Abstract
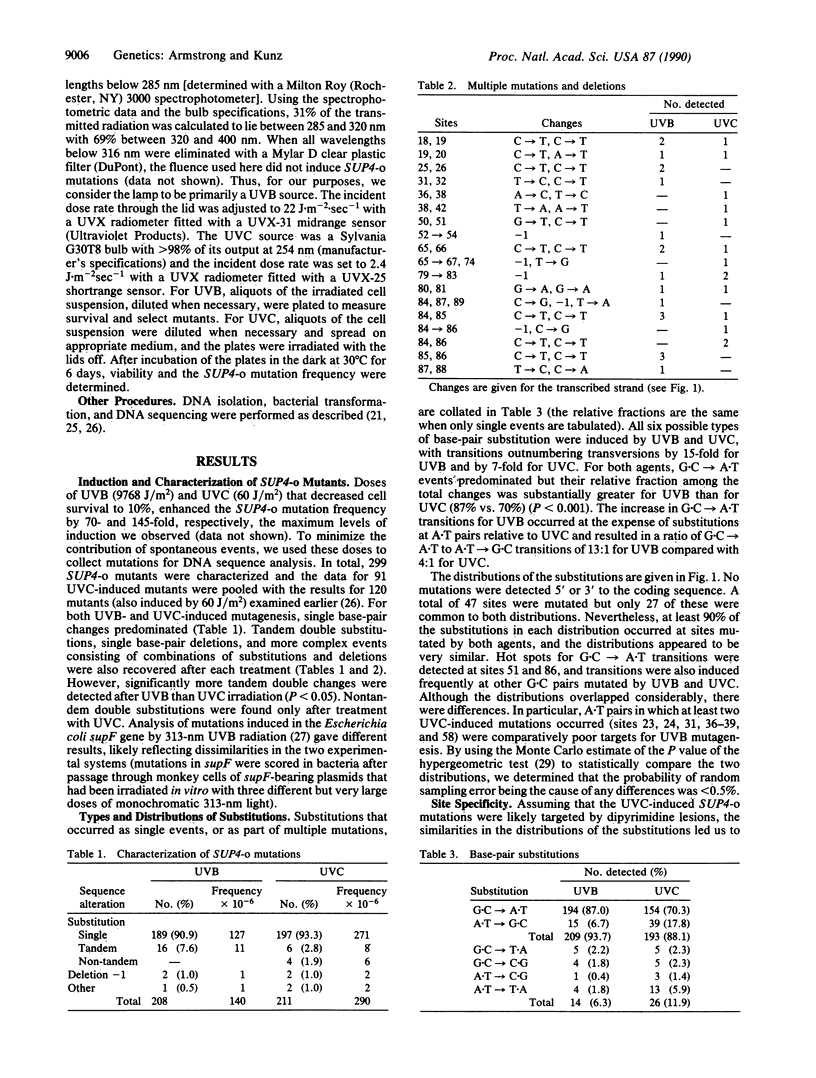
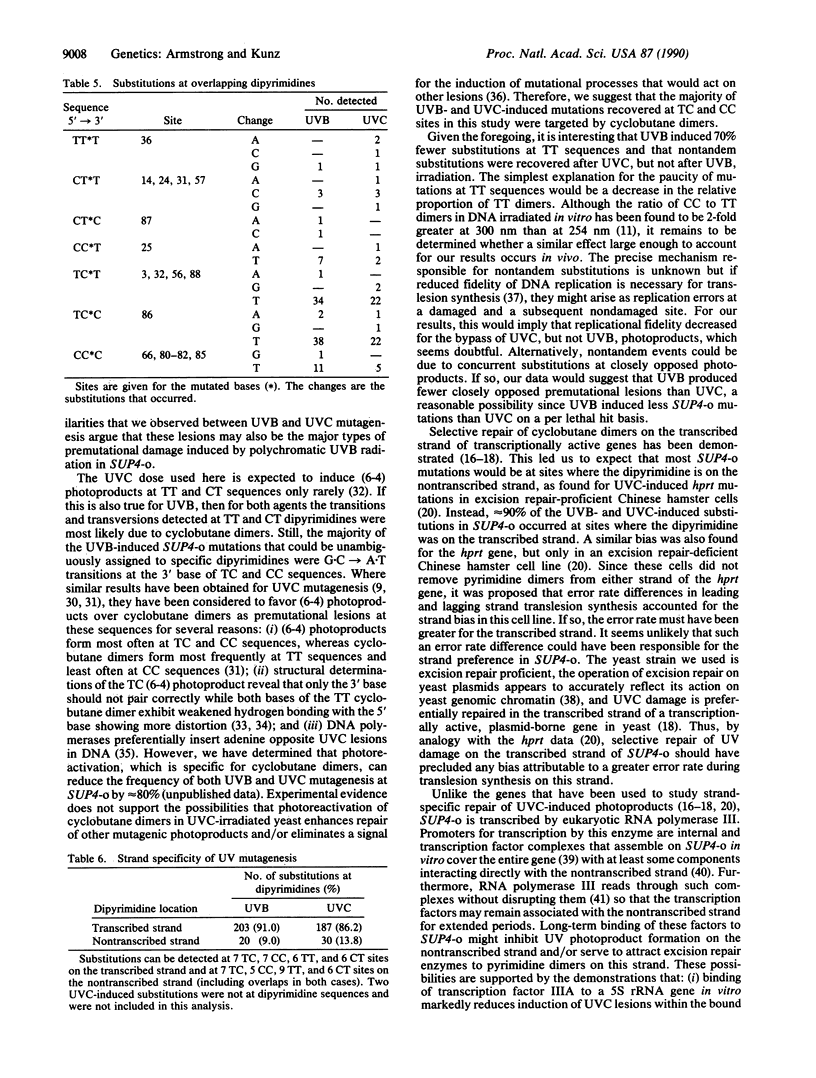
DNA sequencing was used to characterize 208 mutations induced in the SUP4-o tRNA gene of the yeast Saccharomyces cerevisiae by UVB (285-320 nm) radiation. The results were compared to those for an analysis of 211 SUP4-o mutations induced by 254-nm UVC light. In each case, greater than 90% of the mutations were single base-pair changes but G.C----A.T transitions predominated and accounted for more of the mutations induced by UVB than UVC. Double substitutions, single base-pair deletions, and more complex events were also recovered. However, UVB induced 3-fold more tandem substitutions than UVC and nontandem double events were detected only after irradiation with UVC. Virtually all induced substitutions occurred at sites where the pyrimidine of the base pair was part of a dipyrimidine sequence. Although the site specificities were consistent with roles for cyclobutane dimers and pyrimidine-pyrimidone(6-4) lesions in mutation induction, preliminary photoreactivation data implicated cyclobutane dimers as the major form of premutational DNA damage for both agents. Intriguingly, there was a preference for both UVB- and UVC-induced mutations to occur at sites where the dipyrimidine was on the transcribed strand.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. T., Skopek T. R. Statistical test for the comparison of samples from mutational spectra. J Mol Biol. 1987 Apr 5;194(3):391–396. doi: 10.1016/0022-2836(87)90669-3. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E. UV mutagenic photoproducts in Escherichia coli and human cells: a molecular genetics perspective on human skin cancer. Photochem Photobiol. 1988 Jul;48(1):59–66. doi: 10.1111/j.1751-1097.1988.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Cole C. A., Forbes P. D., Davies R. E. An action spectrum for UV photocarcinogenesis. Photochem Photobiol. 1986 Mar;43(3):275–284. doi: 10.1111/j.1751-1097.1986.tb05605.x. [DOI] [PubMed] [Google Scholar]
- Eisenstark A. Mutagenic and lethal effects of near-ultraviolet radiation (290-400 nm) on bacteria and phage. Environ Mol Mutagen. 1987;10(3):317–337. doi: 10.1002/em.2850100311. [DOI] [PubMed] [Google Scholar]
- Ellison M. J., Childs J. D. Pyrimidine dimers induced in Escherichia coli DNA by ultraviolet radiation present in sunlight. Photochem Photobiol. 1981 Oct;34(4):465–469. [PubMed] [Google Scholar]
- Enninga I. C., Groenendijk R. T., Filon A. R., van Zeeland A. A., Simons J. W. The wavelength dependence of u.v.-induced pyrimidine dimer formation, cell killing and mutation induction in human diploid skin fibroblasts. Carcinogenesis. 1986 Nov;7(11):1829–1836. doi: 10.1093/carcin/7.11.1829. [DOI] [PubMed] [Google Scholar]
- Epstein J. H. Photocarcinogenesis: a review. Natl Cancer Inst Monogr. 1978 Dec;(50):13–25. [PubMed] [Google Scholar]
- Franklin W. A., Doetsch P. W., Haseltine W. A. Structural determination of the ultraviolet light-induced thymine-cytosine pyrimidine-pyrimidone (6-4) photoproduct. Nucleic Acids Res. 1985 Jul 25;13(14):5317–5325. doi: 10.1093/nar/13.14.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen O. S., Marzouki N., Ruet A., Sentenac A., Fromageot P. Two polypeptide chains in yeast transcription factor tau interact with DNA. J Biol Chem. 1989 May 5;264(13):7505–7511. [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation of products of the 5,6-dihydroxydihydrothymine type by ultraviolet light in HeLa cells. Biochemistry. 1977 Jun 14;16(12):2791–2795. doi: 10.1021/bi00631a032. [DOI] [PubMed] [Google Scholar]
- Hsia H. C., Lebkowski J. S., Leong P. M., Calos M. P., Miller J. H. Comparison of ultraviolet irradiation-induced mutagenesis of the lacI gene in Escherichia coli and in human 293 cells. J Mol Biol. 1989 Jan 5;205(1):103–113. doi: 10.1016/0022-2836(89)90368-9. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Li A. P., Machanoff R. A fluence response study of lethality and mutagenicity of white, black, and blue fluorescent light, sunlamp, and sunlight irradiation in Chinese hamster ovary cells. Mutat Res. 1977 Dec;45(3):333–342. doi: 10.1016/0027-5107(77)90143-9. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Riggs D. L., Negri R., Nguyen L. H., Geiduschek E. P. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol Cell Biol. 1989 Jun;9(6):2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmink J., Boelens R., Koning T., van der Marel G. A., van Boom J. H., Kaptein R. 1H NMR study of the exchangeable protons of the duplex d(GCGTTGCG).d(CGCAACGC) containing a thymine photodimer. Nucleic Acids Res. 1987 Jun 11;15(11):4645–4653. doi: 10.1093/nar/15.11.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Amaudruz F., Tyrrell R. M. Determination of the spectrum of mutations induced by defined-wavelength solar UVB (313-nm) radiation in mammalian cells by use of a shuttle vector. Mol Cell Biol. 1988 Dec;8(12):5425–5431. doi: 10.1128/mcb.8.12.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Kunz B. A., Kohalmi L., Kang X. L., Magnusson K. A. Specificity of the mutator effect caused by disruption of the RAD1 excision repair gene of Saccharomyces cerevisiae. J Bacteriol. 1990 Jun;172(6):3009–3014. doi: 10.1128/jb.172.6.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Pierce M. K., Mis J. R., Giroux C. N. DNA sequence analysis of the mutational specificity of u.v. light in the SUP4-o gene of yeast. Mutagenesis. 1987 Nov;2(6):445–453. doi: 10.1093/mutage/2.6.445. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Ultraviolet-induced reversion of cyc1 alleles in radiation-sensitive strains of yeast. I. rev1 Mutant strains. J Mol Biol. 1978 Jun 15;122(1):1–21. doi: 10.1016/0022-2836(78)90104-3. [DOI] [PubMed] [Google Scholar]
- Machida I., Saeki T., Nakai S. Effects of near-ultraviolet light on mutations, intragenic and intergenic recombinations in Saccharomyces cerevisiae. Mutat Res. 1986 Mar;160(1):11–17. doi: 10.1016/s0027-5107(96)90003-2. [DOI] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Miguel A. G., Tyrrell R. M. Induction of oxygen-dependent lethal damage by monochromatic UVB (313 nm) radiation: strand breakage, repair and cell death. Carcinogenesis. 1983;4(4):375–380. doi: 10.1093/carcin/4.4.375. [DOI] [PubMed] [Google Scholar]
- Mis J. R., Kunz B. A. Analysis of mutations induced in the SUP4-o gene of Saccharomyces cerevisiae by cis-diammine dichloroplatinum(II). Carcinogenesis. 1990 Apr;11(4):633–638. doi: 10.1093/carcin/11.4.633. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Newlon C. S. Yeast chromosome replication and segregation. Microbiol Rev. 1988 Dec;52(4):568–601. doi: 10.1128/mr.52.4.568-601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Moehring M. P., Webb R. B. Ultraviolet action spectra for DNA dimer induction, lethality, and mutagenesis in Escherichia coli with emphasis on the UVB region. Photochem Photobiol. 1984 Nov;40(5):613–620. doi: 10.1111/j.1751-1097.1984.tb05349.x. [DOI] [PubMed] [Google Scholar]
- Pierce M. K., Giroux C. N., Kunz B. A. Development of a yeast system to assay mutational specificity. Mutat Res. 1987 Apr;182(2):65–74. doi: 10.1016/0165-1161(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W. Interactions between yeast photolyase and nucleotide excision repair proteins in Saccharomyces cerevisiae and Escherichia coli. Mol Cell Biol. 1989 Nov;9(11):4767–4776. doi: 10.1128/mcb.9.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L., Glickman B. W. Mechanisms of ultraviolet-induced mutation. Mutational spectra in the Escherichia coli lacI gene for a wild-type and an excision-repair-deficient strain. J Mol Biol. 1987 Nov 20;198(2):187–202. doi: 10.1016/0022-2836(87)90305-6. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Woodhead A. D., Grist E. Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Bedoyan J., Thoma F. DNA repair in a small yeast plasmid folded into chromatin. Nucleic Acids Res. 1990 Apr 25;18(8):2045–2051. doi: 10.1093/nar/18.8.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Thoma F. Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell. 1990 May 18;61(4):675–684. doi: 10.1016/0092-8674(90)90479-x. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Okumoto D. S., Sancar A., Bohr V. A. Preferential DNA repair of (6-4) photoproducts in the dihydrofolate reductase gene of Chinese hamster ovary cells. J Biol Chem. 1989 Oct 25;264(30):18005–18010. [PubMed] [Google Scholar]
- Wang S. S., Hopper A. K. Isolation of a yeast gene involved in species-specific pre-tRNA processing. Mol Cell Biol. 1988 Dec;8(12):5140–5149. doi: 10.1128/mcb.8.12.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Becker M. M. Selective visualization of gene structure with ultraviolet light. Proc Natl Acad Sci U S A. 1988 Feb;85(3):654–658. doi: 10.1073/pnas.85.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Jordan E., Brown D. D. A bacteriophage RNA polymerase transcribes through a Xenopus 5S RNA gene transcription complex without disrupting it. Cell. 1986 Feb 14;44(3):381–389. doi: 10.1016/0092-8674(86)90459-9. [DOI] [PubMed] [Google Scholar]
- Zelle B., Reynolds R. J., Kottenhagen M. J., Schuite A., Lohman P. H. The influence of the wavelength of ultraviolet radiation on survival, mutation induction and DNA repair in irradiated Chinese hamster cells. Mutat Res. 1980 Aug;72(3):491–509. doi: 10.1016/0027-5107(80)90121-9. [DOI] [PubMed] [Google Scholar]



