Abstract.
Histoplasmosis is one of the most common and deadly opportunistic infections among persons living with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome in Latin America, but due to limited diagnostic capacity in this region, few data on the burden and clinical characteristics of this disease exist. Between 2005 and 2009, we enrolled patients ≥ 18 years of age with suspected histoplasmosis at a hospital-based HIV clinic in Guatemala City. A case of suspected histoplasmosis was defined as a person presenting with at least three of five clinical or radiologic criteria. A confirmed case of histoplasmosis was defined as a person with a positive culture or urine antigen test for Histoplasma capsulatum. Demographic and clinical data were also collected and analyzed. Of 263 enrolled as suspected cases of histoplasmosis, 101 (38.4%) were confirmed cases. Median time to diagnosis was 15 days after presentation (interquartile range [IQR] = 5–23). Crude overall mortality was 43.6%; median survival time was 19 days (IQR = 4–69). Mycobacterial infection was diagnosed in 70 (26.6%) cases; 26 (25.7%) histoplasmosis cases were coinfected with mycobacteria. High mortality and short survival time after initial symptoms were observed in patients with histoplasmosis. Mycobacterial coinfection diagnoses were frequent, highlighting the importance of pursuing diagnoses for both diseases.
Introduction
Histoplasmosis is an infection caused by inhalation of the fungus Histoplasma capsulatum from contaminated soil. It is the most prevalent mycosis in Central America, and is challenging to diagnose.1–5 The clinical presentation of histoplasmosis ranges from asymptomatic infection or mild respiratory illness to severe disseminated disease.6 Although histoplasmosis can occur among immunocompetent persons, immunocompromised individuals, such as people living with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) (PWAs), are at high risk of developing disseminated disease. In AIDS patients, mortality due to disseminated histoplasmosis may approach 50%, especially in those with severe manifestations.7 Among those at highest risk, clinical manifestations are nonspecific and similar to those produced by other disseminated infectious diseases,6 which can delay diagnosis and treatment. These symptoms include malaise, fever, anorexia, and weight loss; physical examination often shows hepatosplenomegaly, lymphadenopathy, pallor, and petechiae, and in some patients, ulcerations and/or nodules of skin and mucous membranes.6,8,9 These similarities can delay diagnosis and treatment. Guatemala is considered a hyperendemic country for histoplasmosis, with previous surveys showing a 23–81% range of skin test positivity to the histoplasmin skin test antigen.10 However, reports of clinical disease and outcomes from the country are limited and based mainly on case reports, traveler-associated outbreaks, and one study that evaluated symptoms and risk factors in eight histoplasmosis cases.1,11,12 In 2005, a new assay for detection of Histoplasma antigen in urine was developed and validated in Guatemala.13 During the assay evaluation, we prospectively enrolled patients who had symptoms consistent with histoplasmosis. Herein, we describe clinical presentation and outcomes of disseminated histoplasmosis in patients attending a large urban HIV clinic in Guatemala.
Materials and Methods
Study design and data collection.
A prospective cohort study was conducted among patients attending to an HIV clinic (Clínica Familiar “Luis Ángel García”), housed within a large public hospital in Guatemala City, Guatemala. All HIV-positive patients who were ≥ 18 years of age and visited the clinic between February 2005 and March 2009 were eligible for enrollment as suspected cases if they presented with three of five of the following clinical criteria: fever, pancytopenia, weight loss, skin or mucosal lesions indicative of histoplasmosis, or radiological evidence suggestive of histoplasmosis. Informed consent was obtained from all enrolled participants, and clinic personnel performed a baseline questionnaire and laboratory evaluation at the initial visit. Additional clinical data were collected at subsequent clinic follow-up visits. Clinical and demographic data were collected and stored on-site in a Microsoft® Access database (Microsoft Corporation, Redmond, WA) developed for this study.
Case definition.
A case of histoplasmosis was defined as a PWA who had a sterile-site clinical specimen positive for H. capsulatum by culture, or a urine specimen positive for Histoplasma. The diagnosis of histoplasmosis was made based on the recommendations of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group.9 A case of mycobacterial infection was defined as a PWA who had a sterile-site or sputum culture positive for mycobacteria, or microscopic observation of acid-fast bacilli in tissue.
Laboratory methods.
Specimens analyzed included blood, urine, cerebrospinal fluid (CSF), bone marrow, lymphatic nodes, or skin depending on the patient's presentation. Blood and bone marrow were processed by lysis centrifugation and were subsequently cultured using Mycosel™ (BD, Franklin Lakes, NJ), Sabouraud, and Lowenstein–Jensen media to detect fungi and mycobacteria; it was done locally. Urine was tested for Histoplasma antigen by enzyme-linked immunosorbent assay (ELISA) at Centers of Disease Control and Prevention (Atlanta, GA).13,14 Mycobacteria were detected with Ziehl–Nielsen stain or culture, but speciation was only to Mycobacterium tuberculosis. All CSF and tissue biopsies were stained with Giemsa, Gram, and Ziehl–Nielsen and were subsequently cultured for bacteria, mycobacteria, and fungi as above. Additionally, CSF samples were evaluated for the presence of Cryptococcus spp. by microscopy with India ink stain and/or with the Cryptococcus latex agglutination (BIO-RAD; New South Wales, Gladeswille, Australia). Other diagnostic tests included complete blood cell count, liver function tests, and lactate dehydrogenase (LDH).
Statistical analysis.
Clinical data were analyzed using SAS 9.3. χ2, Fischer's exact, and Wilcoxon rank-sum tests were used to perform bivariate analysis, as appropriate. Kaplan–Meier survival analysis was performed to compare mortality among cases and by infection. Factors associated with survival with a significance level of P < 0.05 were included in the model.
Results
Etiology of infection.
During January 2005–March 2009, 567 patients were evaluated and 387 (68.2%) patients met the inclusion criteria. A total of 263 (67.9%) patients were enrolled, of whom 101 (38.4%) met the case definition for histoplasmosis: 71 (27.0%) had histoplasmosis infection alone, 26 (9.9%) had histoplasmosis and mycobacterial coinfection, and four (1.5%) had histoplasmosis and other coinfections (Table 1). Among the nonhistoplasmosis cases, 38 (14.4%) cases had mycobacterial infections only, six (2.3%) had mycobacterial disease and another coinfection, and 18 (6.8%) had other nonmycobacterial infections and other infections, including cryptococcosis, Pneumocystis pneumonia (PCP), and coccidioidomycosis as well as viral, bacterial, and parasitic infections. A total of 100 (38.0%) patients had no identified etiology of infection.
Table 1.
Etiology of infection among patients suspected to have histoplasmosis, by patient (N = 263 patients) and by disease frequency (N = 218 infections)
| Etiology of infection, by patient (N = 263) | n (%) |
|---|---|
| Histoplasmosis | 101 (38.4) |
| Histoplasmosis alone | 71 (27.0) |
| Histoplasmosis + mycobacterial coinfection | 26 (9.9) |
| Histoplasmosis + other coinfection | 4 (1.5) |
| Mycobacterial disease | 70 (26.6) |
| Mycobacterial alone | 38 (14.4) |
| Mycobacterial + other coinfection | 6 (2.3) |
| Other infection (nonhistoplasmosis, nonmycobacterial) | 18 (6.8) |
| No identified etiology of infection | 100 (38.0) |
| Etiology of infection, by disease frequency (N = 218) | n (%) |
| Histoplasmosis | 101 (46.3) |
| Mycobacterial disease | 70 (32.1) |
| Other fungal disease | 17 (7.8) |
| Cryptococcosis | 9 (4.1) |
| Candidiasis | 3 (1.4) |
| Coccidioidomycosis | 3 (1.4) |
| Pneumocystis pneumonia | 2 (0.9) |
| Parasitic disease | 8 (3.7) |
| Bacterial disease | 3 (1.4) |
| Viral disease | 2 (0.9) |
Parasitic diseases identified include Chagas, Cryptosporidium, Cyclospora, and Toxoplasma. Bacterial diseases include Klebsiella and Shigella. Viral diseases include hepatitis B and hepatitis C.
Medical history and clinical presentation.
Median age and gender were similar among histoplasmosis cases and nonhistoplasmosis cases (age: 35 years versus 31 years, P = 0.15; male gender: 80.2% versus 72.8%, P = 0.18; Table 2). At clinical presentation, no symptoms were significantly different between histoplasmosis cases and nonhistoplasmosis cases; however, gastrointestinal (GI) complaints (66.3% in cases versus 73.5% in nonhistoplasmosis cases; P = 0.22), pulmonary complaints (62.4% versus 61.7%; P = 0.92), and oral or skin lesions were most common. The skin lesions (N = 70 patients) were predominantly ulcers (71%), papules (11.4%), and erythema multiforme (10.6%), whereas oral ulcers (N = 78 patients) were seen in lips (6.0%), bucal mucosa (5.7%), and tongue (5%).
Table 2.
Demographic and clinical characteristics of patients suspected to have histoplasmosis, by etiology of infection identified
| Histoplasmosis (N = 101) | No histoplasmosis (N = 162) | P value | |
|---|---|---|---|
| Characteristic | n (%) | n (%) | |
| Male | 81 (80) | 118 (73) | 0.18 |
| Median age, years (IQR) | 35 (27 - 41) | 31 (28–40) | 0.15 |
| Prior HIV diagnosis | 94 (93) | 158 (98) | 0.08 |
| Prior AIDS diagnosis | 7 (7) | 13 (8) | 0.74 |
| Previous histoplasmosis infection | 5 (5) | 2 (1) | 0.07 |
| Clinical signs and symptoms at first visit | |||
| GI symptoms | 67 (66) | 119 (74) | 0.22 |
| Pulmonary symptoms | 63 (62) | 100 (62) | 0.92 |
| Oral lesions | 27 (27) | 51 (32) | 0.41 |
| Skin lesions | 26 (26) | 44 (27) | 0.80 |
| Palpable lymph nodes | 20 (20) | 39 (24) | 0.42 |
| Neurologic symptoms | 17 (17) | 37 (23) | 0.24 |
| Fever (> 38.0°F) | 25 (25) | 40 (25) | 0.99 |
| Karnofsky score ≤ 50 | 28 (28) | 29 (18) | 0.06 |
| Laboratory markers at first visit (median) | |||
| Median CD4 count (IQR) | 25 (10–57) | 45 (18–98) | 0.02* |
| WBC (cells/μL) | 4,280 | 5,360 | 0.01* |
| Hemoglobin (g/dL) | 9.0 | 10.0 | 0.003* |
| Hematocrit (%) | 27.7 | 29.2 | 0.0032* |
| Platelet count (103/μL) | 181 | 284 | < 0.001* |
| LDH (units/L) | 471 | 333 | 0.002* |
| SGOT (AST) (units/L) | 136 | 68 | 0.004* |
| SGPT (ALT) (units/L) | 48 | 44 | 0.14 |
| Bilirubin (mg/dL) | 0.6 | 0.5 | 0.03* |
| Alkaline phosphatase (units/L) | 381 | 303 | < 0.001* |
| Medications at first visit | |||
| ART | 15 (15) | 25 (15) | 0.90 |
| PCP prophylaxis | 69 (68) | 111 (69) | 0.97 |
| Antifungal therapy | 39 (39) | 35 (22) | < 0.001* |
| Fluconazole | 27 (27) | 28 (17) | 0.07 |
| Amphotericin B | 7 (7) | 4 (3) | 0.08 |
| Itraconazole | 5 (5) | 4 (3) | 0.28 |
AIDS = acquired immune deficiency syndrome; ALT = alanine transaminase ART = antiretroviral therapy; AST = aspartate transaminase; GI = gastrointestinal; HIV = human immunodeficiency virus; IQR = interquartile range; LDH = lactate dehydrogenase; PCP = Pneumocystis pneumonia; SGOT = serum glutamic oxaloacetic transaminase; SGPT = serum glutamic pyruvic transaminase; WBC = white blood cell.
Significant at P < 0.05.
Of 101 patients receiving a diagnosis of histoplasmosis, 91 (90%) had positive H. capsulatum sterile-site cultures, and 62 (61.3%) had positive urine antigen. Laboratory findings are detailed in Table 2. Several laboratory markers were significantly lower in cases than noncases at first visit, including median CD4 T-cell count (25 cells/mm3, range = 10–57; versus 45, range = 18–98; P = 0.02), median white blood cell counts (4,280 cells/μL versus 5,360; P = 0.01), platelet count (181 × 103 cells/μL versus 284; P ≤ 0.001), hemoglobin levels (9.0 g/dL versus 10.0; P = 0.003), and hematocrit levels (27.7% versus 29.2%; P = 0.003). LDH levels were significantly higher among histoplasmosis cases versus nonhistoplasmosis cases (471 units/L versus 333; P = 0.002), as were serum glutamic oxaloacetic transaminase levels (136 units/L versus 68; P = 0.004), bilirubin levels (0.6 mg/dL versus 0.5; P = 0.03), and alkaline phosphatase (381 units/L versus 303; P < 0.001).
Overall, 15 (14.9%) of histoplasmosis cases were receiving antiretroviral therapy (ART) at their initial visit, 69 (68.3%) were on Pneumocystis jirovecii (PCP) prophylaxis, and 39 (38.6%) were on antifungal therapy (Table 2).
Treatment and outcomes.
Median time to diagnosis was 15 days among histoplasmosis cases, compared with 7 days among nonhistoplasmosis cases diagnosed with other infections (P = 0.23). Of the 101 histoplasmosis cases, 18 (17.8%) received empiric antifungal therapy prior to definitive diagnosis of histoplasmosis, and 68 (67.3%) were started on antifungal therapy at diagnosis; median time to treatment was 21 days (interquartile range [IQR] = 7–31). Of the histoplasmosis cases who did not receive antifungal therapy and for whom information was available (N = 21), almost all cases (N = 17, 80%) died before a diagnosis of histoplasmosis was made. ART had been initiated in 15 (14.9%) cases and continued in 53 (53%) cases and 81 (50%) noncases. Detailed data are shown in Table 3.
Table 3.
Treatment and outcomes
| Treatment and outcomes | Histoplasmosis (N = 101) n (%) | No histoplasmosis (N = 162) n (%) | P value |
|---|---|---|---|
| Empiric antifungal therapy | 18 (18) | 21 (13) | < 0.001* |
| Received antifungal therapy | 68 (67) | 63 (40) | < 0.001* |
| Fluconazole | 36 (36) | 45 (28) | 0.18 |
| Amphotericin B | 30 (30) | 19 (12) | < 0.001* |
| Itraconazole | 43 (43) | 18 (11) | < 0.001* |
| On ART after diagnosis | 53 (53) | 81 (50) | 0.70 |
| Outcome | |||
| Median days to diagnosis (IQR) | 15 (5–23) | 7 (1–21) | 0.23 |
| Median days to antifungal treatment (IQR) | 21 (7–31) | 24 (7–33) | 0.95 |
| Crude mortality | 44 (44) | 50 (31) | 0.04 |
| 30-day mortality | 25 (25) | 15 (10) | < 0.001* |
| Median days survival (IQR) | 19 (4–69) | 61.5 (27–105) | < 0.001* |
ART = antiretroviral therapy; IQR = interquartile range.
Significant at P < 0.05.
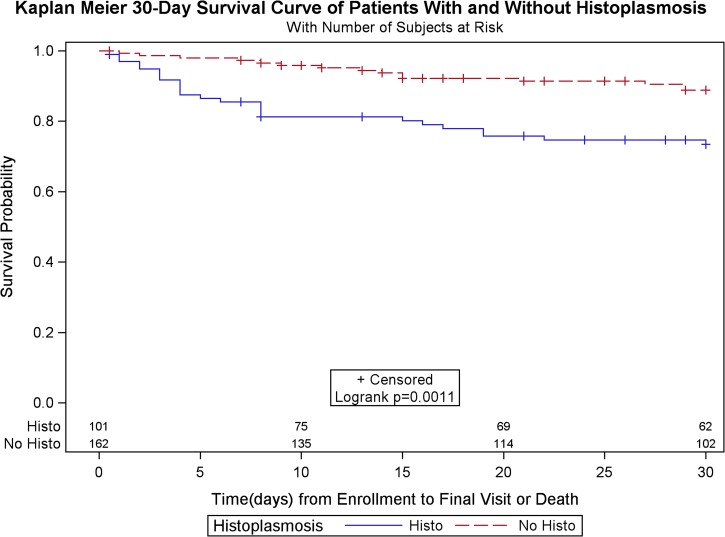
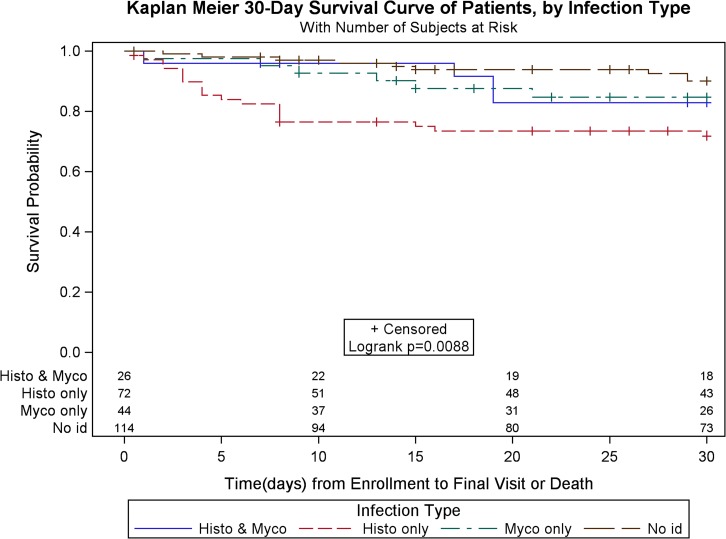
There was a higher crude mortality in patients with histoplasmosis (43.6% among cases versus 30.8% among nonhistoplasmosis cases; P = 0.04), and mortality at 30 days after enrollment was significantly higher among histoplasmosis cases (24.8% versus 9.3%, P < 0.001; Figure 1). Median survival time was lower for histoplasmosis cases (19 days [range = 4–69] versus 61.5 [27–105]; P < 0.001). In comparing survival among patients with histoplasmosis versus patients with other diagnosed infections, median survival was significantly shorter among patients with histoplasmosis, even for those with histoplasmosis and mycobacterial infection. (P = 0.01; Figure 2.). Histoplasmosis patients also had lower CD4 cell counts (median = 32; IQR = 15–59) than nonhistoplasmosis patients (median = 45; IQR = 18–98), whereas patients with both histoplasmosis and mycobacterial infections had lowest CD4 counts (median = 19; IQR = 6–51).
Figure 1.
Kaplan–Meier survival curve of histoplasmosis cases vs. patients without histoplasmosis.
Figure 2.
Kaplan–Meier 30-day survival curve, by infection type.
Discussion
Disseminated histoplasmosis remains an important and deadly opportunistic infection among PWAs. Although Guatemala is a hyperendemic area for histoplasmosis, laboratory detection is limited, and this disease remains underrecognized. This report describes one of the largest cohorts of histoplasmosis patients in Latin America, and is the first study to describe histoplasmosis presentation, coinfection, and outcomes among PWAs in Guatemala.10
Histoplasmosis was diagnosed in 101 (38.4%) of the enrolled patients, and mycobacteria infection in 70 (26.6%). Mycobacterial infection was also the most frequent coinfection in patients with histoplasmosis, with 26 (9.9%) patients. This finding is similar to other reports in some Latin American countries where mycobacterial coinfection was reported in 8–15% of patients with histoplasmosis.15 Other nonmycobacterial infections, including cryptococcosis, PCP, and coccidioidomycosis as well as viral, bacterial, and parasitic infections were also identified, which has been well described among patients with low CD4 cell counts.
Patients coinfected with Histoplasma and Mycobacterium had better survival rates than those infected with Histoplasma alone; yet median CD4 counts were lower among patients with both infections (19 versus 32) (Figure 2). A possible explanation for this finding could be that M. tuberculosis regulates the production of several cytokines.16,17 The control of tuberculosis by the host defenses involves a balance between pro-inflammatory and anti-inflammatory responses. Among these, tumor necrosis factor (TNF)-α, interleukin-12, and interferon (IFN)-γ are key to control the dissemination and severity of histoplasmosis.16–18 In patients with active tuberculosis, T cells secreting IFN-γ and TNF-α are frequent.17 On the other hand, the inhibition of TNF-α exacerbates histoplasmosis in mice.18 We hypothesize that in patients with HIV coinfected with M. tuberculosis and Histoplasma, the active M. tuberculosis infection and its effects on the increased production of TNF-α and IFN-γ acts as a semiprotective mechanism against histoplasmosis, a mechanism that is absent in HIV patients infected with Histoplasma alone. Further evidence is required to test this hypothesis. Treatment of these coinfected patients presents special challenges. Rifampin, a first-line drug for treatment of mycobacterial infections, induces cytochrome P450 enzymes that metabolize azoles, effectively reducing azole serum concentrations by more than half, while increasing rifampin concentrations when the two are coadministered.19,20 Thus, rifampin and itraconazole cannot be administered together,21 moxifloxacin, a first-line fluoroquinolone used to treat rifamycin-resistant tuberculosis, does not interact with itraconazole,22 and may be an effective replacement for rifampicin in coinfected patients,15 but is cost-prohibitive in resource-limited settings.
At the time of presentation, over 23% of patients in this study had symptoms consistent with histoplasmosis, and more than one-third of these patients died. These findings are consistent with those in Brazil and French Guiana, where most studies on histoplasmosis in Latin America have been performed.1,3,23,24 In French Guiana in the post–highly active ART (HAART) era, histoplasmosis was the most commonly diagnosed AIDS-defining illness, with an incidence of 15.4/1,000 person years.24 Disseminated histoplasmosis has been estimated to occur in 2–5% of PWAs who live in endemic regions.1
Clinical features of AIDS-associated histoplasmosis in this cohort were similar to those published elsewhere in Latin America, where fever, pulmonary and GI symptoms are most frequent.7,25 A high prevalence of mucosal and skin involvement has been described throughout Latin America and has been attributed to the possible greater virulence of the South American species26–29 as well to delayed diagnosis.3,29 The prevalence of skin lesions in our cohort was greater than in the United States (1–7%) but less than what has been reported in South America (53–93%).3 Our study found no statistically significant differences in clinical symptoms or signs that might aid in distinguishing Histoplasma infections from other diseases; since nonspecific symptoms such as malaise and cough can be confused with tuberculosis, bacterial pneumonia, and other febrile illnesses, clinical diagnosis remains challenging.3,15
Laboratory abnormalities, including pancytopenia, elevated LDH, and cholestatic hepatitis have been previously described as possible predictors of disease and mortality.30–32 We found similar laboratory abnormalities in our bivariate analyses but were unable to evaluate them further in multivariate modeling due to limitations in the data. However, combining clinical data and laboratory markers could be useful in developing a score as a mortality predictor.
Many patients received antifungal therapy if there was clinical suspicion of disease. In patients with histoplasmosis who did not receive initial antifungal therapy and for whom information was available, almost all cases had died before the diagnosis was made. This finding highlights the need for rapid diagnosis to improve treatment management and outcomes. Additionally, at follow-up visits, some patients with histoplasmosis were started on empiric antifungal treatment before diagnosis in response to clinical improvement or because clinicians had excluded other infections such as tuberculosis.
Mortality rates among immunocompromised individuals reported in other South American studies are similar to that found in our study (19–39%)3,27,33,34 and are higher than rates reported in the United States35–38 except in circumstances where PWAs are diagnosed late or are not receiving HAART.7,38 Compared with patients diagnosed with mycobacterial and other opportunistic infections, patients with histoplasmosis had a shorter median survival time, and time to diagnosis neared time to death in many instances. These findings highlight both a need to use faster diagnostic methods and to have a low threshold for empiric treatment of immunocompromised individuals with symptoms and signs consistent with histoplasmosis.
This study is subject to several limitations. Data were not uniformly available for each patient, and histoplasmosis diagnosis using the ELISA assay could not be performed in real time, which could have improved the outcome of some of the patients.
In spite of these limitations, this study provides the first data in Guatemala to describe Histoplasma and Mycobacterium coinfections and outcomes among PWAs. Rapid and accurate diagnostics for both histoplasmosis and mycobacterial infections are essential, since their symptoms are similar and often indistinguishable. To facilitate earlier diagnosis of histoplasmosis, we suggest consideration of a screening algorithm that includes antibody detection in serum, antigen detection in urine, polymerase chain reaction in blood or pulmonary samples, and fungal culture for pulmonary and blood samples. In parallel, tests for M. tuberculosis could be carried out to complement the differential diagnosis. In addition, early presumptive treatments could help to improve patient's survival.
Although histoplasmosis mortality is currently high in many developing countries, a urine Histoplasma antigen assay that is accessible and available throughout Latin America has the potential to drastically improve patient outcomes.
Acknowledgments:
We acknowledge the many individuals in the hospitals and laboratories in the Clínica Familiar “Luis Ángel García,” Hospital General San Juan de Dios, Guatemala, and Corporación para Investigaciones Biológicas, Medellin, Colombia, for their help in identifying cases and isolates, and also thank the following individuals: Roque Miramontes and Mary Brandt, Mycotic Diseases Branch, Centers for Disease Control and Prevention.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M, 2011. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 49: 785–798. [DOI] [PubMed] [Google Scholar]
- 2.Marques SA, Robles AM, Tortorano AM, Tuculet MA, Negroni R, Mendes RP, 2000. Mycoses associated with AIDS in the Third World. Med Mycol 38 (Suppl 1): 269–279. [PubMed] [Google Scholar]
- 3.Couppie P, Aznar C, Carme B, Nacher M, 2006. American histoplasmosis in developing countries with a special focus on patients with HIV: diagnosis, treatment, and prognosis. Curr Opin Infect Dis 19: 443–449. [DOI] [PubMed] [Google Scholar]
- 4.Gómez B, 2011. Histoplasmosis: epidemiology in Latin America. Curr Fungal Infect Rep 5: 199–205. [Google Scholar]
- 5.Iriart X, Blanchet D, Menard S, Lavergne RA, Chauvin P, Adenis A, Cassaing S, Fillaux J, Magnaval JF, Demar M, Carme B, Bessieres MH, Couppie P, Nacher M, Berry A, Aznar C, 2014. A complementary tool for management of disseminated Histoplasma capsulatum var. capsulatum infections in AIDS patients. Int J Med Microbiol 304: 1062–1065. [DOI] [PubMed] [Google Scholar]
- 6.Kauffman CA, 2009. Histoplasmosis. Clin Chest Med 30: 217–225 v. [DOI] [PubMed] [Google Scholar]
- 7.Baddley JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ, Jr, 2008. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis 62: 151–156. [DOI] [PubMed] [Google Scholar]
- 8.Caceres DH, Tobón AM, Cleveland AA, Scheel CM, Berbesi DY, Ochoa J, Restrepo A, Brandt ME, Chiller T, Gómez BL, 2016. Clinical and laboratory profile of persons living with human immunodeficiency virus/acquired immune deficiency syndrome and histoplasmosis from a Colombian hospital. Am J Trop Med Hyg 95: 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JA, Riddell J, Kauffman CA, 2013. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep 15: 440–449. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RL, Dobrovolny CG, 1960. The distribution of histoplasmin sensitivity in Guatemala. Am J Trop Med Hyg 9: 518–522. [DOI] [PubMed] [Google Scholar]
- 11.Segura L, Rojas M, Pelaez N, Shor-Posner G, RosaRe D, Moreno J, Klaskala W, Baum MK, 1997. Disseminated histoplasmosis and human immunodeficiency virus type 1 infection: risk factors in Guatemala. Clin Infect Dis 25: 343–344. [DOI] [PubMed] [Google Scholar]
- 12.Taylor ML, Chavez-Tapia CB, Vargas-Yanez R, Rodriguez-Arellanes G, Pena-Sandoval GR, Toriello C, Perez A, Reyes-Montes MR, 1999. Environmental conditions favoring bat infection with Histoplasma capsulatum in Mexican shelters. Am J Trop Med Hyg 61: 914–919. [DOI] [PubMed] [Google Scholar]
- 13.Scheel CM, Samayoa B, Herrera A, Lindsley MD, Benjamin L, Reed Y, Hart J, Lima S, Rivera BE, Raxcaco G, Chiller T, Arathoon E, Gomez BL, 2009. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum antigenuria in immunocompromised patients. Clin Vaccine Immunol 16: 852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caceres DH, Scheel CM, Tobon AM, Ahlquist Cleveland A, Restrepo A, Brandt ME, Chiller T, Gomez BL, 2014. Validation of an enzyme-linked immunosorbent assay that detects Histoplasma capsulatum antigenuria in Colombian patients with AIDS for diagnosis and follow-up during therapy. Clin Vaccine Immunol 21: 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agudelo CA, Restrepo CA, Molina DA, Tobon AM, Kauffman CA, Murillo C, Restrepo A, 2012. Tuberculosis and histoplasmosis co-infection in AIDS patients. Am J Trop Med Hyg 87: 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djoba Siawaya JF, Ruhwald M, Eugen-Olsen J, Walzl G, 2007. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int J Infect Dis 11: 289–299. [DOI] [PubMed] [Google Scholar]
- 17.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A, 2011. Immunological biomarkers of tuberculosis. Nat Rev Immunol 11: 343–354. [DOI] [PubMed] [Google Scholar]
- 18.Allendoerfer R, Deepe GS, Jr, 1998. Blockade of endogenous TNF-alpha exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol 160: 6072–6082. [PubMed] [Google Scholar]
- 19.Jaruratanasirikul S, Sriwiriyajan S, 1998. Effect of rifampicin on the pharmacokinetics of itraconazole in normal volunteers and AIDS patients. Eur J Clin Pharmacol 54: 155–158. [DOI] [PubMed] [Google Scholar]
- 20.Baciewicz AM, Chrisman CR, Finch CK, Self TH, 2013. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin 29: 1–12. [DOI] [PubMed] [Google Scholar]
- 21.Tucker R, Denning D, Hanson L, Rinaldi M, Graybill J, Sharkey P, Pappagianis D, Stevens D, 1992. Interaction of azoles with rifampin, phenytoin, and carbamazepine: in vitro and clinical observations. Clin Infect Dis 14: 165–174. [DOI] [PubMed] [Google Scholar]
- 22.Stass H, Nagelschmitz J, Moeller JG, Delesen H, 2004. Pharmacokinetics of moxifloxacin are not influenced by a 7-day pretreatment with 200 mg oral itraconazole given once a day in healthy subjects. Int J Clin Pharmacol Ther 42: 23–29. [DOI] [PubMed] [Google Scholar]
- 23.Tobón AM, Franco L, Espinal D, Gómez I, Arango M, Trujillo H, Restrepo A, 1996. Disseminated histoplasmosis in children: the role of itraconazole therapy. Pediatr Infect Dis J 15: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 24.Huber F, Nacher M, Aznar C, Pierre-Demar M, El Guedj M, Vaz T, Vantilcke V, Mahamat A, Magnien C, Chauvet E, Carme B, Couppie P, 2008. AIDS-related Histoplasma capsulatum var. capsulatum infection: 25 years experience of French Guiana. AIDS 22: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 25.Tobon AM, Agudelo CA, Rosero DS, Ochoa JE, De Bedout C, Zuluaga A, Arango M, Cano LE, Sampedro J, Restrepo A, 2005. Disseminated histoplasmosis: a comparative study between patients with acquired immunodeficiency syndrome and non-human immunodeficiency virus-infected individuals. Am J Trop Med Hyg 73: 576–582. [PubMed] [Google Scholar]
- 26.Kasuga T, White TJ, Koenig G, McEwen J, Restrepo A, Castaneda E, Da Silva Lacaz C, Heins-Vaccari EM, De Freitas RS, Zancope-Oliveira RM, Qin Z, Negroni R, Carter DA, Mikami Y, Tamura M, Taylor ML, Miller GF, Poonwan N, Taylor JW, 2003. Phylogeography of the fungal pathogen Histoplasma capsulatum. Mol Ecol 12: 3383–3401. [DOI] [PubMed] [Google Scholar]
- 27.Karimi K, Wheat LJ, Connolly P, Cloud G, Hajjeh R, Wheat E, Alves K, Lacaz Cd Cda S, Keath E, 2002. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. J Infect Dis 186: 1655–1660. [DOI] [PubMed] [Google Scholar]
- 28.Durkin MM, Connolly PA, Karimi K, Wheat E, Schnizlein-Bick C, Allen SD, Alves K, Tewari RP, Keath E, 2004. Pathogenic differences between North American and Latin American strains of Histoplasma capsulatum var. capsulatum in experimentally infected mice. J Clin Microbiol 42: 4370–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldani LZ, Aquino VR, Lunardi LW, Cunha VS, Santos RP, 2009. Two specific strains of Histoplasma capsulatum causing mucocutaneous manifestations of histoplasmosis: preliminary analysis of a frequent manifestation of histoplasmosis in southern Brazil. Mycopathologia 167: 181–186. [DOI] [PubMed] [Google Scholar]
- 30.Graviss EA, Vanden Heuvel EA, Lacke CE, Spindel SA, White AC, Jr, Hamill RJ, 2000. Clinical prediction model for differentiation of disseminated Histoplasma capsulatum and Mycobacterium avium complex infections in febrile patients with AIDS. J Acquir Immune Defic Syndr 24: 30–36. [DOI] [PubMed] [Google Scholar]
- 31.Butt AA, Michaels S, Greer D, Clark R, Kissinger P, Martin DH, 2002. Serum LDH level as a clue to the diagnosis of histoplasmosis. AIDS Read 12: 317–321. [PubMed] [Google Scholar]
- 32.Couppie P, Sobesky M, Aznar C, Bichat S, Clyti E, Bissuel F, El Guedj M, Alvarez F, Demar M, Louvel D, Pradinaud R, Carme B, 2004. Histoplasmosis and acquired immunodeficiency syndrome: a study of prognostic factors. Clin Infect Dis 38: 134–138. [DOI] [PubMed] [Google Scholar]
- 33.Pietrobon D, Negro-Marquínez L, Kilstein J, Galíndez J, Greca A, Battagliotti C, 2004. Disseminated histoplasmosis and AIDS in an Argentine hospital: clinical manifestations, diagnosis and treatment. Enferm Infecc Microbiol Clin 22: 156–159. [DOI] [PubMed] [Google Scholar]
- 34.Putot A, Perrin S, Jolivet A, Vantilcke V, 2015. HIV-associated disseminated histoplasmosis in western French Guiana, 2002–2012. Mycoses 58: 160–166. [DOI] [PubMed] [Google Scholar]
- 35.Wheat LJ, Hackett E, Durkin M, Connolly P, Petraitiene R, Walsh TJ, Knox K, Hage C, 2007. Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin Vaccine Immunol 14: 638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajjeh RA, Pappas PG, Henderson H, Lancaster D, Bamberger DM, Skahan KJ, Phelan MA, Cloud G, Holloway M, Kauffman CA, Wheat LJ, 2001. Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clin Infect Dis 32: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 37.Myint T, Al-Hasan MN, Ribes JA, Murphy BS, Greenberg RN, 2014. Temporal trends, clinical characteristics, and outcomes of histoplasmosis in a tertiary care center in Kentucky, 2000 to 2009. J Int Assoc Provid AIDS Care 13: 100–105. [DOI] [PubMed] [Google Scholar]
- 38.Anderson AM, Mehta AK, Wang YF, Jing Q, Easley K, Nguyen ML, 2010. HIV-associated histoplasmosis in a nonendemic area of the United States during the HAART era: role of migration from endemic areas and lack of antiretroviral therapy. J Int Assoc Physicians AIDS Care (Chic) 9: 296–300. [DOI] [PubMed] [Google Scholar]