Abstract.
We report the results of an investigation into a fatal case of hantavirus pulmonary syndrome (HPS) in Rio de Janeiro State, Brazil, where the disease had not been reported previous to 2015. Following the notification of an HPS case, serum samples were collected from the household members and work contacts of the HPS patient and tested for antibody to hantaviruses. Seroprevalence of 22% (10/45) was indicated for hantavirus out of 45 human samples tested. Blood and tissue samples were collected from 72 rodents during fieldwork to evaluate the prevalence of hantavirus infection, by using enzyme-linked immunosorbent assay IgG, and to characterize the rodent hantavirus reservoir(s), by reverse transcription polymerase chain reaction and sequencing. Antibody prevalence was 6.9%. The circulation of a single genotype, the Juquitiba hantavirus, carried by two rodent species, black-footed pigmy rice rat (Oligoryzomys nigripes) and cursor grass mouse (Akodon cursor), was shown by analysis of the nucleotide sequences of the S segment. Juquitiba hantavirus circulates in rodents of various species, but mainly in the black-footed pigmy rice rat. HPS is a newly recognized clinical entity in Rio de Janeiro State and should be considered in patients with febrile illness and acute respiratory distress.
INTRODUCTION
Hantavirus pulmonary syndrome (HPS) is an emerging infectious disease and represents a major public health problem in the Americas because of its high case fatality rate. The majority of HPS cases in Latin America occur in the southern Cone of South America (e.g., Argentina, Brazil, and Chile). Although considerable knowledge about HPS has accumulated in these countries, with the establishment of surveillance programs, the ability to identify new cases in nonendemic areas is still very challenging.1,2 An HPS diagnosis can easily be missed if the disease is not suspected. HPS recognition, based on the early identification of signs and symptoms, is not easy to achieve and can be misdiagnosed as other prevalent diseases endemic in Latin America, such as dengue fever, leptospirosis, rickettsiosis, and influenza.
Dengue fever is considered as the fastest-spreading vector-borne viral disease. Over the last 50 years, it has affected more than 100 countries in all tropical and subtropical regions of the world.3,4 In Brazil, 1,399,480 suspected cases of Dengue virus (DENV) infection were reported up to 2016, with Rio de Janeiro being the gateway and dispersion area for most serotypes.5,6 The high prevalence of dengue fever in Rio de Janeiro compromises the efficiency of clinical and laboratory diagnosis by health professionals due to the overlap of clinical signs and the unavailability of reliable tests for differential diagnosis.
Cases of HPS have been reported in 16 federated units in Brazil. It is often fatal and is frequently mistaken for acute respiratory distress syndrome, which can have many causes. Since the first outbreak of HPS in 1993, in the city of Juquitiba, 2,020 cases have been registered and six hantavirus genotypes associated with HPS have been described, namely the Juquitiba virus (JUQV) as well as the Araraquara, Castelo dos Sonhos, Laguna Negra, Anajatuba, and Rio Mamore viruses. An additional hantavirus, Araucaria virus, closely related to JUQV, has been associated with HPS cases.7–9
We report the results on the first human case of HPS in Rio de Janeiro State in this article. The clinical condition was initially interpreted as dengue fever, a well-known endemic disease in the state as mentioned above. An eco-epidemiological study was conducted as part of the investigation to assist with the prevention and control measures and to provide important information to guide clinical practice and surveillance.
MATERIALS AND METHODS
Case report.
In May 2015, a 34-year-old male patient presented to a public hospital in the municipality of Rio Claro, Rio de Janeiro State, with a 4-day history of fever, headache, arthralgia, and myalgia. He was treated with oral fluid replacement and symptomatic medication. Two days later, the patient returned, complaining of persistent fever, associated with dyspnea, nausea, and vomiting. On admission, his leukocyte count was 4,000/mm3, platelets 52,000/mm3, hematocrit 46%, alanine aminotransferase 121 U/L, and aspartate aminotransferase 48 U/L. Electrolytes, blood glucose, and renal function were normal.
Twenty-four hours after admission, the patient rapidly progressed to respiratory failure and shock. Cardiopulmonary resuscitation failed and the patient died of respiratory failure 7 days after the onset of the illness. Diagnostic tests for dengue, nonstructural protein 1, and IgM enzyme-linked immunosorbent assay (ELISA) were negative. This scenario raised a clinical suspicion of HPS. The blood samples subsequently were evaluated by serological and polymerase chain reaction (PCR) testing for antibodies to hantaviruses and hantaviral RNA. The ELISA result was positive for IgM against the recombinant nucleocapsid protein of JUQV.10 The viral genome was detected by reverse transcription PCR (RT-PCR) and the virus was identified as the JUQV.
Outbreak investigation.
Soon after the confirmation of the first case of HPS in Rio de Janeiro State, in the municipality of Rio Claro, an outbreak investigation was conducted locally to establish the overall risk of hantavirus infection in the region. This study was approved by the Ethics Committee of FIOCRUZ, under number CAAE 51398415.7.0000.5248.
Study area.
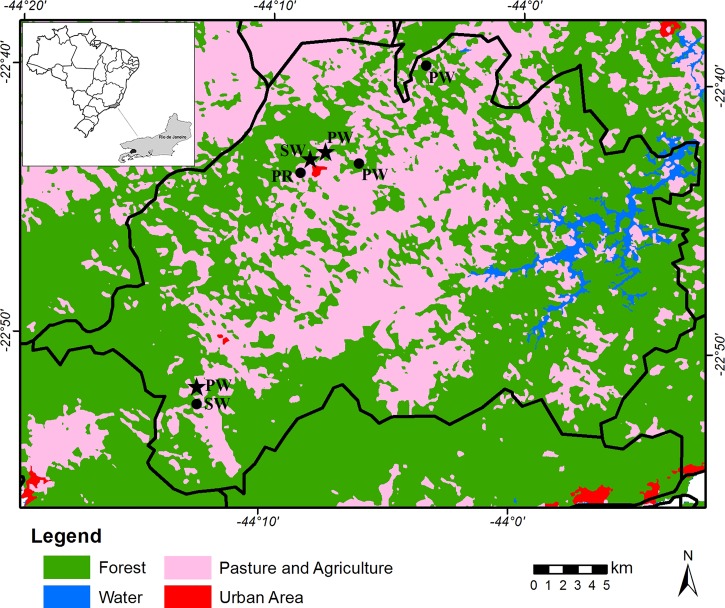
Rio Claro municipality is located in the Atlantic Forest biome, and has 42% original vegetation cover. Despite being one of the least deforested regions in the state, many rural activities are carried out in the area (Figure 1). It is located in midwestern Rio de Janeiro (22°43′23″ S, 44°08′08″ W) 446 m above sea level and 132 km from the city of Rio de Janeiro, with a population of 17,425 inhabitants (20.7 people/km2), most of them living in the urban areas, according to the Brazilian Institute of Geography and Statistics.
Figure 1.
The study areas in Rio Claro municipality, Rio de Janeiro, Brazil, showing the rodent collection sites (black stars, hantavirus positive rodents; black dots, hantavirus negative rodents). PW = patient's workplace; PR = patient's residence; SW = areas surrounding the workplaces. This figure appears in color at www.ajtmh.org.
The patient's house was located in a periurban area, bordered by signalgrass (Brachiaria spp.) and bamboo groves (family Poaceae, subfamily Bambusoideae). In an interview with the patient's family members, it was established that a few days before the onset of symptoms, he handled some old boards that were in the garage of their house. He worked at four chicken farms and slept on the canvas used to cover the poultry feed. There was considerable dust in the places in which he worked.
Human and rodent sampling.
In June 2015, serum samples from 45 healthy residents at the patient's workplace and neighboring residences were collected as part of the outbreak investigation.
Subsequently, a serosurvey was carried out to estimate the prevalence of hantaviruses in rodents captured at sites of probable infection of the patient. Fieldwork involving small mammals was conducted on two occasions in seven trapping areas in June and November 2015; four in the patient's workplace, one in the patient's residence, and two in the surrounding areas (Figure 1). Trapping success was calculated as the number of captured animals per trapping effort multiplied by 100, where the trapping effort is the number of traps set multiplied by the number of nights of capture. Trappings transects were established and traps were placed in forested areas, peridomestic environments, and along the shrub and forest borders. Each capture station was supplied with Sherman (HB Sherman Traps, Tallahassee, FL) and Tomahawk (Tomahawk Live Trap, Hazelhurst, WI) live traps, placed 10 m apart, in linear ground transects of 20 capture stations, which were inspected in the early morning for five consecutive days. The bait was a mixture of bacon, peanut butter, banana, and oatmeal.
Each animal was anesthetized and euthanized in accordance with the Guidelines for the Care and Use of Laboratory Animals of Oswaldo Cruz Foundation (FIOCRUZ), Brazil (license LW-39/14). Blood was collected by heart puncture from each anesthetized rodent. Tissue samples of the liver, spleen, kidney, lung, and heart were collected and stored immediately in liquid nitrogen for further processing. The specimens were then measured, weighted, and sexed. The rodents were identified by morphology and karyological analysis, as previously reported.11,12 The positive specimens were deposited as vouchers with the mammal collection at the National Museum of Federal University of Rio de Janeiro. Other species were housed in the mammal collection of FIOCRUZ. The capture of small mammals was authorized by Chico Mendes Institute for Biodiversity Conservation (permit13373-1).
Serological and molecular tests.
All asymptomatic human serum samples were tested by ELISA for antihantavirus (IgM and IgG) antibodies using the recombinant Andes nucleocapsid protein as the specific antigen.13 The serum samples of the rodents were examined for antihantavirus IgG antibodies.13 Samples that yielded an IgG-positive result were analyzed by RT-PCR for viral genomic partial S segment characterization. Amplification and sequencing were performed using specific primers for the nucleoprotein coding region of the S segment of the viral genome as reported previously.14 Hantavirus genomic sequences obtained from the rodents and the patient were aligned with the S segment sequences from the main Brazilian and South American hantavirus genotypes with the use of ClustalW in the Mega 6 software. Phylogenetic reconstruction was performed under Bayesian inference, using the MrBayes software. Maximum likelihood estimation was also carried out using the Mega 6 software.
RESULTS AND DISCUSSION
The case described here was the first confirmed case of HPS in Rio de Janeiro State. The possible diagnosis of hantavirus infection was only considered on the seventh day of illness when the patient presented with respiratory distress. A clinical diagnosis of HPS is difficult, mainly in the earlier phases of illness, since others infectious diseases such as dengue, rickettsioses, and influenza have overlapping clinical manifestations. In the present case, HPS was misdiagnosed as dengue fever, an endemic arboviral disease in Rio de Janeiro State. Although increased macrovascular permeability and plasma extravasation occur due to both the dengue virus and hantavirus, the rapid administration of intravenous fluids that is recommended for dengue is inappropriate for HPS.
The patient lived in a periurban area surrounded by signalgrass and bamboo, with poor sanitation conditions. He worked in rural areas (chicken farms) surrounded by forests. It is likely that exposure of the patient to rodents occurred, based on these environmental conditions. His case is consistent with the high prevalence of hantavirus antibodies in other households and workplaces in this region. The detection of IgG against hantavirus was noted in 10 samples, seven of them from the patient's workplace (16%). The rate of seroprevalence was 22% (10/45), illustrating that the population studied had previously been exposed to hantaviruses. All of the samples were nonreactive to IgM antibodies. The high rate of seroprevalence (22%) and the absence of cases in Rio Claro municipality and Rio de Janeiro State before 2015 can be explained by inadequate surveillance, the occurrence of mild or asymptomatic infections, and the misdiagnosis of HPS by physicians. Hantavirus infection may well mimic dengue fever, which is frequently seen in this region. It is likely that the most probable HPS cases are labeled as clinical dengue fever, based on clinical and epidemiological criteria only. It is therefore advisable to suspect of hantavirus infection in seronegative dengue cases. Furthermore, HPS is underdiagnosed or misdiagnosed in many regions of Brazil and other Latin American countries.
Seventy-two rodents belonging to six genera were captured as follows: Rodentia Sigmodontinae: 38 Akodon cursor (cursorial grass mouse), 12 Akodon montensis (montane grass mouse), 1 Euryoryzomys russatus (russet euryoryzomys), 1 Nectomys squamipes (atlantic water rat), 11 Oligoryzomys nigripes (black-footed pigmy rice rat), 2 Oxymycterus dasythricus (atlantic forest hocicudo), and 2 Sooretamys angouya (angouya sooretamys); and Rodentia Murinae: 1 Mus musculus (house mouse) and 4 Rattus rattus (house rat). The total capture success was 4% for a capture effort of 1,815 traps. The most abundant species were A. cursor (cursor grass mouse) and A. montensis (montane grass mouse), followed by O. nigripes (black-footed pigmy rice rat). Five (7%) of the rodents were seroreactive to hantaviruses. Four of these were black-footed pigmy rice rats (80%), two of them captured in areas surrounding the patient's workplace and the other two specimens in the vicinity of his house, and one cursor grass mouse seroreactive (20%), captured in the vicinity of the patient's workplace (Figure 1). Four of the samples were RT-PCR positive for hantavirus, of which three were black-footed pigmy rice rats and one was a cursor grass mouse. The JUQV was detected in both species (GenBank accession numbers: KY053840-Oln19539; KY053841-Oln19540; KY053842-Akc19583 and KY053844-Oln19603).
Although the black-footed pygmy rice rat was not the most abundant rodent species in this study, it accounted for 80% of antibody-positive specimens of hantavirus, indicating its important role in the dynamics of the hantavirus cycle in the study area. JUQV was the hantavirus genotype observed in the black-footed pygmy rice rats, as expected, since this species is considered to be the main host of this genotype. This finding was previously reported in Brazil, including in Rio de Janeiro State, as well as in Paraguay.15–17 The black-footed pygmy rice rat occurs in eastern Brazil, in primary and secondary vegetation in Atlantic Forest and Cerrado (savannah-like) biomes.
This is the first time that JUQV has been recognized in a cursor grass mouse, besides previous serological evidence, indicating spillover infection from the main reservoir, the black-footed pygmy rice rat.18 Furthermore, the occurrence of JUQV-like infection in two different genera of hosts suggests a potential adaptation of this genotype to genetically distinct rodent species, as previously observed in the central and southern regions of Brazil, where distinct rodent species, A. montensis, Akodon paranaensis, Oligoryzomys mattogrossae (fornesi), Oligoryzomys Judex, and Thaptomys nigrita have been identified as being naturally infected with JUQV.19–22 In this study, the cursor grass mouse was the most abundant species identified and has adapted to exist in primary and secondary forests, especially in the transition area between Atlantic Forest and Cerrado areas in southeastern Brazil. It is a generalist species that occurs near human habitation, as does the black-footed pygmy rice rat (O. nigripes).23 These findings raise the possibility that the cursor grass mouse acts as a secondary host in this region and probably plays an important role in maintaining the virus in the wild.
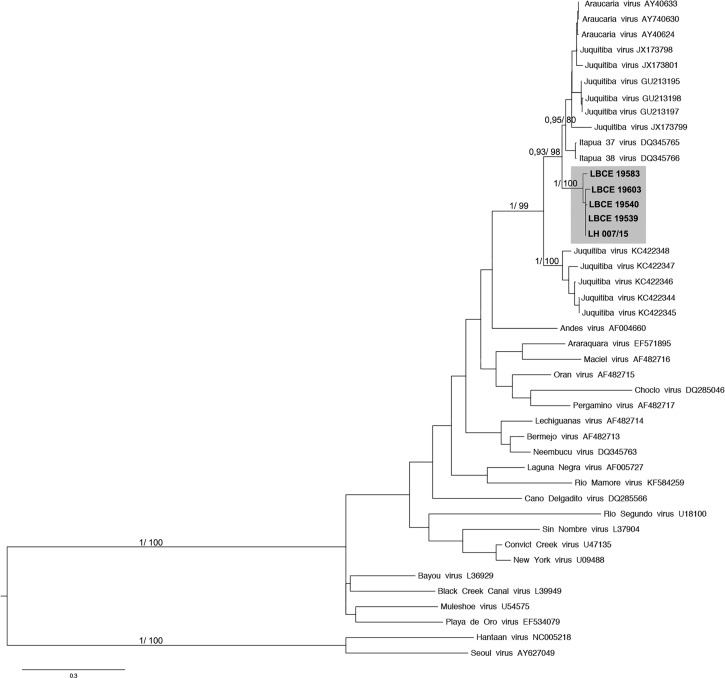
The sequence of the JUQV obtained from the patient (GenBank accession no. KY053843-LH007/15) revealed 98–100% (nt) genetic identity to that of the hantavirus identified in the black-footed pigmy rice rats and cursor grass mouse captured at the study sites, both near the patient's workplace and his residence, clearly marking this area as endemic for hantavirus. According to the phylogenetic analysis of the complete S-segment sequences of JUQV conducted in our previous study,24 the existence of two distinct JUQV clades was demonstrated. The first clade contained the JUQV from two nonendemic areas in Brazil: the states of Espírito Santo and Rio de Janeiro (in the second case in the municipalities of Teresópolis and Valença), and the second clade contained JUQV strains from Argentina, Paraguay, and other endemic areas in Brazil. In fact, the sequences from the patient and rodents from Rio Claro, Rio de Janeiro State, formed a well-supported clade with JUQV strains from known endemic areas, indicating the presence of these two distinct lineages in Rio de Janeiro State (Figure 2). This reinforces the possibility of the risk of hantavirus infection in this area and the need for genetic characterization of the circulating hantaviruses.
Figure 2.
A phylogenetic tree based on the nucleoprotein partial sequences (386 nt) of hantaviruses estimated using maximum likelihood and Bayesian methods. The scale bars indicate an evolutionary distance of 0.3 substitutions per position in the sequence. The numerical value of ≥ 0.7/70.0 at the node indicates the posterior probability (pp) replicates and bootstrap values that support the interior branch. The branch labels include the GenBank® accession number and the viral species or strain and the sequences obtained in this study are shown in the gray box.
Furthermore, the circulation of a pathogenic hantavirus, and the appearance of a single case in an area not previously known to have hantavirus infection, should trigger a comprehensive medical and epidemiological assessment, for analysis of individual risk factors of exposure, and a long-term ecological and environmental study to fully understand the epidemiology and risk of hantaviruses to humans in Rio de Janeiro State. This case report and the relatively high human antibody prevalence that is observed in the region should help physicians to suspect and better diagnose HPS in clinical practices. This would assist with the anticipation of future outbreaks.
Acknowledgments:
We are grateful to staff members of the Rio Claro Municipal Health Secretariat and the Rio de Janeiro State Health Secretariat for logistical support.
REFERENCES
- 1.Pan American Health Organization. World Health Organization , 2013. Epidemiological Alert: Hantavirus Pulmonary Syndrome (HPS). October 17, 2013. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=23388&Itemid=270. Accessed September 15, 2016. [Google Scholar]
- 2.Figueiredo LT, Souza WM, Ferrés M, Enria DA, 2014. Hantaviruses and cardiopulmonary syndrome in South America. Virus Res 187: 43–54. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) , 2013. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Diseases. Geneva, Switzerland: WHO. [Google Scholar]
- 4.Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F, 2016. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the black sea region increases risk of chikungunya, dengue, and zika outbreaks in Europe. PLoS Negl Trop Dis 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secretaria de Vigilância em Saúde/Ministério da Saúde (SVS/MS) , 2016. Monitoramento dos Casos de Dengue, febre de Chikungunya e Febre Pelo Vírus Zika até a Semana Epidemiológica 37 de 2016. Boletim Epidemiológico Volume 47 - n° 34. Brasília: SVS/MS. [Google Scholar]
- 6.Secretaria de Vigilância em Saúde/Ministério da Saúde (SVS/MS) , 2014. Monitoramento dos Casos de Dengue Semana Epidemiológica (SE) 35 e Febre de Chikungunya SE 36 de 2014. Boletim Epidemiológico. Volume 45 - n° 20. Brasília: SVS/MS. [Google Scholar]
- 7.Oliveira RC, Guterres A, Fernandes J, D'Andrea PS, Bonvicino CR, de Lemos ER, 2014. Hantavirus reservoirs: current status with an emphasis on data from Brazil. Viruses 29: 1929–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazilian Ministry of Health, 2016. Confirmed Cases of Hantavirus Diseases. Brazil, Major Regions and Federative Units, 1993 to 2016. Available at: http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/hantavirose. Accessed July 20, 2016.
- 9.Oliveira RC, Cordeiro-Santos M, Guterres A, Fernandes J, de Melo AX, João GA, Novais MA, Travassos da Rosa ES, Vasconcelos PF, Vilges de Oliveira S, de Albuquerque BC, de Lemos ER, 2014. Rio Mamoré virus and hantavirus pulmonary syndrome, Brazil. Emerg Infect Dis 20: 1568–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raboni SM, Levis S, Rosa ES, Bisordi I, Delfraro A, Lemos E, Correia DC, Duarte dos Santos CN, 2007. Hantavirus infection in Brazil: development and evaluation of an enzyme immunoassay and immunoblotting based on N recombinant protein. Diagn Microbiol Infect Dis 58: 89–97. [DOI] [PubMed] [Google Scholar]
- 11.Bonvicino CR, Otazú IB, Vilela JF, 2005. Karyologic and molecular analysis of Proechimys Allen, 1899 (Rodentia, Echimyidae) from the Amazonian region. Arq Mus Nac (Rio de Janeiro) 63: 191–200. [Google Scholar]
- 12.Bonvicino CR, 2011. Diversidade cariotipica em roedores Akodontini do Brasil. Bol Soc Bras Mastozoologia 62: 7–11. [Google Scholar]
- 13.Padula PJ, Rossi CM, Della Valle MO, Martínez PV, Colavecchia SB, Edelstein A, Miguel SD, Rabinovich RD, Segura EL, 2000. Development and evaluation of solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J Med Microbiol 49: 149–155. [DOI] [PubMed] [Google Scholar]
- 14.Guterres A, de Oliveira RC, Fernandes J, Schrago CG, de Lemos ER, 2015. Detection of different South American hantaviruses. Virus Res 210: 106–113. [DOI] [PubMed] [Google Scholar]
- 15.Chu YK, Goodin D, Owen RD, Koch D, Jonsson CB, 2009. Sympatry of 2 hantavirus strains, paraguay, 2003–2007. Emerg Infect Dis 15: 1977–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira RC, Teixeira BR, Mello FC, Pereira AP, Duarte AS, Bonaldo MC, Bonvicino CR, D'Andrea PS, Lemos ER, 2009. Genetic characterization of a Juquitiba-like viral lineage in Oligoryzomys nigripes in Rio de Janeiro, Brazil. Acta Trop 112: 212–218. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira RC, Padula PJ, Gomes R, Martinez VP, Bellomo C, Bonvicino CR, Freire e Lima DI, Bragagnolo C, Caldas AC, D'Andrea PS, de Lemos ER, 2011. Genetic characterization of hantaviruses associated with sigmodontine rodents in an endemic area for hantavirus pulmonary syndrome in southern Brazil. Vector Borne Zoonotic Dis 11: 301–314. [DOI] [PubMed] [Google Scholar]
- 18.Sobreira M, Souza GT, Moreli ML, Borges AA, Morais FA, Figueiredo LT, Almeida AM, 2008. A serosurvey for hantavirus infection in wild rodents from the states of Rio de Janeiro and Pernambuco, Brazil. Acta Trop 107: 150–152. [DOI] [PubMed] [Google Scholar]
- 19.Raboni SM, Hoffmann FG, Oliveira RC, Teixeira BR, Bonvicino CR, Stella V, Carstensen S, Bordignon J, D'Andrea PS, Lemos ER, Duarte dos Santos CN, 2009. Phylogenetic characterization of hantaviruses from wild rodents and hantavirus pulmonary syndrome cases in the state of Parana (southern Brazil). J Gen Virol 90: 2166–2171. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira RC, Gentile R, Guterres A, Fernandes J, Teixeira BR, Vaz V, Valdez FP, Vicente LH, da Costa-Neto SF, Bonvicino C, D'Andrea PS, Lemos ER, 2014. Ecological study of hantavirus infection in wild rodents in an endemic area in Brazil. Acta Trop 131: 1–10. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira BR, Loureiro N, Strecht L, Gentile R, Oliveira RC, Guterres A, Fernandes J, Mattos LH, Raboni SM, Rubio G, Bonvicino CR, dos Santos CN, Lemos ER, D'Andrea OS, 2014. Population ecology of hantavirus rodent hosts in southern Brazil. Am J Trop Med Hyg 91: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guterres A, de Oliveira RC, Fernandes J, Strecht L, Casado F, Gomes de Oliveira FC, D'Andrea PS, Bonvicino CR, Schrago CG, de Lemos ER Sampaio, 2014. Characterization of Juquitiba virus in Oligoryzomys fornesi from Brazilian Cerrado. Viruses 6: 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umetsu F, Pardini R, 2007. Small mammals in a mosaic of forest remnants and anthropogenic habitats—evaluating matrix quality in an Atlantic forest landscape. Landsc Ecol 22: 517–530. [Google Scholar]
- 24.Guterres A, de Oliveira RC, Fernandes J, D'Andrea PS, Bonvicino CR, Bragagnolo C, Guimarães GD, Almada GL, Machado RR, Lavocat M, Elkhoury Mda R, Schrago CG, de Lemos ER, 2013. Phylogenetic analysis of the S segment from Juquitiba hantavirus: identification of two distinct lineages in Oligoryzomys nigripes . Infect Genet Evol 18: 262–268. [DOI] [PubMed] [Google Scholar]