Abstract
Background
Liver X receptor (LXR) is a nuclear receptor presenting in macrophages; it works indispensably in lipid metabolism control and also negatively regulates the expression of inflammatory genes in macrophages. There are many LXR-related studies in adults with metabolic syndrome but rare reports in obese children with obstructive sleep apnea-hypopnea syndrome (OSAHS). The aim of this study was to investigate the expression of LXR, cholesterol ester transfer protein (CETP), and cyclooxygenase-2 (COX-2) genes in obese children with OSAHS compared with obese children without OSAHS and non-obese children.
Material/Methods
Sleep monitoring was conducted in 80 obese children with sleep disorders. Fasting morning blood samples from the 80 obese children and 51 normal children were collected and separated, so that macrophages were obtained after culture. Fluorescence quantitative real-time PCR (RT-PCR) was used to detect expression levels of the LXR, CETP, and COX-2 genes.
Results
LXR, COX-2, and CETP levels in the OSAHS group were higher than those in the other two groups (P<0.05), and the LXR levels in the group of obese children without OSAHS were higher than those in control group (P<0.05). COX-2 expression in the group with moderate to severe OSAHS was higher than that in the group with mild OSAHS (P<0.05). Meanwhile, there were no significant differences in the LXR and CETP levels between the moderate to severe OSAHS group and the mild OSAHS group (P>0.05).
Conclusions
LXR gene expression was significantly increased in obese children with OSAHS. The severity of OSAHS was positively correlated with COX-2 levels.
MeSH Keywords: Acidosis, Respiratory; Liver Abscess; Pediatric Obesity; Sleep Apnea, Obstructive
Background
Along with the increasing seriousness of childhood obesity [1], the morbidity of child obstructive sleep apnea-hypopnea syndrome (OSAHS) also has been increasing year by year. The prevalence of OSAHS has been highly variable according to the estimates of previous studies [2,3]. A early population-based study found that the prevalence of OSAHS in men is 4% and in women it is 2%, with the prevalent rate ranging from 0.7% to 3.3% [4,5]. However, some OSAHS patients don’t present the clinical syndromes, which leads to a higher prevalence, as high as 20% to 30% in the middle-aged population [2,6]. Franklin and Lindberg [7] also reported that the prevalence of daytime sleepiness has increased over time and that OSAHS was discovered in 50% of women and 37% of men in studies from 2013 and 2008, respectively.
OSAHS symptoms affect the growth of children, cause mood disorders, and even affect cognitive abilities and increase the risk of metabolic diseases in some extreme cases. A previous study [8] has shown that macrophages are the major effective cells in the body’s natural immune response, and they play an important role in lipid metabolism and cholesterol efflux. Liver X receptor (LXR) is a nuclear receptor presenting in the macrophages; it works indispensably in lipid metabolism control and also negatively regulates the expression of inflammatory genes in macrophages [9]. Currently, the studies of LXR are much more focused on adult patients, with few relevant reports or studies focused on the pediatric cases. Especially, research studies on OSAHS are rarely reported.
Cholesterol ester transfer protein (CETP) plays a central role in high-density lipoprotein (HDL) metabolism. Increased CETP activity could regulate the anti-inflammatory and anti-oxidant properties of HDL. Therefore, CETP contributes to chronic inflammation and oxidative stress [10,11]. Cyclooxygenase-2 (COX-2) is a key enzyme for the synthesis of eicosanoids. COX-2 is considered to be expressed in the vascular tissues according to the stimulation of pro-inflammatory factors, such as lipopolysaccharides, mitogens, and cytokines [12]. A previous study [13] reported that COX-2 in activated monocytes is of particular relevance in inflammation. Both of the CETP and COX-2 proteins are important for the inflammatory processes; however, there is no evidence showing a direct association between them.
Therefore, the aim of this study was to investigate the expression of LXR, CETP, and COX-2 genes in obese children with OSAHS compared to obese children without OSAHS and non-obese children
Material and Methods
Object of study
Stratified cluster random samples of students selected from eight elementary schools in the four districts of Lanzhou City have been involved in this study as the research subjects in accordance with the International Classification of Sleep Disorders (ICSD). The sleep questionnaire was designed and distributed with full consideration of the local characteristics. The 18-item pediatric obstructive sleep apnea-hypopnea syndrome quality of life questionnaire [14] was used and translated into Chinese, also combining the characteristics of the Chinese population. The OSAHS questionnaire was designed as five parts, including physical suffering, daytime problems, sleep disturbance, caregiver concerns, and emotional distress. According to our previous pre-surgery research, the questionnaire was validated for the specific population in this study.
The OSAHS group consisted of students who had different states of sleep disorder, who were overweight according to body mass index (BMI), and who met diagnostic criteria for OSAHS; students who met diagnostic criteria for obesity were chosen as the obesity without OSAHS group. Another group of non-obese students were chosen as the control group. This study was approved by Lanzhou University Ethics Committee, Lanzhou, China. Written informed consent was obtained from 1 parent, and assent was obtained from each child age ≥7 years.
Standard of diagnosis
Obesity diagnosis
The diagnosis criteria for obesity were chosen according to the standard II of “body mass index reference norm for screening overweight and obesity in Chinese children and adolescents” [15–18]. Briefly, a BMI of 28 was defined as the cut-off point for obesity in males and females aged 18 years. This norm for obesity was specially developed for the Chinese, which is consistent with the ethnic characteristics of the Chinese population [18].
OSAHS diagnosis
All participants underwent polysomnography with a Polywin instrument for overnight monitoring. According to the apnea-hypopnea index (AHI), patients were diagnosed with OSAHS when apnea-hypopnea occurred ≥1 times per hour. Patients were divided into the mild group (1–5 times per hour), the moderate group (5–10 times per hour), and the severe group (>10 times per hour). All of the participants were clinically assessed by the pediatrician.
The following are different states of sleep disorders [19]: obstructive apnea, hypopnea, mouth breathing, snoring, restless sleep, and so forth. Obstructive apnea is characterized by recurrent episodes of complete obstruction of the upper airway (defined as a decrease in airflow greater than 90% from the baseline for more than 10 seconds), and obstructive hypopnea is characterized by repeated episodes of partial upper airway obstruction (defined as a decrease in airflow or chest wall or abdominal excursion greater than 30% from baseline) during sleep [20]. Both apnea and hypopnea result in increased respiratory effects, intermittent hypoxia, disruption of sleep architecture, hypercapnia, and sympathetic activation. We defined the sleep disorder criteria as symptoms that occur at least three times a week and persist for one month.
Exclusive criteria
Patients with morbid obesity, endocrinological disorder-related obesity, and other additional risk factors for obesity, including hypertrophic adenoids/tonsils, cardiac abnormalities, neurological diseases, infectious diseases, and other acute and chronic diseases in children, were excluded [19,21,22].
Experimental method
All participants underwent a 5 mL fasting blood draw in the morning, and the blood was isolated by lymphocyte separation medium. Then, the mononuclear cells were translated into macrophages in vitro. RNA was extracted by using the Trizol method. The quantitative real-time PCR (RT-PCR) method was used to detect LXR, COX-2, and CETP gene expression. LXR forward primer: 5′-TGTTTC TCC GTG TCC TCT GTG T-3′, reverse primer: 5′-CAC CCA ACC CTT TGA CTC TCT T-3′; the product size was 148 bp. COX-2 forward primer: 5′-TCA GAC GCT CAG GAA ATA GAA AC-3′, reverse primer: 5′-GGG GAA GTA TGT AGG AGT TGA AGA-3″; the product size was 208 bp. CETP forward primer: 5′-ATG CCC CTG ACT GCT ACC T-3′, reverse primer: 5′-CCC AAT GTC TCC ATC TGA AAG-3′; the product size was 221 bp. 2-ΔΔct relative quantification was used to test the expression of target genes. The gene expression was calculated according to the following formula: ΔΔCt=(target gene Ct value – reference gene Ct value) test group – (target gene Ct – reference gene Ct value) the mean of the control group. β-actin acted as an internal reference, and the normal control group acted as a standard. 2-ΔΔct shows the mRNA expression level of the target gene in the test group relative to the fold-change in the control group.
Statistical analyses
SPSS 19.0 statistical software was used for all analyses. Descriptive analyses were used to evaluate the mean values and standard deviations of the means. Pearson correlation analysis was used to analyze the correlation. Count data were analyzed by using the χ2 test. P<0.05 was considered as statistically significant.
Results
General characteristics of research data
There were 80 cases of obese children with sleep disorders, and the average age was 9.45±1.78 years. There were 36 cases that met the diagnostic criteria for OSAHS, and 44 cases that did not meet the diagnostic criteria for OSAHS. We also used 51 healthy individuals as the control group. The specific characteristics are listed in Table 1.
Table 1.
Basic data and characteristics of the subjects.
| Characteristics | Values |
|---|---|
| Sleep disorder patients (n) | 80 |
| OSAHA patients (n) | 36 |
| Age (year) | 9.49±1.69 |
| Gender (male: female) | 28: 8 |
| Severity of OSAHS | |
| Mild (n) | 25 |
| Moderate to severe OSAHS (n) | 11 |
| Obesity patients (n) | 44 |
| Age (year) | 9.57±1.75 |
| Gender (male: female) | 37: 7 |
| Normal control individuals (n) | 51 |
| Age (year) | 9.68±1.77 |
| Gender (male: female) | 42: 9 |
Expression of LXR, CETP, and COX-2 in the OSAHS, obesity without OSAHS, and control groups
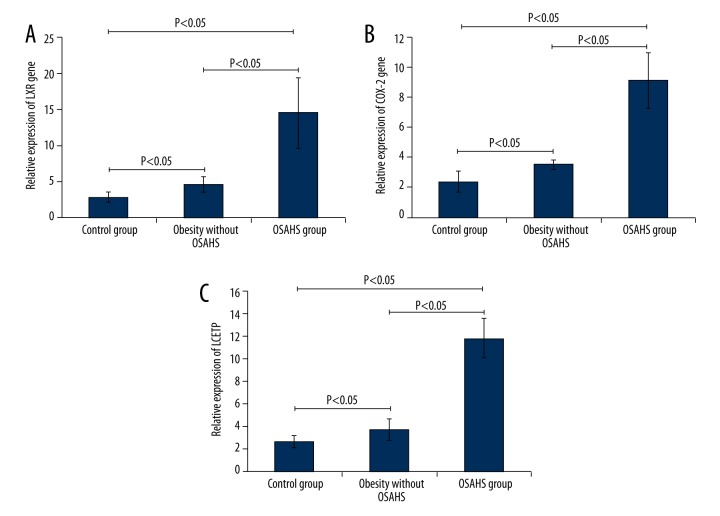
The expression of LXR, CETP, and COX-2 in the different study groups is illustrated in Figure 1. The results indicated that LXR, COX-2, and CETP expression in the OSAHS group was higher compared to that in the obesity without OSAHS group and the control group (Figure 1, P<0.05), and LXR expression in the obesity without OSAHS group was significantly higher compared to that in the control group (Figure 1, P<0.05). However, there were no significant differences in the expression of COX-2 and CETP (Figure 1, P>0.05) between the obesity without OSAHS group and the control group.
Figure 1.
Observation of the expression of LXR, COX-2, and CETP in the control group, the obesity without OSHAS group, and the OSHAS group. (A) Expression of LXR gene in each group. (B) Expression of COX-2 gene in each group. (C) Expression of CETP gene in each group. * P<0.05 indicates that there were significant differences in the gene expression between the marked two groups.
Expression of LXR, CETP, and COX-2 in the mild and moderate to severe groups
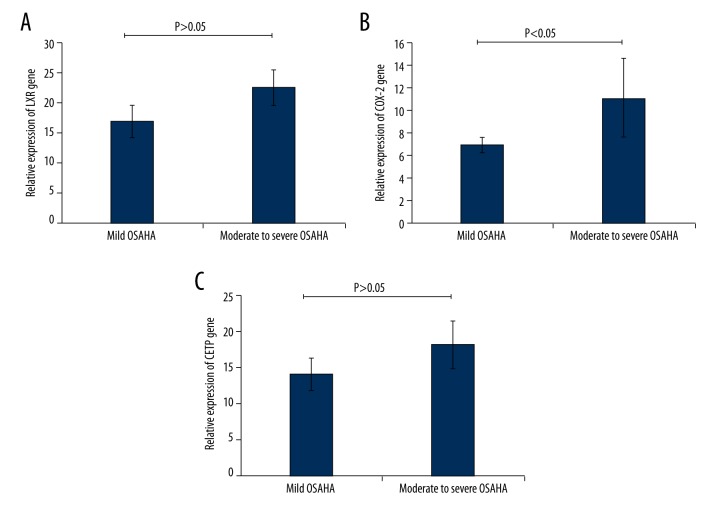
COX-2 expression in the moderate to severe OSAHS group was significantly higher than that in the mild OSAHS group (Figure 2B, P<0.05). However, there were no significant differences in LXR and CETP expression between the mild OSAHS group and the moderate to severe OSAHS group (Figure 2A, 2C, P>0.05).
Figure 2.
Detection of the expression of LXR, COX-2, and CETP in the mild OSAHS group and the moderate or severe OSAHS group. (A) Expression of the LXR gene in the mild OSAHS group and the moderate or severe OSAHS group. (B) Expression of the COX-2 gene in the mild OSAHS group and the moderate or severe OSAHS group. (C) Expression of the CETP gene in the mild OSAHS group and the moderate or severe OSAHS group. * P<0.05 indicates that there were significant differences in the gene expression between the marked two groups.
Severity of OSAHS was positively correlated with expression of COX-2
In order to investigate the correlation between OSAHS severity and COX-2 expression, Pearson correlation analysis was performed. The results indicated that the severity of OSAHS was positively correlated with the expression of COX-2 (Figure 3A, r=0.9032, P<0.05).
Figure 3.
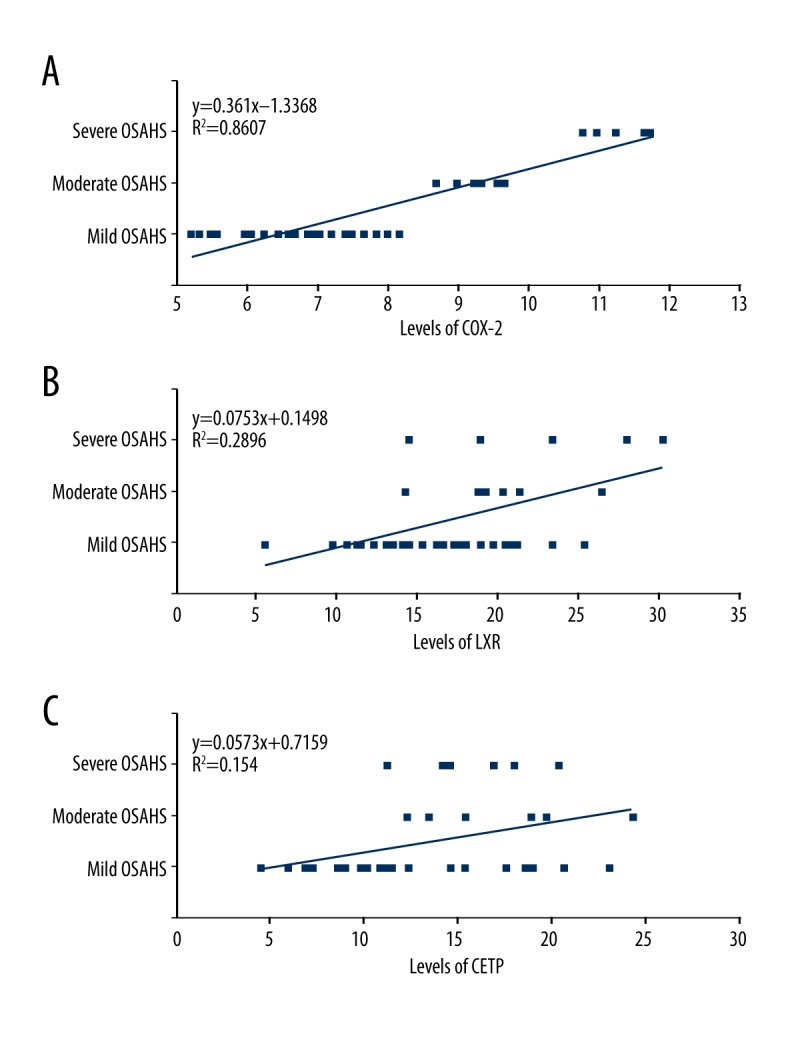
Correlation analysis for the severity of OSAHS and the COX-2, LXR, and CETP levels in OSAHS patients. (A) Correlation between severity of OSAHS and COX-2. (B) Correlation between severity of OSAHS and LXR. (C) Correlation between severity of OSAHS and CETP.
Meanwhile, we also evaluated the correlation between LXR or CETP expression and OSAHS severity. However, the Pearson correlation analysis results showed that there was no significant correlation between LXR expression (Figure 3B, P> 0.05) or CETP expression (Figure 3C, P>0.05) and OSAHS severity.
Discussion
As the sixth risk factor for various diseases and a global economic burden for disease treatment [23], obesity has become a global public health problem that should not be ignored or overlooked. The basic pathological changes of obesity and OSAHS are closely related to insulin resistance and the metabolic syndrome of dyslipidemia [24,25]. OSAHS could be induced by the oxidative stress caused by repeatedly hypoxia and re-oxygenation during sleep. Then, OSAHS would induce expression of inflammation-related susceptibility genes and protein synthesis of biologically active substances, which cause inflammation and metabolic disorders [26] and increase the risk of cardiovascular diseases and metabolic diseases [27]. Therefore, obesity mutually influences both OSAHS and metabolic syndrome.
OSAHS can also have various negative health effects and can cause behavioral consequences in the pediatric population [28], including metabolic derangements (obesity, dyslipidemia, insulin sensitivity, metabolic syndrome) and cardiovascular derangements (hypertension, endothelial dysfunction, chronic inflammation, ventricular size or function abnormalities, elevated pulmonary arterial pressure). Obesity is related to nonalcoholic fatty liver disease, cardiovascular disease, type 2 diabetes, dyslipidemia, insulin resistance, and OSAHS [29]. Previous studies [28–30] have also reported the connection between OSAHS and obesity. Therefore, obesity is clinically significant when investigating the pathological mechanism of OSAHS.
Previous studies showed that the mechanism of the increased risk for adverse consequences in OSAHS or obesity is activation of the systemic inflammatory pathways (such as high-sensitivity C-reactive protein, interferon gamma, tumor necrosis factor alpha, etc.) [31,32]. However, the pathogenetic mechanism of OSAHS has not been fully clarified. Therefore, in this study, we investigated the role of inflammation-associated factors, such as LXR, CETP, and COX-2 protein, in OSAHS pathological processes.
LXR is the transcription factor of the nuclear receptor super-family [33]. The current study found that the target genes under the control of LXR are critical control points for lipid metabolism, transport, transformation, and synthesis, and glucose metabolism associated with the pathological process of various diseases, including cardiovascular disease, organ dysfunction, cancer, inflammation, diabetes, and so forth [34]. CETP is a target gene of LXR, which combines with LXR by CETP gene promoter DR4 structure [35]. Then, CETP expression is activated. COX is a rate-limiting enzyme involved in maintaining the body’s various physiological and pathological features and catalyzes arachidonic acid to generate prostaglandins. There are two types of COX: COX-1 and COX-2. The former structure type, COX-1, also known as a “house-keeping gene” [36], is involved in normal physiological functions such as protecting the gastrointestinal tract, regulating blood flow of the kidneys, and so forth. The latter COX is inducible COX-2, also known as an “inflammatory response gene” [37–39]. After macrophage LXR activation in endogenous ligands, the LXR inhibits pro-inflammatory gene expression of COX-2. Meanwhile, LXR plays an important role in regulating COX-2 expression. COX-2 can’t be detected in most organizations in a normal physiological state. However, when the cells are stimulated by inflammatory signals, growth factors, and various factors, the expression of COX-2 always rapidly increases. Currently, LXR expression and the significance of its target genes in various diseases have become a focus for research [40–43]. Disappointingly, rare reports have emerged in the field of pediatrics about LXR; therefore, the related research about OSAHS and LXR is of great value or significance, and close attention should be paid to it.
This study showed that LXR, COX-2, and CETP expressions in the OSAHS group were higher compared to the obesity without OSAHS group and the control group. This result suggests that the factors of OSAHS and obesity can enhance the gene expression of LXR, CETP, and COX-2 in obese children. COX-2 expression in the moderate to severe OSAHS group was higher compared to the mild OSAHS group, and differences in LXR and CETP levels in the obesity without OSAHS group and the control group were not statistically significant. This result suggests that COX-2 may be a sensitive indicator for lipid metabolism and inflammatory responses. Previous studies [44–46] reported that the LRX is correlated with adult OSAHS and also proved that the metabolic disorders and inflammatory responses not only occur in adult patients with OSAHS, but also occur in children OSAHS. Furthermore, OSAHS is a early risk factor for some related diseases [47,48], which provides a theoretical basis for recent discoveries that cardiovascular and cerebrovascular diseases are occurring in younger and younger patients. The above illustrations suggest that it is critical to further illustrate the importance of prevention and early intervention for childhood obesity and OSAHS [49].
Although this study resulted in some interesting findings, there were also a few limitations. Firstly, the obese children with sleep disorders were selected as the object of study; however, there was lack of research data on obese children without OSAHS. Secondly, no indicators reflect centripetal obesity, such as abdominal circumference and neck circumference data. These deficiencies will be improved and supplemented in our further studies.
Conclusions
LXR gene expression was significantly increased in obese children with OSAHS. The severity of OSAHS was not correlated with the LXR gene expression. However, the severity of OSAHS was positively correlated with the COX-2 levels in OSAHS patients. In future studies, the role of LXR, COX-2, and CETP will be investigated in more detail, and the other markers will also be explored. Furthermore, the future studies will also confirm the validity of the biomarkers in the identification of children with OSAHS.
Abbreviations
- LXR
liver X receptor
- OSAHS
obstructive sleep apnea-hypopnea syndrome
- CETP
cholesterol ester transfer protein
- COX-2
cyclooxygenase-2
- BMI
body mass index
- ICSD
International Classification of Sleep Disorders
- AHI
apnea hypopnea index
Footnotes
Conflict of interests
None.
Source of support: This work was supported by Young Technology Fund Scheme of Gansu Province (Grant No. 099RJYA007) and the Health Industry Research Program of Gansu Province (Grant No. GSWSKY-2014-28)
References
- 1.Zong XN, Li H. Secular trends in prevalence and risk factors of obesity in infants and preschool children in 9 Chinese cities, 1986–2006. PLoS One. 2012;7:e46942. doi: 10.1371/journal.pone.0046942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. Eur J Intern Med. 2012;23:586–93. doi: 10.1016/j.ejim.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Strollo PJ, Rogers RM. Obstructive sleep apnea. N Engl J Med. 1996;334:99–104. doi: 10.1056/NEJM199601113340207. [DOI] [PubMed] [Google Scholar]
- 4.Gislason T, Almqvist M, Eriksson G, et al. Prevalence of sleep apnea syndrome among Swedish men, a epidemiological study. J Clin Epidemiol. 1988;41:571–76. doi: 10.1016/0895-4356(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 5.Cirignotta F, D’Alessandro R, Partinen M, et al. Prevalence of every night snoring and obstructive sleep apneas among 30–69-year-old men in Bologna, Italy. Acta Neurol Scand. 1989;79:366–72. doi: 10.1111/j.1600-0404.1989.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 7.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population, a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 9.Lee SD, Tontonoz P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis. 2015;242:29–36. doi: 10.1016/j.atherosclerosis.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navab M, Ananthramaiah GM, Reddy ST, et al. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Sugano M, Sawada S, Tsuchida K, et al. Low density lipoproteins develop resistance to oxidative modification due to inhibition of cholesteryl ester transfer protein by a monoclonal antibody. J Lipid Res. 2000;41:126–33. [PubMed] [Google Scholar]
- 12.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beloqui O, Paramo JA, Orbe J, et al. Monocyte cyclooxygenase-2 overactivity: A new marker of subclinical atherosclerosis in asymptomatic subjects with cardiovascular risk factor? Eur Heart J. 2005;26:153–58. doi: 10.1093/eurheartj/ehi016. [DOI] [PubMed] [Google Scholar]
- 14.Chervin RD, Hedger K, Dillon JE, et al. Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng-ye J. Body mass index reference norm for screening overweight and obesity in Children and adolescents. Chin J Epidemiol. 2004;25:97–102. [PubMed] [Google Scholar]
- 19.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho V, Crainiceanu CM, Punjabi NM, et al. Calibration model for apnea-hypopnea indices: Impact of alternative criteria for hypopneas. Sleep. 2015;38:1887–92. doi: 10.5665/sleep.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iber C American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22.Dingli K, Assimakopoulos T, Wraith PK, et al. Spectral oscillations of RR intervals in sleep apnoea/hypopnoea syndrome patients. Eur Respir J. 2003;22:943–50. doi: 10.1183/09031936.03.00098002. [DOI] [PubMed] [Google Scholar]
- 23.Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 24.Soylu AC, Levent E, Sarıman N, et al. Obstructive sleep apnea syndrome and anthropometric obesity indexes. Sleep Breath. 2012;16:1151–58. doi: 10.1007/s11325-011-0623-9. [DOI] [PubMed] [Google Scholar]
- 25.Dayyat E, Kheirandish-Gozal L, Capdevila OS, et al. Obstructive sleep apnea in children relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–44. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly A, Dougherty S, Cucchiara A, et al. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33:1185–91. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhushan B, Khalyfa A, Spruyt K, et al. Fatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep Med. 2011;12:666–71. doi: 10.1016/j.sleep.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blechner M, Williamson AA. Consequences of obstructive sleep apnea in children. Curr Probl Pediatr Adolesc Health Care. 2016;46:19–26. doi: 10.1016/j.cppeds.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Goodson BL, Wung SF, Archbold KH. Obstructive sleep apnea hypopnea syndrome and metabolic syndrome: A synergistic cardiovascular risk factor. J Am Acad Nurse Pract. 2012;24:695–703. doi: 10.1111/j.1745-7599.2012.00771.x. [DOI] [PubMed] [Google Scholar]
- 30.Chaicharn J, Lin Z, Chen ML, et al. Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep. 2009;32:927–38. doi: 10.1093/sleep/32.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalyfa A, Sans Capdevila O, Boazza M, et al. Genome-wide gene expression profiling in children with obstructive sleep apnea. Sleep Med. 2009;10:75–86. doi: 10.1016/j.sleep.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Gileles-Hillel A, Alonso-Alonso-Alvarez ML, Kheirandish-Gozal L, et al. Inflammatory markers and obstructive sleep apnea in obese children: the NANOS study. Mediators Inflamm. 2014;2014:605280. doi: 10.1155/2014/605280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann JM, Kliewer SA, Moore LB, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–40. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 34.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–14. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest. 2000;105:513–20. doi: 10.1172/JCI8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crofford L. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl. 1997;49:15–19. [PubMed] [Google Scholar]
- 37.Li B, Li YM, Li X, et al. COX-2 inhibition improves immune system homeostasis and decreases liver damage in septic rats. J Surg Res. 2009;157:43–47. doi: 10.1016/j.jss.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Sheng-Yuan Z, Xiong F, Chen YJ, et al. Molecular characterization of SMN copy number derived from carrier screening and from core families with SMA in a Chinese population. Eur J Hum Genet. 2010;18:978–84. doi: 10.1038/ejhg.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy RC, Chen GH, Tateda K, et al. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L537–43. doi: 10.1152/ajplung.2001.281.3.L537. [DOI] [PubMed] [Google Scholar]
- 40.Sim WC, Park S, Lee KY, et al. LXR-alpha antagonist meso-dihydroguaiaretic acid attenuates high-fat diet-induced nonalcoholic fatty liver. Biochem Pharmacol. 2014;90:414–24. doi: 10.1016/j.bcp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 41.He Q, Pu J, Yuan A, et al. Liver X receptor agonist treatment attenuates cardiac dysfunction in type 2 diabetic db/db mice. Cardiovasc Diabetol. 2014;13:149. doi: 10.1186/s12933-014-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchgessner TG, Martin R, Sleph P, et al. Pharmacological characterization of a novel liver X receptor agonist with partial LXRalpha activity and a favorable window in non-human primates. J Pharmacol Exp Ther. 2014;2:219923. doi: 10.1124/jpet.114.219923. [DOI] [PubMed] [Google Scholar]
- 43.Li N, Wang X, Zhang J, et al. Identification of a novel partial agonist of liver X receptor alpha (LXRalpha) via screening. Biochem Pharmacol. 2014;92:438–47. doi: 10.1016/j.bcp.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Yong-jie Q, Mei-juan X, Qin Y. The expression and meaning of liver X receptor in obstructive sleep apnea-hypopnea syndrome patients. Chinese J Pract Intern Med. 2009;29:4. [Google Scholar]
- 45.Deboer MD, Mendoza JP, Liu L, et al. Increased systemic inflammation overnight correlates with insulin resistance among children evaluated for obstructive sleep apnea. Sleep Breath. 2012;16:349–54. doi: 10.1007/s11325-011-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shamsuzzaman A, Szczesniak RD, Fenchel MC, et al. Glucose, insulin, and insulin resistance in normal-weight, overweight and obese children with obstructive sleep apnea. Obes Res Clin Pract. 2014;8(6):e584–91. doi: 10.1016/j.orcp.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhushan B, Khalyfa A, Spruyt K, et al. Fatty-acid binding protein 4 gene polymorphisms and plasma levels in children with obstructive sleep apnea. Sleep Med. 2011;12:666–71. doi: 10.1016/j.sleep.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Hakim F, Kheirandish-Gozal L, et al. Inflammatory pathways in children with insufficient or disordered sleep. Respir Physiol Neurobiol. 2011;178:465–74. doi: 10.1016/j.resp.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laitinen TT, Pahkala K, Venn A, et al. Childhood lifestyle and clinical determinants of adult ideal cardiovascular health: The Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Princeton Follow-Up Study. Int J Cardiol. 2013;169:126–32. doi: 10.1016/j.ijcard.2013.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]