Abstract.
The increase in severe dengue (SD) cases has caused great impact on public health and has concerned authorities of countries where the disease is endemic and epidemics reach high proportions. The recognition of progression signs of this severe disease during the initial febrile phase can be difficult, since the symptoms are often indistinguishable from other febrile diseases. The aim of this study was to evaluate the clinical manifestations and laboratory findings in patients from two dengue outbreaks and their association with the disease. The study was conducted in patients (n = 153) with signs and symptoms consistent with dengue occurred during two distinct epidemics, 2010 and 2013, in the city of Campos dos Goytacazes, Rio de Janeiro, Brazil. According to the 2009 World Health Organization criteria, patients were classified as dengue without warning signs ([DwoWS] 60.6%, 57/94), dengue with warning signs ([DwWS] 30.9%, 29/94), and SD (4.25%, 4/94). Patients with DwWS/SD presented lower platelet and leukocyte counts and higher transaminase levels when compared with the DwoWS ones. Interestingly, patients from the epidemic of 2010 caused by dengue virus 2 (DENV-2) had lower platelet counts than patients of the 2013 epidemic caused by DENV-4. Furthermore, plasma leakage, gastrointestinal bleeding, and pleural effusion, hallmarks for a more severe disease, were also more frequently observed in those cases. Although previous studies may have extensively reported the wide range of the clinical aspects of dengue, the characterization of DENV-4 is desirable considering the burden of the disease during epidemics, especially for the health units and hospitals performing patient's management.
Introduction
Dengue is currently considered globally as the most important mosquito-borne viral disease, and the incidence has grown dramatically around the world in recent decades. It is estimated that 3.9 billion people in 128 countries are at risk of dengue infection.1 One recent estimate indicates that 390 million dengue infections occur per year, of which 96 million manifest clinically with any disease severity.2
Demographic changes, urbanization, and international travel contribute to the expansion of geographical areas where transmission occurs, and all four dengue virus (DENV) serotypes are now circulating in Asia, Africa, and the Americas.3 Not only is the number of cases increasing as the disease spreads to new areas, but explosive outbreaks are also occurring. Currently, Brazil accounts for approximately 70.0% of reported cases of dengue in the Americas.4
In Brazil, the first dengue epidemic with laboratory confirmation occurred in 1981 in Boa Vista, Roraima, in the North Region of Brazil, where DENV-1 and 4 were isolated.5 However, it was only after DENV-1 was introduced in Rio de Janeiro (RJ) in 1986 that the disease became a nationwide public health problem.6 In 2007–2008, the country experienced the most severe epidemic in terms of morbidity and mortality and severe cases in children due to the DENV-2 reemergence.7 DENV-4 was reintroduced in Brazil in 2010, and in RJ, the first dengue cases were detected in Niteroi in 2011,8 and despite the epidemic caused by DENV-1, DENV-4 could be isolated during the disease surveillance supported by the laboratory diagnosis performed.9
In 2010 and 2013, a total of 1,011,548 and 1,452,489 dengue cases were reported in Brazil, with RJ representing 2.9% (29,824) and 14.7% (213,058) of those cases, respectively.10
Early diagnosis of dengue is critical, since some patients may progress from a mild to a severe disease in a short period.11 Although dengue pathogenesis is not completely clear, it is believed that multiple circulating serotypes; host factors such as secondary infection, comorbidities, genetic polymorphism; and factors related to the virus serotype or genotype would determine the evolution of the clinical forms of dengue.12
The white blood cell (WBC) count can reveal a leukopenia and neutropenia, lymphocytosis with lymphocytic atypia, and monocytosis. Signs and symptoms such as severe abdominal pain and continuous, persistent vomiting, postural hypotension, hepatomegaly, mucosal bleeding, increased hematocrit, or abrupt drop of platelets, are considered as potential hallmarks of the disease severity.13 Repeated monitoring of the platelet count and hematocrit is recommended, as an abrupt decrease in platelet counts is an alarm sign and a significant hematocrit increase is an indirect sign of plasma leakage.14 In this study, we aimed to analyze the laboratory and clinical findings during two distinct epidemics that occurred in 2010 and 2013, caused by DENV-2 and DENV-4, respectively, in Campos dos Goytacazes, RJ, Brazil.
Materials and Methods
Ethics statement.
The samples were collected as part of an ongoing project approved by resolution number CSN196/96 from the Oswaldo Cruz Foundation Ethical Committee in Research (CEP 274/05 and CEP100/00) and by the Ethics Committee of Plataforma Brasil, Fundação Oswaldo Cruz (FIOCRUZ; CAAE 13318113.7.0000.5248). All participants provided a written consent.
Enrollment, data and specimen collection.
The specimens (serum and plasma) analyzed in this study were collected during ongoing epidemics that occurred in 2010 and 2013, in the city of Campos dos Goytacazes. Patients were assisted at the Hospital Plantadores de Cana, where an infectious disease physician collected data on demographic characteristics, symptoms, and physical signs using a structured questionnaire. Dengue-suspected cases (n = 153, 49 in 2010 and 104 in 2013) were obtained during an active surveillance performed by the Viral Immunology Laboratory, Oswaldo Cruz Institute, FIOCRUZ. Laboratory diagnosis was performed by the Flavivirus Laboratory, Regional Reference Laboratory for the Brazilian Ministry of Health, located in RJ. Acute serum samples (up to the 7th day after the disease onset) were subjected to reverse transcription polymerase chain reaction (RT-PCR) and nonstructural protein 1 antigen capture enzyme-linked immunosorbent assay (NS1 ELISA). Convalescent samples (after the 7th day after the onset of the symptoms) were tested using IgM antibody capture ELISA (MAC-ELISA) and IgG-ELISA. Both acute and convalescent serum samples were stored at −70°C. Nonspecific laboratory investigations for all patients were obtained in the hospital in which they were treated and included hemoglobin test, WBC count, platelet count, liver function tests (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), renal function tests, and ultrasonography of abdomen. Each sample was accompanied by an identification form containing demographic information, vital signs, physical examination, complete blood count, liver enzymes, ultrasounds and X-rays findings, history of the previous diseases, and medication use.
Inclusion criteria for World Health Organization dengue case definition.
Dengue-confirmed cases were classified according to the 2009 World Health Organization (WHO) classification13,15 and grouped as follows: dengue without warning signs (DwoWS)—patients living in and/or traveling to dengue-endemic area, presenting fever, and two of the following symptoms: nausea, vomiting, rash, aches, pain, positive tourniquet test, and leukopenia; dengue with warning signs (DwWS)—dengue patients with any of the following warning signs: abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, liver enlargement > 2 cm, and an increase in hematocrit concurrent with rapid decrease in platelet count; severe dengue (SD)—dengue patients presenting at least one of the following: severe plasma leakage (leading to shock and fluid accumulation with respiratory distress), severe bleeding evaluated by clinicians, severe involvement of liver by AST or ALT > 1,000 U, central nervous system with impaired consciousness, and severe involvement of the heart and other organs. Non-dengue cases, excluded after a negative result by all dengue laboratory diagnosis, were considered in this study as other febrile diseases (OFD).
Dengue infection laboratory diagnosis.
Laboratory-positive dengue infection was defined as patients experiencing a febrile illness consistent with dengue according to WHO criteria, and infection confirmed based on the results obtained by the laboratory diagnostic assays: RT-PCR, MAC-ELISA, NS1 ELISA, and IgG-ELISA.
Serological diagnosis.
Anti-DENV Ig.
The Panbio dengue IgM Capture ELISA (Brisbane, Australia) was used for the qualitative detection of anti-DENV IgM antibodies in serum for case confirmation according to the manufacturer's instructions.
The IgG-ELISA previously described by Miagostovich and others16 was performed for the characterization of dengue immune response as primary or secondary infections in dengue cases previously confirmed by virus isolation, RT-PCR, and/or MAC-ELISA. Alternatively, the IgG Select Dx kit (Focus Diagnostics, Carlsbad, CA) was used for characterization of dengue immune response as primary and secondary. In brief, the acute samples presenting an NS1 and/or RT-PCR-positive result with a negative IgG detection were considered as primary infections, and acute samples presenting an NS1 and/or RT-PCR-positive result with a positive IgG detection were classified as secondary ones.
NS1 ELISA. For the NS1 antigen capture, the Platelia™ Dengue NS1 Ag-ELISA Kit (Bio-Rad Laboratories, Marnes-La-Coquette, France) was used according to the manufacturer's protocol. All NS1-negative samples were submitted to an NS1 immune complex dissociation protocol previously described.17 All samples were tested between 1 and 9 days after disease onset.
Molecular methods.
The viral RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions and stored at −70°C for DENV detection and typing. The RT-PCR for detecting and typing DENV was performed as described previously.18 Those samples (OFD) tested negative by all dengue laboratory diagnosis were also subjected to an RT-PCR for detection of other flaviviruses and alphaviruses according to a previously published protocol.19 Aiming to further exclude DENV infection in all negative cases (OFD), samples were tested by using Simplexa™ Dengue Kit, a real-time RT-PCR assay (Focus Diagnostics, Cypress, CA), according to the manufacturer's protocol, for qualitative detection and typing of DENV.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism software, version 6.0 (GraphPad Software Inc., San Diego, CA). The Kruskal–Wallis nonparametric test and Dunn's multiple comparison test were used to compare the differences between study groups (OFD, DwoWS, and DwWS/SD). Fisher's test was used to compare clinical and laboratory characteristics between patient groups. Correlation was estimated by Spearman regression analysis. Values of P < 0.05 were considered as significant for all statistical analysis.
Results
The 2010 epidemic.
During the 2010 epidemic that occurred in Campos dos Goytacazes, a total of 49 dengue-suspected cases were investigated. DENV infection was confirmed in 49.0% (24/49) of the cases, and 51.0% (25/49) were characterized as OFD. By RT-PCR and MAC-ELISA, 66.7% (16/24) of the cases were confirmed and 45.8% (11/24) by NS1 ELISA (Table 1).
Table 1.
Laboratory confirmation of dengue-suspected cases analyzed from epidemics that occurred in 2010 and 2013, in Campos dos Goytacazes, Rio de Janeiro, Brazil
| Dengue laboratory diagnostic methods | Year |
|
|---|---|---|
| 2010 (n = 24) | 2013 (n = 70) | |
| Positive/tested (%) | Positive/tested (%) | |
| MAC–ELISA | 16/24 (66.7) | 44/70 (62.8) |
| NS1 ELISA | 11/24 (45.8) | 27/70 (38.5) |
| RT-PCR (serotypes) | 16/24 (66.7) (DENV-2; 16/16) | 34/70 (48.5) (DENV-4; 34/34) |
DENV = dengue virus; ELISA = enzyme-linked immunosorbent assay; MAC = IgM antibody capture; NS1 = nonstructural protein 1; RT-PCR = reverse transcription polymerase chain reaction.
All positive cases detected occurred between the 1st and 8th day of disease. According to the 2009 WHO clinical criteria, confirmed cases (n = 24) were classified as DwoWS (54.0%, 13/24), DwWS (21.0%, 5/24), and SD (12.5%, 3/24) (Table 2). Males were more affected than females; however, irrespective of gender, the mean age of patients was 34 years. DENV-2 was the only infecting serotype identified in 66.7% (16/24) of the cases. Secondary infections were prevalent (91.1%, 18/24) when compared with the primary ones (8.9%, 3/24). Hospitalizations occurred in 45.8% (11/24) of the cases, and 41.6% (10/24) of the patients presented comorbidity (Table 2).
Table 2.
Characteristics of dengue-suspected cases from epidemics that occurred in 2010 and 2013, in Campos dos Goytacazes, Rio de Janeiro, Brazil
| Epidemic year (total of cases analyzed) |
||
|---|---|---|
| 2010 (n = 49) | 2013 (n = 104) | |
| Positive/tested (%) | Positive/tested (%) | |
| Case characteristics | ||
| Dengue | 24/49 (49.0) | 70/104 (67.3) |
| Mean age (in years) | 34 | 37 |
| Gender (male:female) | 29:20 | 23:62 |
| Case classification* | ||
| DwoWS | 13/24 (54.0) | 44/70 (63.0) |
| DwWS | 5/24 (21.0) | 24/70 (34.2) |
| SD | 3/24 (12.5) | 1/70 (1.4) |
| Infecting serotype | ||
| DENV-2 | 16/24 (66.7) | 0/70 |
| DENV-4 | 0/24 | 34/70 (48.6) |
| Type of infection | ||
| Primary | 3/24(8.9) | 11/70 (15.7) |
| Secondary | 18/24 (91.1) | 59/70 (84.2) |
| Factors associated with a more severe disease | ||
| Hospitalization | 11/24 (45.8) | 16/70 (22.9) |
| Abdominal pain | 10/24 (41.6) | 15/70 (21.4) |
| Plasma leakage | 5/24 (20.8) | 3/70 (4.3) |
| Bleeding | 6/24 (25.0) | 4/70 (5.7) |
| Pleural effusion | 4/24 (16.6) | 0/70 |
| Increased hematocrit with rapid decrease of platelets | 4/24 (16.6) | 4/70 (5.7) |
| Comorbidity | 10/24 (41.6) | 27/70 (38.5) |
| Arterial hypertension | 4/10 (40.0) | 18/27 (66.7) |
| Diabetes mellitus | 1/10 (10.0) | 2/27 (7.4) |
| Other comorbidities | 5/10 (50.0) | 16/27 (59.2) |
DENV = dengue virus; DwWS = dengue with warning signs; DwoWS = dengue without warning signs; SD = severe dengue.
Due to the lack of information for the 2009 World Health Organization clinical classification, three cases were not classified in 2010, and one case was not classified in the 2013 epidemic.
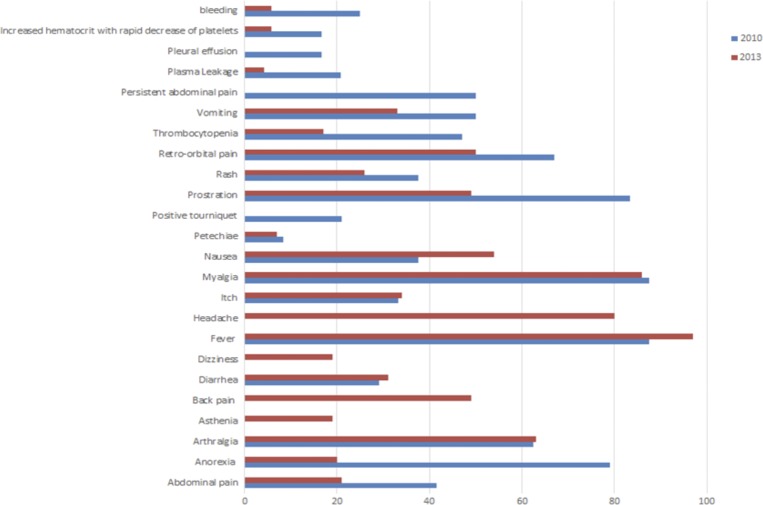
The main signs and symptoms of patients were fever (87.5%), myalgia (87.5%), prostration (83.3%), anorexia (79.1%), retro-orbital pain (67%), arthralgia (62.5%), vomiting (50.0%), thrombocytopenia (47.0%), nausea (37.5%), rash (37.5%), itch (33.3%), diarrhea (29.1%), positive tourniquet (21.0%), and petechiae (8.3%). The most common warning signs of DwWS and SD patients were hemorrhagic manifestations (60.0%, 6/10), persistent abdominal pain (50.0%, 5/10), pleural effusion (40.0%, 4/10), and increased hematocrit with rapid decrease of platelets (40.0%, 4/10) (Figure 1).
Figure 1.
Major signs and symptoms of dengue cases. This figure appears in color at www.ajtmh.org.
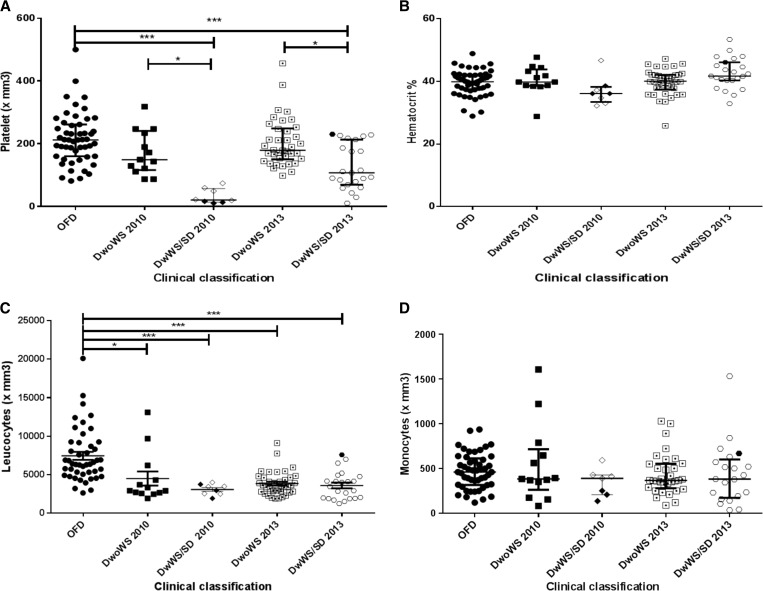
It was observed that the DwWS/SD patients from the epidemic that occurred in 2010 and mainly caused by DENV-2 had significantly lower leukocytes and platelet counts (P < 0.001) when compared with OFD patients; however, no statistically significant differences between monocyte count and hematocrit was observed (Figure 2). Thrombocytopenia was observed in 41.6% (10/24) of the total number of confirmed dengue cases, with 20.0% (2/10) occurring in DwoWS patients and 80.0% (8/10) in DwWS/SD ones. The platelet count < 50,000/mm3 was observed in 75% (6/8) of the DwWS/SD patients.
Figure 2.
Hematological parameters of dengue-positive cases according to clinical classification and epidemic, Campos dos Goytacazes, Rio de Janeiro, Brazil. (A) Platelet, (B) hematocrit, (C) leukocytes, and (D) monocytes. SD/2010 ( ) and SD/2013 (
) and SD/2013 ( ). DwoWS = dengue without warning signs; DwWS = dengue with warning signs; OFD = other febrile disease; SD = severe dengue. P values * < 0.05, ** < 0.01, and *** < 0.001 were considered statistically significant.
). DwoWS = dengue without warning signs; DwWS = dengue with warning signs; OFD = other febrile disease; SD = severe dengue. P values * < 0.05, ** < 0.01, and *** < 0.001 were considered statistically significant.
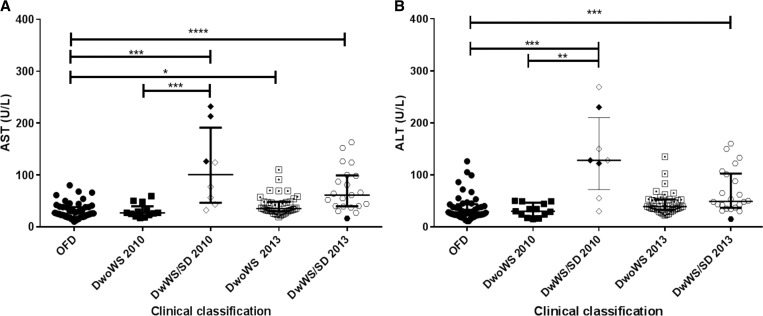
The analysis of the AST revealed high levels in 33.3% (8/24) of patients during the 2010 epidemic, of these, 62.5% (5/8) were DwWS/SD patients and 37.5% (3/8) were DwoWS ones. The high AST levels in DwWS/SD patients were statistically significant when compared with the OFD patients (P < 0.001) (Figure 3). The ALT analysis revealed elevated levels in 37.5% (9/24) of the dengue cases, 50.0% (4/8) DwoWS patients and 50.0% (4/8) DwWS/SD, and no significant differences among the groups were observed.
Figure 3.
Biochemical s of dengue-positive cases according to the clinical classification and epidemic, Campos dos Goytacazes, Rio de Janeiro, Brazil. (A) AST and (B) ALT. SD/2010 ( ) and SD/2013 (
) and SD/2013 ( ). AST = aspartate aminotransferase; ALT = alanine aminotransferase; DwoWS: dengue without warning signs; DwWS = dengue with warning signs; OFD = other febrile disease; SD = severe dengue. P values * < 0.05, ** < 0.01, *** < 0.001, and **** < 0.0001 were considered statistically significant.
). AST = aspartate aminotransferase; ALT = alanine aminotransferase; DwoWS: dengue without warning signs; DwWS = dengue with warning signs; OFD = other febrile disease; SD = severe dengue. P values * < 0.05, ** < 0.01, *** < 0.001, and **** < 0.0001 were considered statistically significant.
The 2013 epidemic.
In 2013, a total of 104 dengue-suspected cases were analyzed, and 67.0% (70/104) were confirmed and 33.0% (34/104) of the cases were considered as OFD. Using MAC-ELISA, 62.8% (44/70) of the infections were confirmed, and RT-PCR confirmed 48.5% (34/70) of the cases and the NS1 ELISA 38.5% (27/70) (Table 1). However, when the serum samples of DENV-4 cases negative by NS1 ELISA were heat dissociated, the test sensitivity increased in 18.5%. According to the 2009 WHO clinical criteria, confirmed cases (n = 70) were classified as DwoWS (63.0%, 44/70), DwWS (34.2%, 24/70), and SD (1.4%, 1/70) (Table 2).
In the 2013 epidemic, females were more affected than males; however, irrespective of gender, the mean age of patients was 37 years. DENV-4 was the only infecting serotype identified in 48.6% (34/70) of the cases. Secondary infections were prevalent (84.2%, 59/70) when compared with the primary ones (15.7%, 11/70). Hospitalizations occurred in 22.9% (16/70) of the cases, and 38.5% (27/70) of the patients presented comorbidity. Moreover, abdominal pain, plasma leakage, bleeding, and pleural effusion, the signs involved in a more severe disease, were less frequently observed than in the 2010 epidemic (Table 2).
The main signs and symptoms of patients were fever (97%), myalgia (86%), headache (80%), arthralgia (63%), nausea (54%), retro-orbital pain (50%), back pain (49%), prostration (49%), abdominal pain (37%), itch (34%), vomiting (33%), diarrhea (31%), rash (26%), anorexia (20%), dizziness (19%), asthenia (19%), thrombocytopenia (17%), and petechiae (7%) (Figure 1).
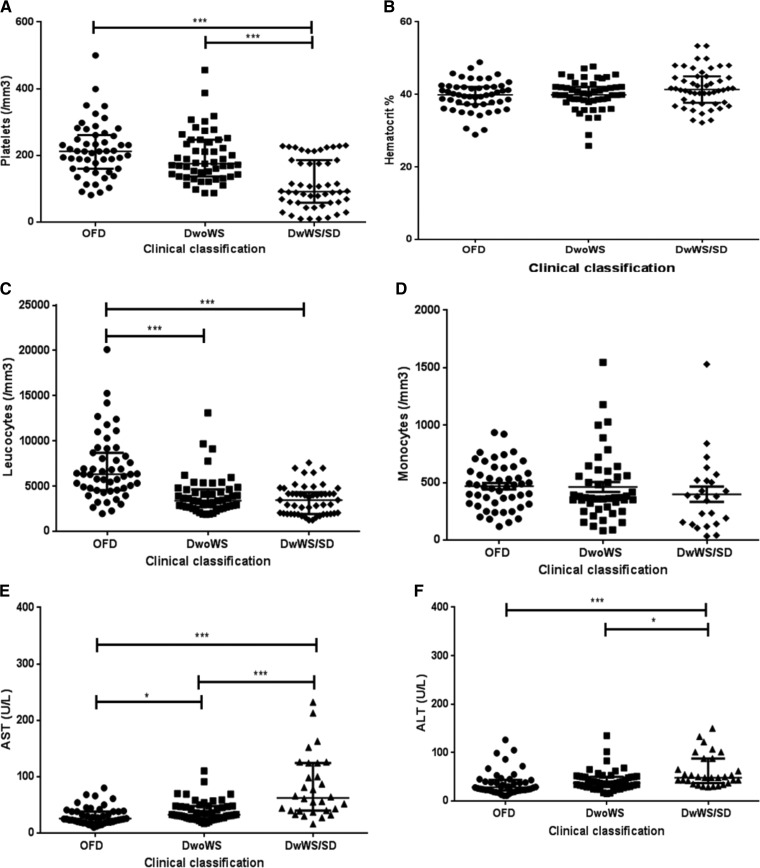
DwWS/SD patients from the epidemic that occurred in 2013 and mainly caused by DENV-4 had significantly lower platelet and leukocyte counts (P < 0.001) when compared with OFD patients. Furthermore, the differences in the platelet counts between the DwoWS and DwWS/SD were also significant (P < 0.05) (Figure 2).
Thrombocytopenia was observed in 17.1% (12/70) of the total number of dengue cases, of these, 8.3% (1/12) were DwoWS patients and 91.7% (11/12) were DwWS/SD ones. Low platelet counts were observed in 25.0% (3/12) of patients, all with DwWS. Both DwoWS and DwWS/SD patients presented lower counts than the OFD patients (P < 0.001); however, no significant difference was observed in relation to the hematocrit and monocyte values (Figure 5).
Figure 5.
Hematological and biochemical findings of dengue-positive cases according to the clinical classification, Campos dos Goytacazes, Rio de Janeiro, Brazil. (A) Platelets, (B) hematocrit, (C) leukocytes, (D) monocytes, (E) AST, and (F) ALT. ALT = alanine aminotransferase; AST = aspartate aminotransferase; DwoWS: dengue without warning signs; DwWS = dengue with warning signs; OFD = other febrile disease; SD = severe dengue. P values * < 0.05, ** < 0.01, and *** < 0.001 were considered statistically significant.
During the 2013 epidemic, increased AST was observed in 42.8% (30/70) of dengue cases, 53.3% (16/30) were patients with DwoWS and 46.6% (14/30) were DwWS ones, whereas the increased ALT levels were also observed in 42.8% (30/70) of the cases, 50% (15/30) were DwoWS cases and 50% (15/30) were DwWS ones.
Both high AST levels observed in DwoWS and DwWS/SD patients in 2013 were statistically significant when compared with the OFD patients (P < 0.05 and P < 0.0001, respectively); however, no significant differences were observed between DwoWS and DwWS/SD cases (Figure 3). Elevated levels of ALT were statistically significant on DwWS/SD patients on 2013 when compared with the OFD cases (P < 0.001) (Figure 3).
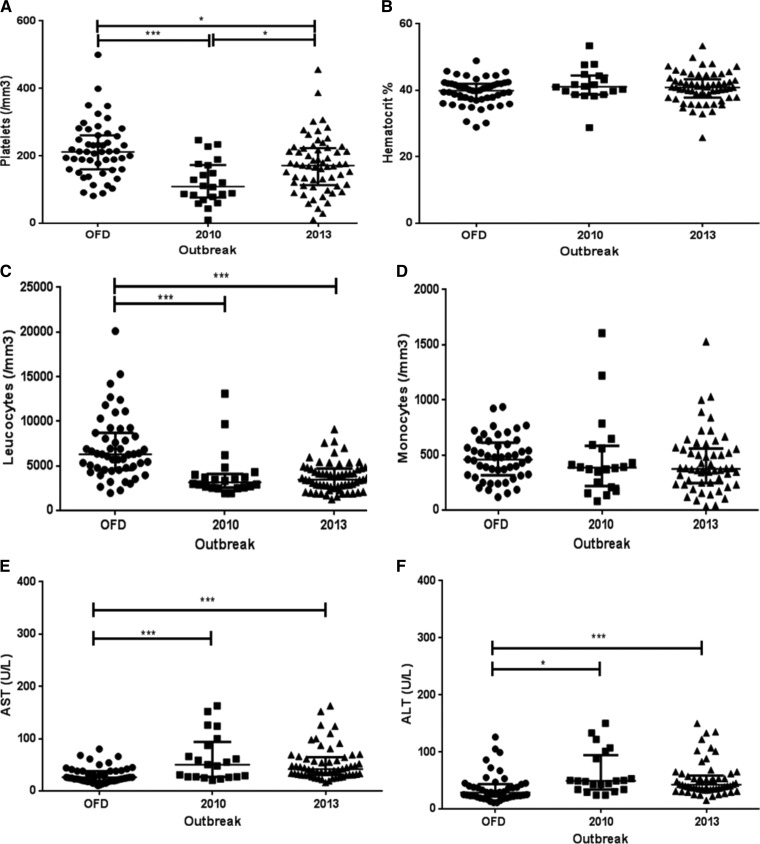
In an overall overview, independently of the clinical classification, the lower platelet count observed in the 2010 epidemic caused by DENV-2 was statistically significant when compared with that observed in the cases occurred in the 2013 epidemic (P < 0.05). Likewise, the lower leukocyte count observed in both epidemics was significant, when compared with the OFD cases (P < 0.001). Moreover, in both epidemics, both AST and ALT levels were higher when compared with the OFD group (Figure 4). Independently of the epidemic year, patients presenting DwWS/SD showed lower platelet and leukocyte counts considering both DwoWS and OFD groups (P < 0.001) and higher AST and ALT levels (Figure 5).
Figure 4.
Hematological and biochemical findings of dengue-positive cases according to the epidemics, Campos dos Goytacazes, Rio de Janeiro, Brazil. (A) Platelets, (B) hematocrit, (C) leukocytes, (D) monocytes, (E) AST and (F) ALT. AST = aspartate aminotransferase; ALT = alanine aminotransferase; OFD = other febrile disease. P values * < 0.05, ** < 0.01, and *** < 0.001 were considered statistically significant.
Discussion
In Brazil, dengue transmission has been occurring continuously since 1986, with occurrence of outbreaks and epidemics, usually associated with the introduction of new serotypes in previously unaffected areas or serotype reemergences. The years of 2010 and 2013 were characterized by two of the largest dengue epidemics reported in the country, when about 1 and 1.4 million cases were reported, respectively. Moreover, despite the introduction of DENV-4 in 2010, this serotype was prevalent in 2013 and was responsible mostly for the mild cases.10 In this study, dengue infection was confirmed in 61.4% (94/153) of suspected cases that occurred in two distinct epidemics in 2010 and 2013, in the city of Campos do Goytacazes, revealed the circulation of DENV-2 and DENV-4, respectively, and distinct hematological and biochemical characteristics.
According to the 2009 WHO criteria, patients were classified as DwoWS (60.6%, 57/94), DwWS (30.9%, 29/94), and SD (2.1%, 2/94). Early recognition of warning signs is important for the appropriate management of patients to prevent deaths from dengue. Furthermore, those markers are important since they occur before the onset of severe disease.20 In 2010, when DENV-2 was the prevalent serotype, the number of hospitalizations (45.8%) was higher than during the 2013 epidemic (22.9%), corroborating previous studies, as it has been shown that infections caused by DENV-2 and DENV-3 are two times more likely to result in a more severe disease than the infection by DENV-4.21 Most of the hospitalizations (80.8%, 21/26) occurred in the acute phase of the disease between the 1st and 7th day after the onset of illness, and 96.2% (25/26) were due to DwWS/SD.
Considering the epidemiological situation of the country, the immune response characterization is important as secondary infections are also related to a more severe disease. In fact, in this study, 81.9% (77/94) of patients had secondary dengue infection, and a high prevalence of secondary infections in the country has been reported in high proportions previously.22–24
When comparing the hematologic parameters in dengue patients from this study, it was observed that regardless of their clinical status, patients of the 2010 epidemic, caused mainly by DENV-2, had lower platelet counts than patients of the 2013, caused mainly by DENV-4 (Figure 4). It was shown that DENV-2 induced more severe thrombocytopenia than DENV-4, corroborating the study by Thomas and others,25 who conducted a retrospective study comparing signs and symptoms of patients infected with different DENV serotypes, reporting differences in the virulence between the serotypes.
Several hypotheses have been formulated to elucidate the possible mechanisms responsible for thrombocytopenia in dengue. Murgue and others26 suggested that DENV affects the bone marrow progenitor cells by inhibiting their function. Other studies reported that the virus indirectly diminishes the proliferative capacity of hematopoietic cells.27 Indeed, there is evidence that DENV induced bone marrow hypoplasia in the acute phase of the disease,28 and in vitro studies have demonstrated that DENV-4 replicates in progenitor cells of human bone marrow by changing their proliferative capacity.29 Other studies reported that DENV infection induces platelet consumption due to increased lysis by complement system and apoptosis. Moreover, the involvement of antiplatelet antibodies has also been demonstrated.30,31
In the 2010 and 2013 epidemics, leukopenia was observed in dengue patients compared with OFD cases (Figure 4). The analysis of leukocytes according to the clinical classification, independently of the epidemic year, has shown a decreased count in DwWS/SD patients compared with DwoWS, however without significant differences (Figure 5). Some studies show that in the early phase of the disease, in both primary and secondary infections, a decrease in leukocyte counts and increase of atypical lymphocytes may be related to the supression of progenitor cells from bone marrow.32
Liver involvement is one of the characteristics of DENV infection. The liver changes, including hepatomegaly and increases in transaminases, have been described in both cases of dengue fever and dengue hemorrhagic fever.33–35 In SD, the occurrence of fulminant hepatic failure has been the cause of death in children.36 Mechanisms of liver damage in dengue may be related to the direct effects of the virus or as a consequence of the host immune response in the liver, leading to circulatory impairment, metabolic acidosis, hypotension, hypoxia, and/or vascular leakage.37 Our results showed that 41.5% (39/94) of patients had transaminase levels above normal, and from those, 51.3% (20/39) were DwWS/SD patients. Variations in transaminase levels during dengue are not fully understood. In this study, there were no significant statistical differences in the levels of AST/ALT between epidemics.
The recognition of hematological and biochemical differences between patients with dengue fever and other diseases is of great importance in identifying patients who may develop severe disease. The initial clinical phase of dengue is often confused with OFD, raising doubts at the time of clinical management and surveillance of the disease. However, the hematological differences and cytokine levels could be used as an early tool to differentiate dengue from other febrile illnesses. Moreover, it was also demonstrated that prediction of the possible onset of thrombocytopenia is possible by using algorithms.38
By comparing the data analyzed in this study, we showed a clear difference in hematological and biochemical findings between patients infected with DENV-2 and DENV-4, with different clinical forms of the disease. The recognition of these parameters helps to ensure that support measures can be taken to prevent increased morbidity and mortality from dengue. Further studies are needed to improve those observations that may impact on the practice of medicine during dengue epidemics, especially in tropical areas where medical resources are deficient and where such epidemics may generate large economic impacts.
Acknowledgments:
We thank the medical staff and nurses of Plantadores de Cana Hospital–Reference Center for Dengue, Campos dos Goytacazes, Rio de Janeiro, Brazil. We also thank the staff from the Flavivirus Laboratory and Viral Immunology Laboratory, Oswaldo Cruz Institute, Oswaldo Cruz Foundation.
REFERENCES
- 1.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI, 2012. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6: e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI, 2013. The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW, 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8 (Suppl): S7–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization (PAHO) , 2016. Number of Reported Cases of Dengue and Severe Dengue (SD) in the Americas, by Country: Epidemiological Week/EW 5 February 3, 2016. Washington, DC: PAHO. [Google Scholar]
- 5.Osanai CH, Travassos da Rosa AP, Tang AT, do Amaral RS, Passos AD, Tauil PL, 1983. Dengue outbreak in Boa Vista, Roraima. Preliminary report [in Portuguese]. Rev Inst Med Trop Sao Paulo 25: 53–54. [PubMed] [Google Scholar]
- 6.Schatzmayr HG, Nogueira RM, Travassos da Rosa AP, 1986. An outbreak of dengue virus at Rio de Janeiro: 1986. Mem Inst Oswaldo Cruz 81: 245–246. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira MG, Costa Mda C, Barreto F, Barreto ML, 2009. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica 25 (Suppl 1): S7–S18. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RM, Eppinghaus AL, 2011. Dengue virus type 4 arrives in the state of Rio de Janeiro: a challenge for epidemiological surveillance and control. Mem Inst Oswaldo Cruz 106: 255–256. [DOI] [PubMed] [Google Scholar]
- 9.Heringer M, Nogueira RM, de Filippis AM, Lima MR, Faria NR, Nunes PC, Nogueira FB, dos Santos FB, 2015. Impact of the emergence and re-emergence of different dengue viruses' serotypes in Rio de Janeiro, Brazil, 2010 to 2012. Trans R Soc Trop Med Hyg 109: 268–274. [DOI] [PubMed] [Google Scholar]
- 10.Secretaria de Vigilância em Saúde/Ministério da Saúde (SVS/MS) , 2014. Casos de Dengue. Brasil, Grandes Regiões e Unidades Federadas, 1990 a 2014. Brasília, Brazil: SVS/MS [Google Scholar]
- 11.Secretaria de Vigilância em Saúde/Ministério da Saúde (SVS/MS) , 2011. Dengue: diagnóstico e manejo clínico—adulto e criança, 4th edition Brasília, Brazil: Secretaria de Vigilância em Saúde, Diretoria Técnica de Gestão/Ministério da Saúde. [Google Scholar]
- 12.Rothman AL, 2004. Dengue: defining protective versus pathologic immunity. J Clin Invest 113: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) , 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 14.Teixeira MG, Barreto ML, 2009. Diagnosis and management of dengue. BMJ 339: b4338. [DOI] [PubMed] [Google Scholar]
- 15.Faria NRC, Solorzano VEF, Nogueira RMR, Chouin-Carneiro T, Nunes PCG, Simoes JBS, Nogueira FB, Lima MRQ, Pinto LMO, Kubelka CF, da Cunha RV, de Azeredo EL, dos Santos FB, 2016. Dengue epidemics in two distinct periods reveal distinct epidemiological, laboratorial and clinical aspects in a same scenario: analysis of the 2010 and 2013 epidemics in Mato Grosso do Sul, Brazil. Trans R Soc Trop Med Hyg 110: 228–236. [DOI] [PubMed] [Google Scholar]
- 16.Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V, 1999. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol 14: 183–189. [DOI] [PubMed] [Google Scholar]
- 17.Lima MRQ, Nogueira RMR, de Filippis AMB, Nunes PCG, de Sousa CS, da Silva MH, dos Santos FB, 2014. A simple heat dissociation method increases significantly the ELISA detection sensitivity of the nonstructural-1 glycoprotein in patients infected with DENV type-4. J Virol Methods 204: 105–108. [DOI] [PubMed] [Google Scholar]
- 18.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV, 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 30: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Morais Bronzoni RV, Baleotti FG, Ribeiro Nogueira RM, Nunes M, Moraes Figueiredo LT, 2005. Duplex reverse transcription-PCR followed by nested PCR assays for detection and identification of Brazilian alphaviruses and flaviviruses. J Clin Microbiol 43: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, Janisch T, Kroeger A, Lum LC, Martinez E, Siqueira JB, Thuy TT, Villalobos I, Villegas E, Wills B, European Union, World Health Organization (WHO-TDR) supported DENCO Study Group , 2011. Multicentre prospective study on dengue classification in four south-east Asian and three Latin American countries. Trop Med Int Health 16: 936–948. [DOI] [PubMed] [Google Scholar]
- 21.Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, Jarman RG, Green S, Rothman AL, Cummings DA, 2010. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis 4: e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nogueira RM, Schatzmayr HG, de Filippis AM, dos Santos FB, da Cunha RV, Coelho JO, de Souza LJ, Guimaraes FR, de Araujo ES, De Simone TS, Baran M, Teixeira G, Miagostovich MP, 2005. Dengue virus type 3, Brazil, 2002. Emerg Infect Dis 11: 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilarde AO, Turchi MD, Siqueira JB, Feres VC, Rocha B, Levi JE, Souza VA, Boas LS, Pannuti CS, Martelli CM, 2008. Dengue and dengue hemorrhagic fever among adults: clinical outcomes related to viremia, serotypes, and antibody response. J Infect Dis 197: 817–824. [DOI] [PubMed] [Google Scholar]
- 24.Honorio NA, Nogueira RMR, Codeco CT, Carvalho MS, Cruz OG, Magalhaes Mde A, de Araujo JM, de Araujo ES, Gomes MQ, Pinheiro LS, da Silva Pinel C, Lourenco-de-Oliveira R, 2009. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 3: e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas L, Verlaeten O, Cabié A, Kaidomar S, Moravie V, Martial J, Najioullah F, Plumelle Y, Fonteau C, Dussart P, Césaire R, 2008. Influence of the dengue serotype, previous dengue infection, and plasma viral load on clinical presentation and outcome during a dengue-2 and dengue-4 co-epidemic. Am J Trop Med Hyg 78: 990–998. [PubMed] [Google Scholar]
- 26.Murgue B, Cassar O, Guigon M, Chungue E, 1997. Dengue virus inhibits human hematopoietic progenitor growth in vitro. J Infect Dis 175: 1497–1501. [DOI] [PubMed] [Google Scholar]
- 27.Basu A, Jain P, Gangodkar SV, Shetty S, Ghosh K, 2008. Dengue 2 virus inhibits in vitro megakaryocytic colony formation and induces apoptosis in thrombopoietin-inducible megakaryocytic differentiation from cord blood CD34+ cells. FEMS Immunol Med Microbiol 53: 46–51. [DOI] [PubMed] [Google Scholar]
- 28.Tsai JJ, Jen YH, Chang JS, Hsiao HM, Noisakran S, Perng GC, 2011. Frequency alterations in key innate immune cell components in the peripheral blood of dengue patients detected by FACS analysis. J Innate Immun 3: 530–540. [DOI] [PubMed] [Google Scholar]
- 29.Nakao S, Lai CJ, Young NS, 1989. Dengue virus, a flavivirus, propagates in human bone marrow progenitors and hematopoietic cell lines. Blood 74: 1235–1240. [PubMed] [Google Scholar]
- 30.Lin CF, Wan SW, Cheng HJ, Lei HY, Lin YS, 2006. Autoimmune pathogenesis in dengue virus infection. Viral Immunol 19: 127–132. [DOI] [PubMed] [Google Scholar]
- 31.Hottz ED, Oliveira MF, Nunes PC, Nogueira RM, Valls-de-Souza R, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza PT, Bozza FA, 2013. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J Thromb Haemost 11: 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jameel T, Mehmood K, Mujtaba G, Choudhry N, Afzal N, Paul RF, 2012. Changing haematological parameters in dengue viral infections. J Ayub Med Coll Abbottabad 24: 3–6. [PubMed] [Google Scholar]
- 33.Nguyen TL, Nguyen TH, Tieu NT, 1997. The impact of dengue haemorrhagic fever on liver function. Res Virol 148: 273–277. [DOI] [PubMed] [Google Scholar]
- 34.Trung DT, Thao LTT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B, 2010. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg 83: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee LK, Gan VC, Lee VJ, Tan AS, Leo YS, Lye DC, 2012. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. PLoS Negl Trop Dis 6: e1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A, Sarkar D, Chakraborty S, Chaudhuri J, Ghosh P, Chakraborty S, 2013. Profile of hepatic involvement by dengue virus in dengue infected children. N Am J Med Sci 5: 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itha S, Kashyap R, Krishnani N, Saraswat VA, Choudhuri G, Aggarwal R, 2005. Profile of liver involvement in dengue virus infection. Natl Med J India 18: 127–130. [PubMed] [Google Scholar]
- 38.Tanner L, Schreiber M, Low JG, Ong A, Tolfvenstam T, Lai YL, Ng LC, Leo YS, Thi Puong L, Vasudevan SG, Simmons CP, Hibberd ML, Ooi EE, 2008. Decision tree algorithms predict the diagnosis and outcome of dengue fever in the early phase of illness. PLoS Negl Trop Dis 2: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]