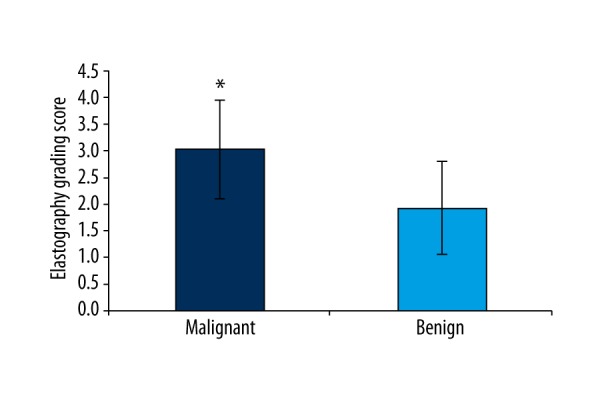
Quantitative analysis of elastography grading score for lung lesions proven benign or malignant. * Comparison with benign lung lesions, P<0.05.
Figure 3.
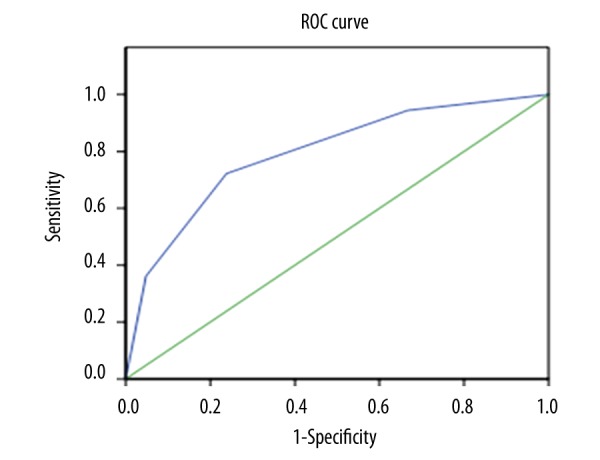
Receiver operating characteristic curve for elastography grading score.
Comparison of elastography grading score versus EBUS B mode features for distinguishing benign from malignant lung lesions
Comparison of ROC curves revealed that the AUCs of distinct boundary, homogeneous echogenicity, hypoechoic, no air bronchogram, and elastography grading score were 0.728, 0.639, 0.718, 0.685, and 0.793, respectively. Compared with all other ultrasound parameters surveyed, the elastography grading score was the best diagnostic parameter for distinguishing benign from malignant lung lesions. The elastography grading score showed more favorable results due to its sensitivity, specificity, PPV, NPV, and accuracy (Figure 4 and Table 3).
Figure 4.
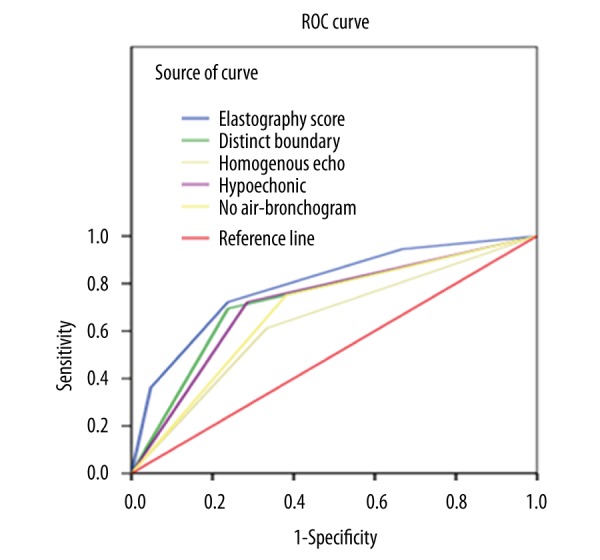
Receiver operating characteristic curve comparison of elastography grading score against conventional endoscopic ultrasound criteria.
Table 3.
Diagnostic values of different ultrasound parameters.
| Ultrasound parameters | Sensitivity | Specifiity | PPV | NPV | Accuracy | AUC |
|---|---|---|---|---|---|---|
| Distinct boundary | 69.4% (25/36) | 76.2% (16/21) | 83.3% (25/30) | 59.3% (16/27) | 71.9% (41/57) | 0.728 |
| Homogenous | 61.1% (22/36) | 66.7% (14/21) | 75.9% (22/29) | 50.0% (14/28) | 63.2% (36/57) | 0.639 |
| Hypoechonic | 72.2% (26/36) | 71.4% (15/21) | 81.3% (26/32) | 60.0% (15/25) | 71.9% (41/57) | 0.718 |
| No air-bronchogram | 75.0% (27/36) | 61.9% (13/21) | 77.1% (27/35) | 59.1% (13/22) | 70.2% (40/57) | 0.685 |
| Elastography score | 72.2% (26/36) | 76.2% (16/21) | 83.4% (26/31) | 61.5% (16/26) | 73.7% (42/57) | 0.793 |
Discussion
Lung cancer is the leading cause of cancer-related deaths. Its morbidity and mortality have grown rapidly in past decades. The correct distinction of lung lesions is important for treatment of this disease. The application of ultrasound in lung diseases was regarded as unsuitable in the past because the bony structures of the thorax and the gas in the lungs make the ultrasound decay rapidly. However, studies showed that when lung lesions involve pleural or pulmonary consolidation, ultrasound can perform well [5]. In recent years, ultrasound has been used in the diagnosis of pleural effusion, subpleural lesions, and atelectasis. For central lung lesions, due to the influence of thoracic bone and lung gas, conventional ultrasound does not work well. With the development of ultrasound bronchoscopy, the ultrasonic probe is placed in front of the bronchoscope. It can reach the lesion directly through the airway, decrease the influence of gas in the lung, and clearly display lung lesions around the large airway. By using high-frequency technology, the improvement in image resolution greatly improved the diagnostic value of ultrasound. In this study, we used ultrasound bronchoscopy to evaluate central lung lesions. Our data showed that the conventional ultrasound images including hypoechogenicity, distinct boundary, homogeneous echogenicity, and no air bronchogram presented statistical significance in the identification of benign and malignant lung lesions, which is consistent with previous data published by Kurimoto’s group, who performed EBUS ultrasound examination in 124 patients with peripheral pulmonary lesions and found that the endobronchial ultrasonography in peripheral lung cancers had numerous characteristics, including clear borderline, internal hypoechoic echo, and homogeneous pattern [6]. In our study, the diagnostic value of distinct boundary was the highest, and the AUC was 0.728. The sensitivity and specificity of benign and malignant lung lesions diagnosis were 69.4% and 76.2%, respectively. The distinct boundary has been shown to be associated with the malignant tumor expansive growth, and as the tumor growing fast, the surrounding lung tissue becomes a thin layer of collapse zone. A clear boundary between the tumor and lung tissues can be displayed on the ultrasound image, and the malignant tumors usually have low and homogeneous pattern echo [7]. The existence of these characteristics may be attributed to the relatively simple composition of tumors [8]; therefore, the accuracy of a single ultrasound image in the diagnosis of malignant lung lesions is not high (63.2–71.9%).
Elastography is an imaging modality used to demonstrate tissue stiffness. Its use is mainly based on the fact that stiffer tissues have lower strains, and compared with softer tissues, the stiffer tissues deform less under compression [9]. Real-time tissue elastography is a new technology that converts an echo signal into a real-time color image, in which color coded from red to blue represents the tissue from the soft to hard [10]. Malignant tumors are usually hard, and this characteristic allows the identification of malignant lesions by elastosonography. Elastography was initially developed for evaluation of lesions accessible from the body surface, such as breast cancer diagnosis or assessment of thyroid and prostate nodules [11–13]. Recently, elastography has also been used for staging of liver fibrosis [14]. With the development of endoscopic ultrasound technology, ultrasound elastography is now being used increasingly in the diagnosis of digestive system diseases.
Endoscopic ultrasound elastosonography was originally used for the differential diagnosis of pancreatic lesions, and later this technology was applied to differentiate benign tissues from malignancies in pancreatic masses, with a sensitivity and specificity of 92.3% and 80%, respectively. In conventional ultrasound, the diagnostic sensitivity and specificity for pancreatic masses determination are 92.3% and 68.9%, respectively. All data indicated that endoscopic ultrasound elastography had more advantages than conventional ultrasound in the diagnosis of malignant pancreatic masses [15]. Compared with use in the diagnosis of digestive system diseases, the application of endoscopic ultrasound elastosonography in the diagnosis of lung mass is less common, and it is now still in the stage of feasibility study. To date, only 1 study [16] reported the value of lung transthoracic ultrasound elastography imaging and guided biopsies in subpleural cancer. In that study, researchers performed transthoracic ultrasound elastography in 95 patients, and reported that the elasticity of malignant pulmonary nodules was lower than that of benign nodules. The elasticity of squamous cell carcinoma was the lowest in malignant pulmonary nodules. Transthoracic ultrasound elastography has been shown to improve the accuracy and yield of percutaneous lung biopsy. Here, we applied EBUS elastography to investigate the central lung lesions, and showed that endobronchial ultrasound elastography was technically feasible and tracheobronchial cartilage did not interfere with image acquisition in most cases. In addition, our data showed that the elastography score of malignant lung lesions was significantly higher than that of benign lesions. Compared with all of the conventional EBUS criteria surveyed, the elastography grading score showed the highest ROC AUC. An elastography grading score of ≥2.5 was selected as the diagnostic standard. The diagnostic accuracy, specificity, sensitivity, positive predictive value, and negative predictive value were73.7%, 76.2%, 72.2%, 83.4%, and 61.5%, respectively. Compared with the characteristics of conventional lung lesions B mode imaging, elastography technology presents obvious advantages in the diagnosis of benign and malignant lung lesions.
Elastography is mainly used to detect the hardness of tissues. However, its application can be affected if the lung lesions are calcified or necrotic. For example, 3 cases of tuberculous lesions with calcification and fibrous tissue hyperplasia were misdiagnosed as malignant lesions in our study. In addition, when the malignant lung lesions appeared necrotic, bleeding, or liquefied, the hardness distribution may be uneven and the elastography score may also be reduced, which can lead to false-negative results. Thus, if the internal structure of the lung mass is altered, the accuracy of the endobronchial ultrasound elastography score may be limited. In this case, it is necessary to assist diagnosis through combination with EBUS B mode features and EBUS-TBNA results. BUS elastography is currently not ready to replace EUBS-TBNA in any of its indications. Pathological diagnosis is the criterion standard to determine the nature of a lung lesion. However, EBUS elastography may be useful for making clinical decisions, such as whether biopsy is necessary for a patient. For the cases with elastography grading score ≥2.5 highly suspected of malignancy, EBUS-TBNA should be performed to get a definitive diagnosis. During the process of EBUS-TBNA, the area of blue on the elastic image maybe punctured first to improve the positive diagnosis rate. For the cases with elastography grading score <2.5 highly suspected of being benign, especially those considered benign by cytology, one can reduce the number of EBUS-TBNA punctures, as well as the damage and complications.
Several technical challenges were encountered during the study. Firstly, elastography relies on the movement and compression deformation of observed tissues. Although having a pronounced effect on mediastinal lymph nodes [17], breathing exercises and vascular pulsations often had a less pronounced effect on compression deformation of lung lesions, particularly in patients with larger lesions. This often requires the operator to press a button on the ultrasonic bronchoscope with the frequency of 3–5 times per second to realize the elastic imaging. Secondly, since a high-frequency transducer is used in EBUS elastography, the depth of penetration is limited, and only the smaller lesions can be imaged. It is difficult to perform elastic imaging of lesions with diameter more than 5 cm. Thirdly, due to the existence of breathing exercises and vascular pulsations, it is impossible to obtain completely identical elastic recordings. Potential solutions to this problem are selection of image frames with a relatively stable pressure curve and amplitude, and increasing of the dynamic recording to minimize errors.
Conclusions
In conclusion, our data indicate that EBUS elastography of central lung lesions is a non-invasive technique that can be performed reliably, may be helpful in the prediction of malignant lung lesions during BUS-TBNA, and is more accurate than traditional EBUS imaging.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: This work was supported by the grant from the Health Bureau of Nantong (No. WQ2015002)
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Jung Y, Bae Y. Clinical application of a color map pattern on shear-wave elastography for invasive breast cancer. Surg Oncol. 2016;25:44–48. doi: 10.1016/j.suronc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Dudea SM, Botar-Jid C. Ultrasound elastography in thyroid disease. Med Ultrason. 2015;17:74–96. doi: 10.11152/mu.2013.2066.171.smd. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa MK, Kubota A, Hanamura H, Furukawa M. [Clinical application of real-time tissue elastography to head and neck cancer – evaluation of cervical lymph node metastasis with real-time tissue elastography]. Nippon Jibiinkoka Gakkai Kaiho. 2007;110:503–5. doi: 10.3950/jibiinkoka.110.503. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35:250–61. doi: 10.1097/01.CCM.0000260674.60761.85. [DOI] [PubMed] [Google Scholar]
- 6.Kunmoto N, Murayama M, Ynshioka S, Nishisaka T. Analysis of the internal structure ofperipheral pulmnnary lesions using endobronchial ultrasonography. Chest. 2002;122:1887–94. doi: 10.1378/chest.122.6.1887. [DOI] [PubMed] [Google Scholar]
- 7.Chao TY, Lie CH, Chung YH, et al. Differentiating peripheral pulmonarylesions based on images of endobronchial ultrasonography. Chest. 2006;130:1191–97. doi: 10.1378/chest.130.4.1191. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Chen Z. [Pathological basis of air bronchogram examined by endobronchial ultrasound in patients with peripheral lung cancer]. Zhong Guo Fei Ai Za Zhi. 2010;13:424–31. doi: 10.3779/j.issn.1009-3419.2010.05.09. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey H. Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity. Radiologe. 2003;43:850–55. doi: 10.1007/s00117-003-0943-2. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich CF, Saftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2014;83:405–14. doi: 10.1016/j.ejrad.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Rubaltelli L, Corradin S, Dorigo A. Differential diagnosis of benign and malignant thyroid nodules at elastosonography. Ultraschall Med. 2009;30:175–79. doi: 10.1055/s-2008-1027442. [DOI] [PubMed] [Google Scholar]
- 12.Kagoya R, Monobe H, Tojima H. Utility of elastography for differential diagnosis of benign and malignant thyroid nodules. Otolaryngol Head Neck Surg. 2010;143:230–34. doi: 10.1016/j.otohns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Sadigh G, Carlos RC, Neal CH, Dwamena BA. Accuracy of quantitative ultrasoundelastography for differentiation of malignant and benign breast abnormalities: A meta-analysis. Breast Cancer Res Treat. 2012;134:923–31. doi: 10.1007/s10549-012-2020-x. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Zhang C, Li J. Diagnostic accuracy of real-time shear wave elastography for staging of liver fibrosis: A meta-analysis. Med Sci Monit. 2016;22:1349–59. doi: 10.12659/MSM.895662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannini M, Botelberge T, Bories E, et al. Endoscopic ultrasound elastography for evaluation of lymphnodes and pancreatic masses: A multicenter study. World J Gastroenterol. 2009;15:1587–93. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperandeo M, Trovato FM, Dimitri L, et al. Lung transthoracic ultrasound elastography imaging and guided biopsies of subpleural cancer: A preliminary report. Acta Radiol. 2015;56:798–805. doi: 10.1177/0284185114538424. [DOI] [PubMed] [Google Scholar]
- 17.He HY, Huang M, Zhu J, et al. Endobronchial ultrasound elastography for diagnosing mediastinal and hilar lymph nodes. Chin Med J. 2015;128:2720–25. doi: 10.4103/0366-6999.167296. [DOI] [PMC free article] [PubMed] [Google Scholar]