Abstract.
Northwestern Argentina is endemic for soil-transmitted helminths, and annual deworming programs are carried out in prioritized areas. High prevalence of Strongyloides stercoralis was reported in this area; therefore, control programs including ivermectin are being evaluated. The NIE-enzyme linked immunosorbent assay (ELISA) was used for this purpose. In this community trial, two groups of patients, classified according to housing and living conditions were evaluated. Simultaneous with baseline survey, Group 1 was moved to new households with access to improved water and sanitation facilities (W and S), where deworming (MDA, massive drug administration) took place within 1 month; whereas Group 2 received MDA but remained living with unimproved W and S. The mean time interval between baseline and the follow-up was 331 days for Group 1 and 508 for Group 2. Anti-NIE levels were measured for each individual before and after interventions and follow-up optical density (OD) ratios were calculated to quantify the variation. A significant decrease of the anti-NIE levels between baseline and follow-up was observed in both groups. Nonetheless, the number of patients that achieved the cure criteria (OD ratio < 0.6) was higher in Group 1 than Group 2 with values of 72.7% (24/33) and 45.0% (18/40), respectively (P = 0.0197). Our results support the conclusion that a combined intervention including deworming and improvements in life conditions is more effective, in terms of the proportion of subjects cured than deworming alone. Furthermore, we found that NIE-ELISA is a useful test for assessing the response to treatment and to evaluate the outcome of control intervention programs.
INTRODUCTION
Strongyloidiasis is one of the soil-transmitted helminth (STH) infections, which are among the most common infections around the world affecting the poorest and most deprived communities frequently in developing zones.1,2 Strongyloides stercoralis infection is globally distributed, affecting an estimated 30–370 million people worldwide. It is associated with poverty and the lack of basic services and sanitary conditions and has been recognized as one of the most neglected of the neglected tropical diseases (NTDs) (not officially listed as NTDs).3–6 It is particularly endemic in tropical and subtropical areas like northern Argentina, where prevalence rates as high as 30–50% have been reported, with socioeconomic, and environmental conditions favorable for its transmission and persistence.7–9
The particularities of the life cycle of S. stercoralis, which include an autoinfective step where L3 larvae reinfect the host through the intestinal mucosa and the perianal skin, can lead to persistent and lifelong infections, which could develop into fatal complications like hyperinfection syndrome and disseminated infection. In this scenario, anthelminthic therapy should be curative; therefore, the evaluation of response to treatment and management of strongyloidiasis have become a major concern in strongyloidiasis, which needs to be studied and more deeply addressed.5,10–12
Nowadays, ivermectin is the treatment of choice for strongyloidiasis. Its superior efficacy compared with albendazole and its better safety profile compared with thiabendazole have been demonstrated. Furthermore, there is some evidence that endemic communities might benefit from regular ivermectin administration in populations where it was provided for onchocerciasis and lymphatic filariasis control, but further evaluations are needed to demonstrate the impact and effectiveness of mass drug administration addressed particularly to S. stercoralis. Currently, ivermectin donations are limited to the control of onchocerciasis and also to programs for the control of lymphatic filariasis in areas coendemic with onchocerciasis.5,13
The coproparasitological tests that are conventionally used to diagnose Strongyloides (direct smear, formalin ether concentration, Baermman, and agar plate culture) are not sensitive enough, particularly in chronic infections where a high percentage of the cases are not detected.3,12,14–16
Because of the problems with conventional parasitological tests, efforts have focused on the development and evaluation of novel serological techniques, but these show variable sensitivity and specificity depending on the source of antigen and the population tested. Enzyme-linked immunosorbent assay (ELISA) appears to be a convenient alternative for diagnosis; ELISA methods using crude antigen preparations of Strongyloides spp. achieve excellent sensitivity, but have some shortcomings, due to difficulties in antigen preparation as well as cross-reactions with other helminth infections.12,14,15,17
These tests are also being implemented in nonendemic regions for follow-up (F/U) evaluation after treatment and have shown that IgG levels against S. stercoralis tend to decline over time after treatment, but in the cases of endemic population the serological evaluation has not been evaluated in depth. Nonetheless, there is considerable variability between results depending on the antigen used, the intervals of time after treatment and the drug chosen for treatment.12,16,18–26
The development of an ELISA based on a 31-kDa recombinant antigen, NIE, isolated from a S. stercoralis L3 cDNA library, has been reported to overcome the difficulties of the crude larval extracts, beingovercome sensitive (75.4% [67.5–83.3]) without cross-reactivity with other helminthic infections (specificity 94.8% [90.7–98.9]) as well as being easily purified and produced in large scale.8,27,28 Furthermore, NIE-ELISA and luciferase immunoprecipitation system were recently evaluated as part of a clinical trial, and NIE-ELISA proved to be the best indicator of seroconversion after successful therapy compared also to an in-house indirect fluorescent antibody technique and two commercial serologic tests.29,30
In our study, the NIE-ELISA test was used to assess the serological response to treatment in endemic areas. This study was conducted as part of a deworming project against STHs in a hyperendemic area of northwestern Argentina, where annual interventions of massive drug administration (MDA) are carried out not only with albendazole (as it is commonly executed) but also with ivermectin, in the context of a research intervention carried out by the group at Universidad Nacional de Salta, the Provincial primary care program and the nongovernmental Fundacion Mundo Sano. The aim of this study was to evaluate the serological response to interventions (MDA program versus a MDA + W and S program) through NIE-ELISA among other parameters.
MATERIALS AND METHODS
This study was conducted as part of a MDA program against STH conducted by the Instituto de Investigaciones en Enfermedades Tropicales-Universidad Nacional de Salta in collaboration with Fundación Mundo Sano and the Primary Health Care System. For this program, free treatment with ivermectin (200 μg/kg of body weight) and albendazole (400 mg), both in a single dose, was delivered to the entire study population.
The study included two urban and one rural communities from Pichanal, Orán, and Tartagal, all pertaining to Salta Province. The characteristics of the area and study population were previously detailed by Echazú and others.6 From September 2010 to March 2014, fecal and venous blood samples were randomly collected (through a random generated list with the household as the unit of randomization) for coproparasitological tests (sedimentation concentration, Baermann, Harada Mori and McMaster, as described elsewhere),31 complete blood counts (CBCs), and a serological assay (NIE-ELISA).
The study population samples were classified in two groups according to the World Health Organization/United Nations International Children’s Emergency Fund recommendation for monitoring sanitation and water supply: Group 1 (G1): Simultaneous with baseline (B/L) survey, was moved to new households at 2 km from the original settlement (through a Provincial program initiated for safety reasons to accommodate a major gas duct) with access to improved water and sanitation facilities (W and S) where deworming took place within 1 month (MDA) (G1 = MDA + W and S); whereas Group 2 (G2) remained living with unimproved W and S (G2 = MDA alone). No other significant differences in terms of geographic, socioeconomic, cultural, or behavioral conditions were identified between communities in both groups.
The second (F/U) serum sample was collected for posttreatment surveillance and to evaluate the outcomes by NIE-ELISA and CBC.
NIE-enzyme linked immunosorbent assay.
The antibody levels were measured in all serum samples by an ELISA test using the recombinant antigen, NIE, for the diagnosis, estimation of prevalence of S. stercoralis infection, and to monitor treatment outcomes.
The NIE-ELISA was performed as has been previously described,32 with minor modifications. A final concentration of 0.125 μg/well/100 μL NIE antigen in buffer coating (pH = 9.6) was added to polystyrene plates and incubated at 4°C overnight, afterward, this the plate was blocked with phosphate-buffered saline (PBS)–Tween 20-5% skim milk. Sera were added at a dilution of 1:100 followed by Human anti IgG-Fc (Jackson Immuno Research, West Grove, PA) in a dilution of 1:20,000. Finally, tetramethylbenzidine substrate was added and allowed to react for 15 minutes and read at 450 nm. Each step was incubated at 37°C for 1 hour, and plates were washed five times with PBS–Tween 0.05% in between. Patient sera were tested in duplicate and means of results were calculated and corrected for background reactivity (no serum added).
The cutoff value was optimized by constructing receiver operating curves (ROCs) based on ELISA results from 20 patients with parasitological evidence of larvae as positive controls and 20 healthy controls from Salta Capital, a nonendemic area. NIE-ELISA achieved a specificity and sensibility of 85% and the area under the curve ROC = 0.92, parameter interpreted according to Swets (1988)33 as a high accuracy test. The cutoff was selected on basis of this optimal combination of sensitivity and specificity.
Follow-up.
Serum samples were stored at −20°C and tested paired in parallel on the same run to eliminate run-to-run variation. To monitor the response to the intervention, we measured the anti-NIE antibody levels (optical density [OD]) and the eosinophil count (absolute eosinophil count cell/μL) at B/L and F/U. To quantify the variation in anti-NIE antibody levels, a F/U OD ratio (ODF/U/ODB/L) was calculated as the posttreatment values divided by the B/L ones for each subject, following the criteria of Kobayashi and others.34 An OD ratio < 0.6 was considered to be an effective response to treatment (serological cure).
Statistical analysis.
For the assessment of the change between B/L and F/U, the changes in mean antibody levels (OD) were assessed by the Wilcoxon matched pairs test, and the difference in OD ratios between the two groups was analyzed by a Mann–Whitney test for intergroup comparisons of continuous data. Differences between specified patient groups were analyzed by the Kruskal–Wallis test and differences in proportions of patients who seroconverted and achieved the cure criteria (OD ratio < 0.6) were evaluated though χ2 test. Results were considered statistically significant with P < 0.05. The analyses were performed using software Prism 5 GraphPad Demo (San Diego, CA) and Info stat, version 1.1. (Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina) and EPIDAT3.1 (http://dxsp.sergas.es).
Ethical approval.
Ethical approval was obtained from the Bioethics Committee of College of Physicians of Salta Province, Argentina (N°14.200), and the Faculty of Health Sciences from the Universidad Nacional de Salta. Participation was voluntary and an informed consent was obtained from all patients involved in the study.
RESULTS
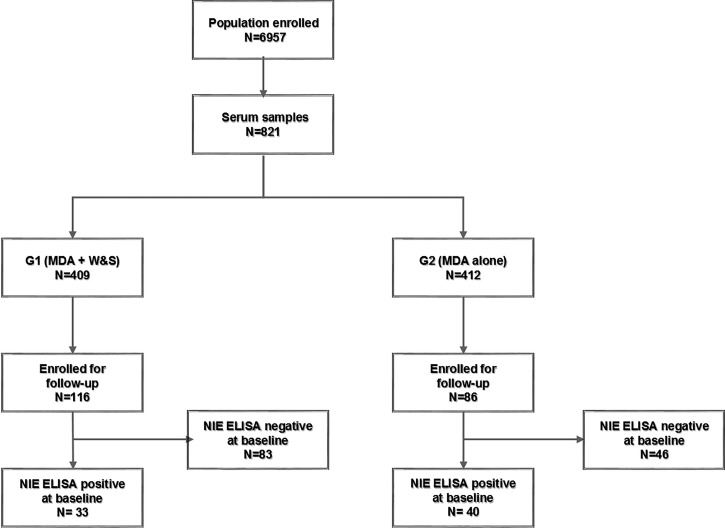
The population under treatment in the three communities included 6,957 individuals; the randomly selected survey sample group included 821 blood samples at B/L, 409 belonging to G1 and 412 to G2. Of these, the F/U group of samples included 116 and 86 individuals with paired samples in Groups 1 and 2, respectively (Figure 1). The main characteristics of the population are summarized in Tables 1 and 2.
Figure 1.
Flow chart. The flow of participants involved in the project.
Table 1.
Population monitored through NIE-ELISA with paired samples
| G1 (MDA + W and S intervention) (N = 116) | G2 (MDA intervention) (N = 86) | P value | |
|---|---|---|---|
| Age (year) | 10 (6.3–13)* | 14 (83–2.3)* | 0.0056 |
| Female sex (%) | 56.9 (66/116) | 67.4 (58/86) | 0.1452 |
| Interval between B/L and F/U (days) | 331 (330–395)* | 508 (282–549) | < 0.0001 |
| Incident cases (%)† between B/L and F/U | 1.2 (1/83) | 8.7 (4/46) | 0.0347 |
B/L = base line; ELISA = enzyme linked immunosorbent assay; F/U = follow-up; G1 = Group 1; G2 = Group 2; IQR = interquartile range; MDA = massive drug administration; W and S = water and sanitation facilities.
Median (IQR).
Based on ELISA-NIE.
Table 2.
Parasitological and hematologic findings
| G1 |
G2 |
|||||
|---|---|---|---|---|---|---|
| B/L | F/U | P value | B/L | F/U | P value | |
| Stst community seroprevalence by NIE ELISA | 20.05% (82/409) | 20.5% (104/508) | 0.8740 | 44.90% (185/412) | 42.25% (139/329) | 0.4693 |
| Stst paired samples seroprevalence by NIE ELISA | 28.4% (33/116) | 8.6% (10/116) | 0.0002 | 46.5% (40/86) | 27.9% (24/86) | 0.0176 |
| Eosinophilia (%)* | 64.3% (72/112) | 42.5% (48/113) | 0.0010 | 50.0% (42/84) | 54.8% (46/84) | 0.5366 |
| Eosinophilia count† | 593.5 (400.8–990.0) | 411.5 (211.5–931.3) | 0.0036 | 536.9 (326.5–1120) | 568.0 (354.5–905.0) | 0.7867 |
| STH‡ | 58.3% (7/12) | 11.8% (4/34) | 0.0030 | 40.9% (29/71) | 19.5 (8/41) | 0.0231 |
| Strongyloides stercoralis‡ | 16.7% (2/12) | 2.9% (1/34) | 0.1623 | 18.3% (13/71) | 4.8% (2/41) | 0.1612 |
| Hookworms‡ | 8.3% (1/12) | 2.9% (1/34) | 0.4548 | 21.1% (15/71) | 7.3% (3/41) | 0.6550 |
| Trichuris trichura‡ | 0% (0/12) | 0% (0/34) | – | 2.8% (2/71) | 0% (0/41) | 1.000 |
| Ascaris lumbricoides‡ | 41.7% (5/12) | 0% (0/34) | 0.0006 | 8.5% (6/71) | 14.6% (6/41) | 0.3507 |
B/L = base line; ELISA = enzyme linked immunosorbent assay; F/U = follow-up; G1 = Group 1; G2 = Group 2; IQR = interquartile range; STH = soil-transmitted helminth.
> 500 cell/μL.
Median (IQR).
Coproparasitologial diagnosis of subjects who had provide fecal samples.
The mean interval between B/L and the F/U measurements was 331 days for G1 and 508 days for G2. The number of patients who had a NIE-ELISA positive at B/L (from the 116 and 86 individuals with paired serum samples) was 33 for G1 and 40 for G2. See details of sample selection (Figure 1). Of the patients NIE-ELISA negative at B/L, 1.2% (1/83) became positive at the time of F/U in G1 and 8.7% (4/46) in G2; a significant difference in the incidence between groups P = 0.0347.
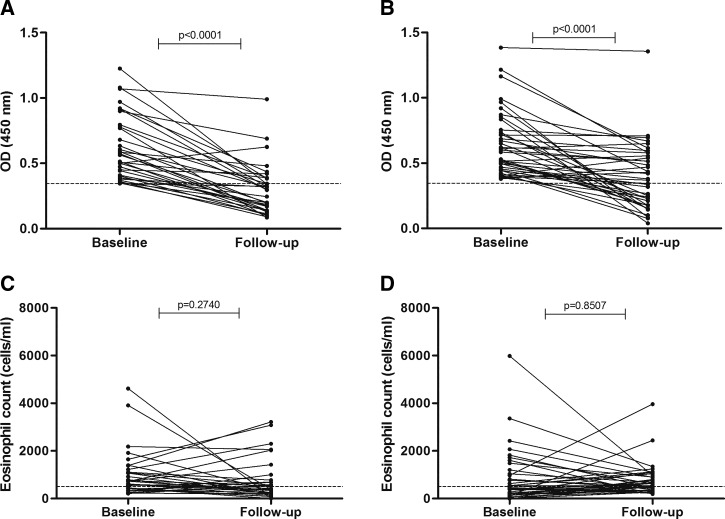
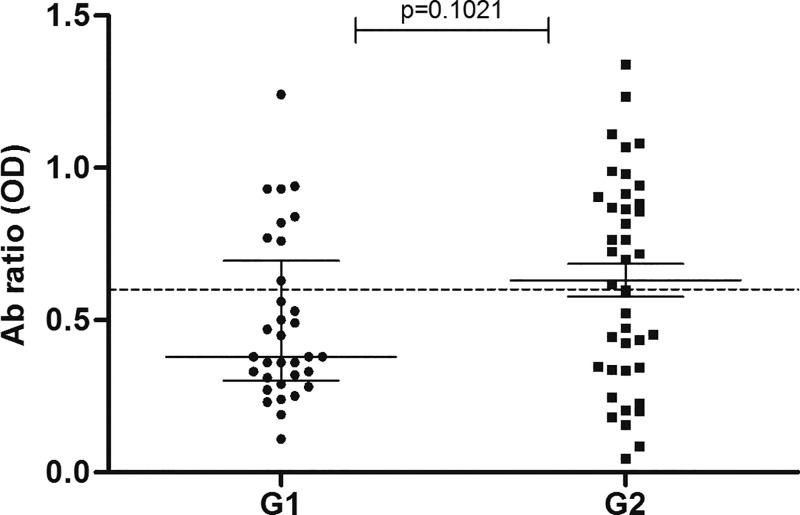
Comparing anti-NIE antibody levels between B/L and F/U within groups (B/L G1 versus F/U G1 and B/L G2 versus F/U G2), we observed a significant decrease (P < 0.0001) in both groups (Figure 2). In the comparison between groups, we found no significant difference in anti-NIE antibodies levels between B/L (B/L G1 versus B/L G2) and F/U (F/U G1 versus F/U G2) (Figure 2) or in the ratio of antibodies between groups (P > 0.05) (OD ratio G1 versus OD ratio G2) (Figure 3).
Figure 2.
Comparison of base line and follow up. Serum anti-NIE values (OD) in (A) G1 (MDA + W and S) (N = 33) and (B) G2 (MDA alone) (N = 40), the dashed line represents the cutoff value. Absolute eosinophil count before and after treatment in (C) G1 and (D) G2. GI = Group 1; G2 = Group 2; MDA = massive drug administration; OD = optical density; W and S = water and sanitation facilities.
Figure 3.
Change in antibody levels after treatment. The values are represented as OD ratio of baseline values divided by follow-up vales in G1 (MDA + W and S) and G2 (MDA alone). An OD ratio < 0.6 is considered as cure. GI = Group 1; G2 = Group 2; MDA = massive drug administration; OD = optical density; W and S = water and sanitation facilities.
Nonetheless, the cure rates were different in each group in terms of the proportion of individuals who reached the cure criteria for G1 (72.7% [24/33]) and G2 (45.0% [18/40]). Table 3 summarizes the results of the two F/U groups.
Table 3.
Compared results of patients enrolled for follow-up at G1 (MDA + W and S) and G2 (MDA alone)
| G1 (N = 33) | G2 (N = 40) | P value | |
|---|---|---|---|
| Serological cure (OD ratio < 0.6) | 72.7% (24/33) | 45.0% (18/40) | 0.0197 |
| Eosinophil count decrease | 42.4% (14/33) | 30% (12/40) | 0.3296 |
G1 = Group 1; G2 = Group 2; OD = optical density; MDA = massive drug administration; W and S = water and sanitation facilities.
We found no correlation between eosinophil count and anti-NIE Ab (OD), nor between the decrease of antibody levels and eosinophil count (OD ratio with eosinophil ratio). No association was found between the age of the subjects with the B/L levels of anti-NIE and the decrease of antibodies (OD ratio); neither with eosinophilia in both groups.
DISCUSSION
Because of the potential risk of developing fatal complications and the autoinfective cycle that allows for perpetuation of infections, strongyloidiasis requires the complete eradication of the parasite. Therefore, efforts should be directed toward evaluation of the response to therapy, especially in endemic areas like northwestern Argentina.7,8,11 The evaluation of antibody levels among population from nonendemic countries as well as migrants has shown good results, though the information available from endemic populations is still limited.16,18–25,29,34–36
In the present study, an ELISA test using the recombinant antigen NIE was applied with the aim to evaluate the serological response to interventions in groups with differential access to water and sanitation.
After treatment, both groups showed a marked decline in antibody levels and had a significant difference between the B/L and F/U time points. Given, the considerable heterogeneity in the level of reactivity among patients and the different B/L prevalence between groups, we considered that the optimal way to evaluate the change in anti NIE-antibodies between B/L and F/U was calculating a F/U ratio as was suggested by Kobayashi and others.34 Using this approach, we observed that 72.7% of G1 could be considered cured in 1 year, and 45.0% of the G2 in 508 days. On the other hand, the proportion of people who seroreverted (fall under the cutoff) was very similar to the proportion of people who reached the serological cure criteria (OD ratio < 0.6), demonstrating the consistency and robustness of the NIE-ELISA technique. Our findings support others that have shown that following therapy the serum antibodies levels decrease slowly over time.22,23,29,34
Even though the general mean OD values were not different between the groups (G1 [MDA + W and S] versus G2 [MDA alone]), the proportion of people who reached the cure criteria by NIE-ELISA test was highly different between them. These findings lead us to presume a decreased risk of reinfection in G1 as a result of the change in the living conditions regarding water and sanitation, since the same drug treatment was applied to both G1 and G2. Furthermore, the statistically significant difference in the percentage of incident cases between groups could be a result of this change in conditions. This is consistent with the association between the presence and prevalence of STH infection and unimproved water and sanitation that has been described in previous studies.6,37,38
However, there are alternative explanations for this difference that should be taken into account: for instance, the prevalence of STH and particularly S. stercoralis was different between study communities at B/L. Therefore, the risk of reinfection could be not only higher in G2 due to the lack of sanitary improvements or a larger environmental burden developed to the higher B/L prevalence but also could exist different factors not evaluated in this work that can influence the antibodies levels found at F/U.
The results in G1 are similar to the findings of two novel evaluations using NIE antigen by ELISA in nonendemic areas where the rate of cure was 71–73% between 9 and 18 months.29,39 In G2, however, the cure rate was lower than G1 despite the fact that the same treatment was applied. An alternative explanation to this difference, which constitutes a limitation of this study is the longer interval between B/L and F/U in G2, which could potentially allow more time for reinfection. The differences in the intervals between surveys should be understood in the context of a substudy performed within a large intervention trial guided by the times and priorities of the Provincial Primary Care service.
F/U time is another factor that affects the treatment outcome, and it is difficult to define an appropriate time interval to measure antibody and detect a significant decrease in antibody level. Most authors assessed a F/U period of 9–21 months,18,19,21–23,29,34 and observed that the largest drop occurs between the 6 and 9 months, suggesting that ideal F/U time could be 6–12 months. An evaluation of the NIE-ELISA against another four tests with a composite reference standard, through the application of a mixed model for predicting the drop of antibodies, supports the conclusion that 1 year is necessary to observe seroreversion.29
This is similar to the findings in the G1 after approximately 331 days after treatment where the life conditions were improved. Here, 72.7% of patients got cured, whereas in G2 it was only 45%. Nonetheless in this work, it was not possible to ensure that the reinfection factor was avoided in either group and it would be necessary in future works to have a better appreciation of the precise drug efficacy without having reinfections as a confounding factor.
Despite the fact that eosinophil count has a good positive predictive value, and some studies found an association between a decrease in the eosinophil count, decrease of antibodies, and cure,19,20,35 our results differ from those studies. Our study population is endemic for STH and other nematode infections such as toxocariasis7; therefore, the elevated and persistent eosinophilia could be triggered by parasites other than S. stercoralis. Besides, a good comparison between groups was not possible since the G2 had a higher number of people with polyparasitosis than G1.
This study has some limitations due the heterogeneous ages of the patients between groups; and mainly due to the loss of subjects for the time of F/U since the study area is a border area with an active labor-related migration. Because of the nature of this work, we were able to have only one follow-up point and we could not obtain fecal samples of all the subjects of study. It would be ideal for further studies to extend the F/U period and the number of collection times to ensure that each patient had a serological and coproparasitological control.
The progress in the diagnosis and treatment monitoring of S. stercoralis infection, along with a better understanding of the benefit of sanitary improvements, would contribute to the creation of guidelines for the control of endemic strongyloidiasis. In this study, we observed a marked decline in anti-NIE antibody levels following deworming. Our results support the body of evidence that serology is useful for the evaluation of interventions and treatment outcomes. The use of NIE-ELISA as a tool to evaluate the serological response to treatment in endemic areas is feasible and represents a potentially significant contribution to improve the monitoring of public health interventions against S. stercoralis.
Acknowledgments:
We thank to the Primary Health Service of the Province of Salta for the collaboration. Also we thank Thomas Nutman, National Institute of Health (NIH), Bethesda, MD, for gently conceding the NIE antigen for this work.
REFERENCES
- 1.Socias E, Fernandez A, Gil JF, Krolewiecki AJ, 2014. Geohelmintiasis en la Argentina una Revisión Sistemática. Med(Buenos Aires) 74: 29–36. [PubMed] [Google Scholar]
- 2.World Health Organization , 2011. The Soil Transmitted Helminth Infections. Geneva, Switzerland: World Health Organization, 366. [Google Scholar]
- 3.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo SS, Verweij JJ, Magnussen P, 2009. Strongyloidiasis: the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103: 967–972. [DOI] [PubMed] [Google Scholar]
- 4.Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, Gotuzzo E, Mena MA, Chiodini PL, Anselmi M, Moreira J, Albonico M, 2013. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7: e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albonico M, Becker S, Odermatt P, Angheben A, Anselmi M, Amor A, Barda B, Buonfrate D, Cooper P, Gétaz L, Keiser J, Khieu V, Monster A, Muñoz J, Requena-Méndez A, Savioli L, Speare R, Steinmann P, van Lieshout L, Utzinger J, Bisoffi Z, StrongNet Working Group , 2016. StrongNet: an International network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 10: e0004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echazú A, Bonanno D, Juarez M, Cajal SP, Heredia V, Caropresi S, Cimino RO, Caro N, Vargas PA, Paredes G, Krolewiecki AJ, 2015. Effect of poor access to water and sanitation as risk factors for soil-transmitted helminth infection: selectiveness by the infective route. PLoS Negl Trop Dis 9: e0004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taranto NJ, Cajal SP, De Marzi MC, Fernández MM, Frank FM, Brú AM, Minvielle MC, Basualdo JA, Malchiodi EL, 2003. Clinical status and parasitic infection in a Wichí Aboriginal community in Salta, Argentina. Trans R Soc Trop Med Hyg 97: 554–558. [DOI] [PubMed] [Google Scholar]
- 8.Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, Juarez M, Di Paolo A, Tapia L, Acosta N, Lee R, Lammie P, Abraham D, Nutman TB, 2010. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol 17: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altcheh J, Fernandez G, Guarnera E, Gutierrez N, Pizzi H, Taranto N, 2007. Geohelmintiasis en la Republica de Argentina. Buenos Aires, Argentina: REMEDIAR-Minist Salud la Nación, 1–116. [Google Scholar]
- 10.Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli A-F, Levecke B, Socias E, Arias LM, Sosa N, Abraham D, Cimino R, Echazú A, Crudo F, Vercruysse J, Albonico M, 2013. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis 7: e2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mejia R, Nutman TB, 2012. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis 25: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J, 2013. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7: e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriquez-Camacho C, Gotuzzo E, Echevarria J, Clinton White A, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN, 2016. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev, doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levenhagen M a, 2014. Costa-Cruz JM. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Trop 135: 33–43. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui AA, Berk SL, 2001. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 16.Conway DJ, Lindo JF, Robinson RD, Bundy DAP, 1995. Towards effective control of Strongyloides stercoralis. Parasitol Today 11: 420–423. [Google Scholar]
- 17.Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, Chen X, 2014. Strongyloidiasis: an insight into its global prevalence and management. PLoS Negl Trop Dis 8(8): e3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato Y, Kobayashi J, Shiroma Y, 1995. Serodiagnosis of strongyloidiasis. The application and significance. Rev Inst Med Trop Sao Paulo 37: 35–41. [DOI] [PubMed] [Google Scholar]
- 19.Loutfy MR, Wilson M, Keystone JS, Kain KC, 2002. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg 66: 749–752. [DOI] [PubMed] [Google Scholar]
- 20.Wehner J, Kirsch C, Kagawa F, Jensen W, Campagna A, Wilson M, 1994. The prevalence and response to therapy of Strongyloides stercoralis in patients with asthma from endemic areas. Chest 106: 762–766. [DOI] [PubMed] [Google Scholar]
- 21.Page WA, Dempsey K, McCarthy JS, 2006. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans R Soc Trop Med Hyg 100: 1056–1062. [DOI] [PubMed] [Google Scholar]
- 22.Karunajeewa H, Kelly H, Leslie D, Leydon J, Saykao P, Biggs B, 2006. Parasite-specifi c IgG response and peripheral blood eosinophil count following albendazole treatment for presumed chronic strongyloidiasis. J Travel Med 13: 84–91. [DOI] [PubMed] [Google Scholar]
- 23.Biggs B, Caruana S, Mihrshahi S, Jolley D, Leydon J, Chea L, Nuon S, 2009. Short report: management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hygeine 80: 788–791. [PubMed] [Google Scholar]
- 24.Salvador F, Sulleiro E, Sánchez-Montalvá A, Saugar JM, Rodríguez E, Pahissa A, Molina I, 2014. Usefulness of Strongyloides stercoralis serology in the management of patients with eosinophilia. Am J Trop Med Hyg 90: 830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, Gobbo M, Bisoffi G, Gobbi F, 2011. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl Trop Dis 5: e1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindo JF, Atkins NS, Lee MG, Robinson RD, Bundy DAP, 1996. Short report: Long-term serum antibody isotype responses to Strongyloides stercoralis filariform antigens in eight patients treated with ivermectin. Am J Trop Med Hyg 55: 474–476. [DOI] [PubMed] [Google Scholar]
- 27.Ravi V, King TP, Andersen JF, Nutman TB, Neva FA, 2005Strongyloides stercoralis recombinant NIE antigen shares epitope with recombinant Ves v 5 and Pol a 5 allergens of insects. Am J Trop Med Hyg 72: 549–553. [PubMed] [Google Scholar]
- 28.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB, 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis 198: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Muñoz J, Nutman TB, 2015. Accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Muñoz J, Nutman TB, 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia LS, 2007. Diagnostic Medical Parasitology FAQ’s. Spinboro, OH: Hardy Diagnostics. [Google Scholar]
- 32.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA, 2002. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol 125: 73–81. [DOI] [PubMed] [Google Scholar]
- 33.Swets JA, 1988. Measuring the accuracy of diagnostic systems. Science 240: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi J, Sato Y, Toma H, Takara M, Shiroma Y, 1994. Application of enzyme immunoassay for post chemotherapy of human strongyloidiasis. Diagn Microbiol Infect Dis 18: 19–23. [DOI] [PubMed] [Google Scholar]
- 35.Grove DI, 1982. Treatment of strongyloidiasis with thiabendazole: an analysis of toxicity and effectiveness. Trans R Soc Trop Med Hyg 76: 114–118. [DOI] [PubMed] [Google Scholar]
- 36.Satoh M, Toma H, Sato Y, Kikuchi M, Takara M, Shiroma Y, Kiyuna S, Hirayama K, 1999. Production of a high level of specific IgG4 antibody associated with resistance to albendazole treatment in HLA-DRB1*0901-positive patients with strongyloidiasis. Am J Trop Med Hyg 61: 668–671. [DOI] [PubMed] [Google Scholar]
- 37.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J, 2012. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 9: e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC, 2014. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med 11: e1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rascoe LN, Price C, Shin SH, McAuliffe I, Priest JW, Handali S, 2015. Development of Ss-NIE-1 recombinant antigen based assays for immunodiagnosis of strongyloidiasis. PLoS Negl Trop Dis 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]